Back to Journals » Journal of Inflammation Research » Volume 17
Comparison of Cytokine RANTES/CCL5 Inflammation in Apical Periodontitis and in Jawbone Cavitations – Retrospective Clinical Study
Authors Vasconcelos e Cruz J , Notter F, Schick F, Lechner J
Received 20 October 2023
Accepted for publication 28 December 2023
Published 5 January 2024 Volume 2024:17 Pages 67—80
DOI https://doi.org/10.2147/JIR.S442693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Joana Vasconcelos e Cruz,1,2 Florian Notter,3 Fabian Schick,3 Johann Lechner4
1Dental Materials, Egas Moniz School of Health & Science, Caparica, Portugal; 2Dental Materials, Centro de Investigação Interdisciplinar Egas Moniz (CiiEM), Caparica, Portugal; 3Dental Surgeon, Clinic for Integrative Dentistry, Munich, Germany; 4Head, Clinic for Integrative Dentistry, Munich, Germany
Correspondence: Johann Lechner, Gruenwalder Str. 10A, Munich, 81547, Germany, Tel +49 89 697 0129, Fax +49 89 692 5830, Email [email protected]
Background: Apical periodontitis (AP) is one of the most common endodontic diseases associated with osteo destructive cytokine production. The literature also reports cytokine studies in fatty degenerative osteonecrotic bone marrow defects (BMDJ/FDOJ) independent of AP.
Objective: We compare the RANTES/CCL5 (R/C) chemokine production between AP and BMDJ/FDOJ. For both pathologies, the R/C expression was also compared to radiographic diagnosis in 2D-OPG, 3D-CBCT/DVT.
Material and Methods: Postoperative samples were collected and divided in three different groups: HB (healthy jawbone) (n=19), APs (n=19), and BMDJ/FDOJ (n=7). The R/C expression was evaluated using multiplex analysis. In addition, two clinical cases from AP and BMDJ/FDOJ groups were randomly selected and radiographic diagnosis in 2D-OPG and 3D-CBCT/DVT was compared to TAU measurements and R/C expression in AP and in BMDJ/FDOJ.
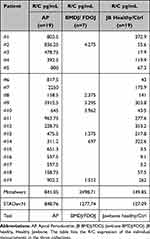
Results: BMDJ/FDOJ showed the highest R/C expression (2498.71 pg/mL), followed by AP (841.85 pg/mL) and HB (149.85 pg/mL) (AP vs BMDJ/FDOJ = p=0.01; AP vs HB = p=< 0.01; BMDJ/FDOJ vs HB = p=< 0.01). In both clinical cases, the radiographic findings depict the AP areas in OPG and CBCT/DVT, in contrast to the BMDJ/FDOJ areas. Conversely, the systemic immunological R/C expressions are threefold and fivefold excessive in both cases.
Discussion: AP is recognized as a pathology requiring treatment, while the pathogenesis of BMDJ/FDOJ is controversially discussed in the literature, despite stronger potential systemic immunological effects (breast cancer (case 1) and multiple sclerosis (case 2)). The inadequate radiographic representation of reduced bone density in BMDJ/FDOJ areas could be a reason for this contradiction.
Conclusion: The data presented provide the first quantitative analysis of R/C expression in AP and BMDJ/FDOJ. BMDJ/FDOJ showed high R/C expression than AP, besides the diagnostic through radiographs being extremely poor. To cover this imprecision, a radiation-free TAU device is available.
Keywords: bone marrow, RANTES/CCL5, apical periodontitis, osteonecrosis, dental radiography, ultrasonography
Graphical Abstract:
Introduction
Apical periodontitis (AP) is one of the most common endodontic diseases that dentists and endodontists are confronted with every day in their practice. Studies show more than 200 thousand root canals are being performed every year in a European country.1 Modern sophisticated radiology shows these osteolytic apical processes very accurately and Cone-beam computed tomography/Digital volume tomography (CBCT/DVT) even more so. AP is therefore a recognised pathological phenomenon and its removal or healing is one of the fundamental tasks in dentistry.2 However, AP is a very complex pathology with many factors involved. It is well known in dental research that the interactions of various inflammatory molecules and messengers can influence and alter the condition and progression of the disease and contribute to the degradation of the periradicular tissue. Therefore, the periapical inflammatory response provides a model suitable for studying the pathogenesis of many systemic diseases where the regulation of cytokine expression plays an important role.3
Studies of cytokine production, cytokine function, and periodontal inflammation specific to chemokine RANTES also known as C–C Motif Chemokine Ligand 5 (CCL5) expression (R/C) in periodontium have been found.4–8 While R/C expression in periodontally damaged tissue has been widely studied in the recent literature,4–8 corresponding studies of R/C levels in cysts and granulomas have been lacking. At the same time, research shows that there is no significant difference in cytokine expression between cysts and granulomas, but there is a clear difference compared to healthy gingiva.9 Therefore, some authors group cysts and granulomas together under the term “apical periodontitis” (AP).9 Notably, numerical data on the levels of R/C expression in AP are lacking, notwithstanding several studies have demonstrated the osteo- destructive presentation of AP through chemokine expression.7,8 In pathologies such as periapical tissue destruction, which are usually defined as non-acutely infected granulomas, to the best of our knowledge, multiplex testing for R/C expression has never been performed.
However, there are increasing reports in the literature of bone marrow defects in jawbone (BMDJ) and fatty degenerative osteonecrotic jawbone (FDOJ) that show singularly high expressions of the proinflammatory chemokine R/C in multiplex analyses.10–12
The study aims to answer some questions:
- The R/C expression is different between AP and BMDJ/FDOJ.
- The BMDJ/FDOJ pathologies are under-estimated in R/C expression or neglected in comparison to the pathogenic relations of an AP.
- The different clinical diagnosis and imaging obtained on the RX can justify the lack of BMDJ/FDOJ diagnosis.
- Are conventional radiographic methods a suitable way of diagnosing BMDJ/FDOJ pathologies?
Materials and Methods
Study Participants
In order to answer the questions raised above, three groups were created:
- Group 1: HB (healthy bone) – Control group, where it was collected 19 postoperative samples of healthy jawbones and the R/C expression was analysed.
- Group 2: AP (Apical Periodontitis) – Experimental group, where 19 apical lesions were collected and the R/C expression was analysed.
- Group 3: BMDJ/FDOJ – Experimental group, where seven BMDJ/FDOJ lesions were collected and the R/C expressions were analysed.
In addition, two clinical cases of groups 2 and 3 were randomly selected and the radiographic diagnostics in 2D-OPG and 3D-CBCT/DVT, the measurements obtained in TAU and R/C expression in AP and in BMDJ/FDOJ were compared. The clinical samples were provided by patients undergoing surgical treatment at the authors’ clinic. Each patient expressed an interest in determining whether chronic inflammation in the jawbone was present and, if so, associated with a pre-existing chronic immune disorder or systemic disease.
The present study was carried out as a retrospective case–control study and was classified as such by the Institute for Medical Diagnostics, Nikolaistr. 22, D-12247 Berlin (IMD-Berlin), according to DIN EN 15198/DIN EN 17025 and received exemption. All patients provided their written informed consent (as outlined in the PLOS consent form), to participate in this study. The study presented here is patient-centered; the samples and data were obtained directly in the course of routine clinical practice and the normal medical care of the patients and evaluated retrospectively. Institutional approval was not required to publish the case details. This study was conducted in accordance with the Declaration of Helsinki.
The samples and data were obtained during routine clinical practice, and normal medical care of the patients was performed. Medication used to alleviate any sensitivities was not stopped. The use of medications to treat systemic diseases was not considered an exclusion criterion. The exclusion criteria included the use of cortisone and bisphosphonates due to their effects on bone metabolism. The samples analyzed were collected during normal procedures of an oral surgery performed at the Clinic for Integrative Dentistry, Munich, Germany. Healthy jawbone samples were collected during the drill procedure for an implant placement and with patient consent.
The presence of BMDJ/FDOJ and AP was evaluated preoperatively for each patient through an OPG (orthopantomography), Cone-beam computed tomography/Digital volume tomography (CBCT/DVT), and trans-alveolar ultrasound device (TAU).
Cytokine Analysis (R/C Expression)
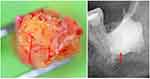
AP and BMDJ/FDOJ samples (Figure 1) were extracted to a volume of up to 0.5 cm3 and stored in a sterile collection tube (Starstedt Micro-Tube Ref. 72.692.005) right after extraction. Tubes were sealed and kept at −20°C until shipment to the laboratory (IMD berlin, Germany). Tissue samples are mechanically processed upon arrival at the laboratory, taken up in 200 µL of protease buffer (Complete Mini Protease Inhibitor Cocktail, Roche, D), and homogenized. The homogenate was centrifuged for 15 min at 13,400 rpm; the supernatant was removed and centrifuged for additional 25 minutes at 13,400 rpm. Millipore Human Cytokine/Chemokine Panel I (MPXHCYTO-60K, Millipore GmbH, Schwalbach, D) is used, according to manufacturer’s protocol, to determine R/C concentration from the supernatant of the tissue homogenate. Luminex 200 TMcombined with xPotent© software (Luminex, Austin, TX, USA) is used to perform the results readout.
Clinical Cases Analysis
Two clinical cases were randomly selected from groups 2 and 3 and the clinical history of each patient was collected. The complementary diagnosis methods used were OPG, CBCT/DVT, and TAU. We observed and compared the R/C expression results obtained from the collected samples (AP and FDOJ).
To illustrate the clinical differences in the representation of chronic osteo destructive processes in the jawbone, we contrast the R/C expressions in AP and in BMDJ/FDOJ with the corresponding radiographs and TAU measurements of bone density in two cases.
Case #1
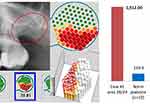
The first case describes a patient with newly diagnosed breast cancer (BC) on the left side. The comparison of the radiographic image of the apical region of 26 teeth and the edentulous alveolar region at 28/29 teeth clearly documents the different pathological imaging of OPG and CBCT/DVT (Figure 2). Here, the CBCT/DVT shows the AP at tooth 26 and the dissolution of the cancellous bone structure in area of 28 teeth. As part of the surgical restoration, tooth 26 was removed, area 28/29 was surgically freed from fatty degenerative osteolysis and both the AP from 26 teeth and a sample of the BMDJ/FDOJ from 28/29 were sent to the laboratory for multiplex analysis according to the procedures described above.
Case #2
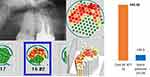
The case #2 describes a patient with a suspected diagnosis of multiple sclerosis (MS). The comparison of the radiographic image of the apical region of 16 teeth and the edentulous alveolar region at 18/19 extracted teeth clearly documents the different pathological imaging of OPG and CBCT/DVT (Figure 3). The CBCT/DVT shows the AP in tooth 16 and the partial dissolution of the cancellous bone structure in area 18.
As part of the surgical reconstruction, tooth 16 was removed, area 18/19 was surgically cleared of fatty degenerative osteolysis and both the AP and a sample of BMDJ/FDOJ from 18/19 were sent to the laboratory for multiplex analysis.
Statistical Analysis
All statistical procedures were performed using statistical software IBM SPSS version 26.0 (IBM Corporation, Armonk, NY, USA). Descriptive statistical analyses of the R/C expression in Group 1 (HB), Group 2 (AP) and Group 3 (BMDJ/FDOJ) were performed. Assessment of whether non-parametric or parametric testing would be more appropriate for the analysis was performed through analysis of data distribution, means, and medians. Mann–Whitney U-test for independent samples was used to determine the differences between groups. All tests were conducted at a significance level of 1%.
Results
Comparisons of R/C Expression Results Between Group 1 (HB), Group 2 (AP) and Group 3 (BMDJ/FDOJ)
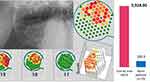
Figure 4 shows the results of the R/C multiplex analysis (pg/mL) of group 1 (HB n=19), group 2 (AP n=19) and group 3 (BMDJ/FDOJ n=7) where BMDJ/FDOJ presented a significantly higher R/C expression compared to the other groups (HB p<0.01; AP p=0.01). Figure 5 compares the distribution and scatter of the individual measurements among the groups. Table 1 lists the mean values and standard deviations (SD ±) of the R/C expression of the individual measurements in all samples collected.
 |
Figure 4 Results of the R/C multiplex examination (pg/mL) of 19 AP samples (in blue), of 7 BMDJ/FDOJ samples (in yellow) and of 19 healthy jaw bones (in green). |
Results and Comparison of R/C Expression in AP and BMDJ/FDOJ in Two Clinical Cases
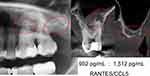
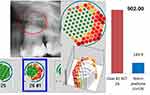
Case #1 shows a notable discrepancy: although radiographic imaging of the alveolar areas at 28/29 shows no clear pathology, R/C expression results revealed a significantly higher value (1512 pg/mL) than in the apical AP sample of the 26 teeth (902 pg/mL) with p=0.01 (Figure 2). Figure 6 summarises all three levels of findings (OPG + TAU + R/C) for the 26 teeth comparatively with healthy bone, while Figure 7 represents the alveolar region at 28/29.
Case #2 also shows a notable discrepancy: although radiographic imaging of the alveolar areas at 18/19 shows no clear pathology, R/C expression shows a significantly higher value (3928 pg/mL) than in the apical AP sample of 16 teeth (645 pg/mL) (Figures 3, 8 and 9).
Discussion
AP is recognized as an inflammatory pathology characterized by an increase in inflammatory cytokines that requires treatment. Its systemic effects, which are related to irritations of the innate and acquired immune systems, are extensively discussed in the literature.4–9
One of the cytokines present in AP lesions is R/C. R/C is a chemokine protein that plays a crucial role in immune system regulation and inflammation. R/C is involved in recruiting immune cells, such as T cells and monocytes, to sites of infection or injury within the body and also interacting with specific receptors on immune cells initiates signaling pathways that contribute to immune responses and various inflammatory processes.4–9
Several studies have been published in the literature where they demonstrate that the jawbone can contain degenerative osteonecrotic lesions (FDOJ) characterized by being an important source of R/C overexpression,10,13,14 however, the AP continues to be more studied and given greater emphasis in dentistry community.4–9
Elevated expression of R/C has been observed in various systemic disorders, including autoimmune diseases, atherosclerosis, and certain viral infections. In addition, the relationship between breast cancer and R/C has already been studied and published in the literature.15,16 Thus, the hypothesis that FDOJ may serve as a trigger of BC progression through R/C overexpression is set by the authors, who thus inspire clinicians to be aware of FDOJ in BC cases.
Regarding the MS, we also found some evidence link with this systemic disease and “silent inflammation” derived from medullary degeneration in the jawbone.17–19 The authors suspect that BMDJ/FDOJ may serve as a trigger for MS progression via R/C overexpression. As such, the dental and medical communities should be made aware of BMDJ/FDOJ in cases of MS.17–19
Understanding the intricate involvement of R/C in systemic diseases and its connection to maxillary bone expression provides valuable insights for potential diagnostic and therapeutic strategies aimed at mitigating the impact of these conditions.
In the present study, we compare these expression levels in postoperatively collected samples from AP with those from BMDJ/FDOJ areas.
Our data support the notion that multiplex studies of AP and BMDJ/FDOJ pathologies exhibit significant R/C expression. However, the data also indicate that the intensity of R/C expression is three times higher in BMDJ/FDOJ compared to AP (2498.71 vs 841.05). In contrast to these findings, the systemic disease relevance of BMDJ/FDOJ and the destruction it causes are still largely unrecognized and disregarded in mainstream medicine and dentistry.
These surprising results led us to question why there has not been given relevance to BMDJ/FDOJ pathology in the dental community? This study aims to clarify this issue and seeks to analyse through two clinical cases if the difficulty in diagnosis could be one of the attributed causes. No one doubts the existence of osteodestructive AP pathologies including their possible systemic immunological links. The need for their therapeutic removal is undisputed due to the associated proven health risk via cytokine and chemokine expression. In contrast to the clear radiographic visualisation of AP processes, BMDJ/FDOJ processes are not10–12 or difficult to diagnose in conventional 2D and 3D radiology.20 Their existence has therefore been under discussion for decades.21 The possible pathogenetic effects of an R/C signalling cascade chronically expressed from local BMDJ/FDOJ areas in its scientifically proven systemic inflammatory induction are ignored or misjudged in medical and dental diagnoses. The comparison of AP diagnosis and pathology and BMDJ/FDOJ diagnosis and pathology shows a clear imbalance to the disadvantage of the BMDJ/FDOJ processes, although the systemically-immunologically relevant R/C expression in the BMDJ/FDOJ areas is considerably higher in the data we collected, by a factor of 3, than in the AP processes. The reason for this obviously lies in the unequal intensity of representation of both processes in dental radiographs: While the existence and potential pathology of the AP are undisputed due to the clear radiographic representation, this does not apply to the BMDJ/FDOJ.
Why is radiography continuing to be the only accepted medium of diagnosis? We need to improve our diagnostic tools for these highly damaging systemic pathologies. We should no longer look at AP as a local inflammation as R/C affects the whole body. In addition, if the X-ray and DVT images are the only diagnostic method, probably some initial AP could go unnoticed.
In order to close the diagnostic gap in the detection of BMDJ/FDOJ processes revealed here, it is therefore obvious to use an additional measurement technique to objectively visualise the cryptic BMDJ/FDOJ areas that are difficult to locate with radiography alone. There are already numerous scientific studies and publications on the effectiveness and practical applicability of transalveolar ultrasound sonography (TAU).22–24 With the development of a novel TAU device, this diagnostic gap could be closed.25,26 The application of the TAU device opens up insight into potential BMDJ/FDOJ pathologies that have a stronger systemic-immunological effect than AP but still lead a pathogenetic shadow existence in the context of osteo destructive and immunologically relevant processes in the jawbone.
The clinically proven TAU device generates an ultrasonic pulse by an extraoral transmitter and guides the pulse through the jawbone, which is then recorded and measured by an ultrasound receiver intraorally.
There is a widespread “diagnostic gap” in dentistry in the recognition of chronic inflammatory BMDJ/FDOJ areas with over-regulated R/C chemokine functions. Measurement of bone density with ultrasounds, in contrast to OPG and CBCT/DVT radiographic procedures, provides objective imaging of reduced bone density in the BMDJ/FDOJ areas. Evaluating jawbone density is the primary function of ultrasounds with the advantage of being a radiation-free tool.
Conclusions
To the best of our knowledge and considering the limitations of this study, we can conclude that:
- Besides the AP, there are other processes in the jaw that are characterised by increased R/C expression. These sources of chronically overdriven R/C expression are chronic osteolytic-destructive bone areas with reduced bone density (BMDJ/FDOJ), which has been little recognised or disputed to date.21
- The possible pathogenetic underestimation of these BMDJ/FDOJ areas with increased R/C expression is due to the insufficient radiographic visualisation – also in CBCT/DVT - of these bone marrow defects.20
- Radiographically clearly visible AP resulting in bone destruction is recognised as systemic inflammatory areas with cytokine expression and function and is therefore generally recognised as oral pathologies and therapeutically cleared in the health interest of the patient.
- In contrast, BMDJ/FDOJ areas are not recognised as pathologies worthy of treatment, although our data show that R/C expression in BMDJ/FDOJ areas can be several times higher than in AP.
- If the systemic-immunological association of local R/C expressions is taken into consideration (Case #1 breast cancer/Case #2 multiple sclerosis) BMDJ/FDOJ areas may negatively affect health more than AP processes.27–29
The data shown here confirm our hypothesis that therapy based only on radiographic procedures (Periapical X-ray, OPG, and CBCT/DVT) is more likely to promote treatments that only remove a subordinate AP pathology, leaving far more pathogenically potent BMDJ/FDOJ areas. Ideally, all forms should be considered: X-rays, CBCT/DVT, and TAU as well as multiplex analysis to confirm the presence of R/C. We welcome good endodontic therapy that, when properly performed, leaves no image on X-ray, CBCT/DVT, or TAU, as well as R/C levels within the norm. These tests should be done around previously treated teeth for hidden and undetected AP pathologies.
Besides these limitations, this work demonstrates the existence of BMDJ/FDOJ processes and raises questions regarding their health relevance. Also, the study emphasizes the difficulty of the diagnosis of this pathology, which could be the reason for their misrecognition by medicine and dentistry. New approaches and the development and spread of new diagnostic tools like ultrasound are completely needed.
Also, further investigations and larger multi-centre studies are necessary to achieve even more evidence regarding the issues raised.
Acknowledgments
The authors thank all patients who participated in this study, as well as all the staff who contributed directly or indirectly to the study.
Disclosure
CaviTAU® (Munich, Germany), the company that designed the new TAU-n apparatus and associated software, provided these tools without charge for the purposes of this study. The ultrasonography procedure was carried out at the Clinic for Integrative Dentistry Munich. CaviTAU® and the Clinic for Integrative Dentistry are in ongoing discussions regarding numerous collaborative arrangements to further improve and verify the new TAU apparatus, CaviTAU®, as it is introduced to the market. The corresponding author is the holder of a patent used in CaviTAU®. Dr Johann Lechner reports non-financial support from DDHT, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Bjørndal L, Reit C. The annual frequency of root fillings, tooth extractions and pulp-related procedures in Danish adults during 1977–2003. Int Endodontic J. 2004;37(11):782–788. doi:10.1111/j.1365-2591.2004.00879.x
2. Orstavik D. Radiographic evaluation of apical periodontitis and endodontic treatment results: a computer approach. Int Dent J. 1991;41(2):89–98. PMID: 2032742.
3. Braz-Silva PH, Bergamini ML, Mardegan AP, et al. Inflammatory profile of chronic apical periodontitis: a literature review. Acta Odontol Scand. 2019;77:3, 173–180. doi:10.1080/00016357.2018.1521005
4. Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79(8):1548–1555. doi:10.1177/00220345000790080401
5. Gemmell E, Carter CL, Seymour GJ. Chemokines in human periodontal disease tissues. Clin Exp Immunol. 2001;125(1):134–141. doi:10.1046/j.1365-2249.2001.01511.x
6. Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res. 2001;36(3):194–203. doi:10.1034/j.1600-0765.2001.360309.x
7. Garlet G, Martins W
8. Emingil G, Atilla G, Hüseyinov A. Gingival crevicular fluid monocyte chemoattractant protein-1 and RANTES levels in patients with generalized aggressive periodontitis. J Clin Periodontol. 2004;31:829–834. doi:10.1111/j.1600-051X.2004.00584.x
9. Hadziabdic N, Kurtovic-Kozaric A, Frkatovic A, et al. Quantitative analysis of CCL5 and ep300 in periapical inflammatory lesions. Acta Medica Academica. 2019;48(2):129–139. doi:10.5644/ama2006-124.251
10. Lechner J, Rudi T, von Baehr V. Osteoimmunology of tumor necrosis factor-alpha, IL-6, and RANTES/CCL5: a review of known and poorly understood inflammatory patterns in osteonecrosis. Clin Cosmetic Invest Dentistry. 2018;10:251–262. doi:10.2147/CCIDE.S184498
11. Lechner J, Schulz T, von Baehr V. Immunohistological staining of unknown chemokine RANTES/CCL5 expression in jawbone marrow defects—osteoimmunology and disruption of bone remodeling in clinical case studies targeting on predictive preventive personalized medicine. EPMA Journal. 2019;1–14. doi:10.1007/s13167-019-00182-1
12. Lechner J, von Baehr V, Zimmermann B. osteonecrosis of the jaw beyond bisphosphonates: are there any unknown local risk factors? Clin Cosmet Investig Dent. 2021;13:21–37. doi:10.2147/CCIDE.S288603
13. Lechner J, von Baehr V. Hyperactivated signaling pathways of chemokine RANTES/CCL5 in osteopathies of jawbone in breast cancer patients—case report and research. Breast Cancer. 2014;8:89–96. doi:10.4137/BCBCR.S15119
14. Lechner J, Schuett S, von Baehr V. Aseptic-avascular osteonecrosis: local “silent inflammation” in the jawbone and RANTES/CCL5 overexpression. Clin Cosmetic Invest Dentistry. 2017;9:99–109. doi:10.2147/CCIDE.S149545
15. Walens A, DiMarco AV, Lupo R, Kroger BR, Damrauer JS, Alvarez JV. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. Elife. 2019;8:e43653. PMID: 30990165; PMCID: PMC6478432. doi:10.7554/eLife.43653
16. Derossi DR, Amarante MK, Guembarovski RL, et al. CCL5 protein level: influence on breast cancer staging and lymph nodes commitment. Mol Biol Rep. 2019;46(6):6165–6170. PMID: 31691056. doi:10.1007/s11033-019-05051-8
17. Hvas J, McLEAN C, Justesen J. Perivascular T cells express the pro-inflammatory chemokine RANTES mRNA in multiple sclerosis lesions. Scand J Immunol. 1997;46(2):195–203. doi:10.1046/j.1365-3083.1997.d01-100.x
18. Lechner J, von Baehr V, Schick F. RANTES/CCL5 signaling from jawbone cavitations to epistemology of multiple sclerosis – research and case studies. Degener Neurol Neuromuscul Dis. 2021;11:41–50. doi:10.2147/DNND.S315321
19. Pittaluga A. CCL5-glutamate cross-talk in astrocyte-neuron communication in multiple sclerosis. Front Immunol. 2017;8:1079. PMID: 28928746; PMCID: PMC5591427. doi:10.3389/fimmu.2017.01079
20. Lechner J. Validation of dental X-ray by cytokine RANTES – comparison of X-ray findings with cytokine overexpression in jawbone. Clin Cosmetic Invest Dentistry. 2014;6:71–79. doi:10.2147/CCIDE.S69807
21. Sekundo C, Wiltfang J, Schliephake H, et al. Neuralgia-inducing cavitational osteonecrosis – a systematic review. Oral Dis. 2021;00:1–20. doi:10.1111/odi.13886
22. Al-Nawas B, Grotz KA, Kann P. Ultrasound transmission velocity of the irradiated jaw bone in vivo. Clin Oral Invest. 2001;5:266–268. doi:10.1007/s00784-001-0133-4
23. Njeh C, Hans D, Fuerst C, Gluer C, Genant HK. Quantitative Ultrasound. Assessment of Osteoporosis and Bone Status. United Kingdom: Martin Dunitz. Ltd; 1999.
24. Bouquot J, Martin W, Wrobleski G. Computer-based thru-transmission sonography (CTS) imaging of ischemic osteonecrosis of the jaws – a preliminary investigation of 6 cadaver jaws and 15 pain patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:550.
25. Lechner J, Zimmermann B, Schmidt M, von Baehr V. Ultrasound sonography to detect focal osteoporotic jawbone marrow defects: clinical comparative study with corresponding Hounsfield units and RANTES/CCL5 expression. Clin Cosmet Investig Dent. 2020;12:205–216. doi:10.2147/CCIDE.S247345
26. Lechner J, Zimmermann B, Schmidt M. Focal bone-marrow defects in the jawbone determined by ultrasonography—validation of new trans-alveolar ultrasound technique for measuring jawbone density in 210 participants. Ultrasound Med Biol. 2021;12. doi:10.1016/j.ultrasmedbio.2021.07.012
27. Lechner J, von Baehr V. Chemokine RANTES/CCL5 as an unknown link between wound healing in the jawbone and systemic disease: is prediction and tailored treatments in the horizon? EPMA Journal. 2015;6:10. doi:10.1186/s13167-015-0032-4
28. Mori F, Nisticò R, Nicoletti CG, et al. RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Mult Scler. 2016;22(11):1405–1412. PMID: 26733422. doi:10.1177/1352458515621796
29. Wilson AJ, Murphy WA, Hardy DC. Transient osteoporosis: transient bone marrow edema? Radiology. 1988;167(3):757–760. doi:10.1148/radiology.167.3.3363136
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.