Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Comparison of Clinical Profiles, Demographics, and Surgical Outcomes of 25-Gauge Vitrectomy for Proliferative Diabetic Retinopathy in Young Adults with Type 1 or Type 2 Diabetes
Authors Zhang M , Zhang J, Xu G, Ruan L, Huang X
Received 27 March 2023
Accepted for publication 19 May 2023
Published 29 June 2023 Volume 2023:16 Pages 1967—1975
DOI https://doi.org/10.2147/DMSO.S412157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Meng Zhang,1,2,* Juan Zhang,1,2,* Gezhi Xu,1,2 Lu Ruan,1,2 Xin Huang1,2
1Department of Ophthalmology, Eye & ENT Hospital, Fudan University, Shanghai, 200031, People’s Republic of China; 2Institute of Eye Research, Eye & ENT Hospital, Fudan University, Shanghai, 200031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Huang, Department of Ophthalmology, Eye & ENT Hospital, Fudan University, 83 Fenyang Road, Shanghai, 200031, People’s Republic of China, Tel +86-021-64377134, Fax +86-021-64377151, Email [email protected]
Purpose: Proliferative diabetic retinopathy (PDR) is a leading cause of poor vision in young adults. This study sought to evaluate the clinical characteristics and outcomes of primary vitrectomy for PDR in young adults.
Patients and Methods: Medical data were retrospectively collected at a large ophthalmology hospital in China. We analyzed data for 99 patients (140 eyes) aged < 45 years with T1D or T2D who underwent primary vitrectomy for PDR-related complications.
Results: There were 18 patients with T1D and 81 patients with T2D. The proportion of males was significantly greater than that of females in both groups. The T1D group had a longer duration of diabetes (P = 0.008), younger age at primary vitrectomy (P = 0.049), and lower body mass index (P < 0.001) than the T2D group. The proportion of eyes with rhegmatogenous retinal detachment (RRD) was greater but the proportion of eyes with traction retinal detachment (TRD) was lower in the T1D group than in the T2D group. The final best-corrected visual acuity (BCVA) improved or remained stable in 100% and 85.3% of eyes and decreased in 0% and 14.7% of eyes in the T1D and T2D groups, respectively. After surgery, the incidence of postoperative complications was significant greater in the T2D group than in the T1D group (P = 0.045). Factors influencing the final visual acuity included preoperative BCVA in both groups, the duration of diabetes (P = 0.031) and preoperative FVP (P = 0.004) in the T1D group, and preoperative RRD (P < 0.001) and postoperative NVG (P < 0.001) in the T2D group.
Conclusion: In this retrospective study, young adults with T2D who underwent vitrectomy had worse final visual acuity and more complications than young adults with T1D.
Keywords: diabetic retinopathy, vitrectomy, type 1 diabetes, type 2 diabetes
Introduction
Diabetes mellitus (DM) is a chronic disease worldwide. With the improvement of living standards and the change of lifestyle, the incidence of DM is increasing each year, and patients are being diagnosed at a younger age.1 Comprehensive data show that the prevalence of diabetes among young adults in China is increasing rapidly.2,3
Due to the early age of onset and aggressive course of disease, young adults with diabetes are prone to serious diabetes-related complications, placing a heavy burden on patients, their families, and society. According to the etiology, DM in young adults can be divided into three categories: type 1 diabetes (T1D), type 2 diabetes (T2D), and other types of DM.4 The prevalence of T2D in young Asian adults is greater than that of T1D (The incidence of T2D is about five times to twice as that of T1DM); the opposite pattern is observed in Caucasians.5–7
Pars plana vitrectomy (PPV) is the preferred surgical method for the treatment of severe proliferative diabetic retinopathy (PDR). It can help to clear the vitreous hemorrhage (VH), relieve vitreoretinal traction, and seal the retinal holes caused by traction. However, PPV is more challenging in young patients because severe active fibrovascular proliferation (FVP) is more common and there is a high incidence of postoperative VH and neovascular glaucoma (NVG).8 In high-risk patients, preoperative administration of anti-vascular endothelial growth factor (VEGF) drugs can reduce intraoperative and postoperative bleeding, which is conducive to surgery and can improve postoperative visual acuity.9
Previous studies have shown that young adults with T2D have almost double the risk of developing retinopathy and develop PDR sooner than young adults with T1D.1 Although the clinical manifestations and outcomes of vitrectomy for severe PDR in older patients have been widely reported, few studies have focused on severe PDR in young adults, despite its increasing prevalence in recent years.10 Therefore, we performed this retrospective study to evaluate the clinical characteristics and surgical outcomes of severe PDR in young adults with T1D or T2D treated at an ophthalmology hospital in China.
Materials and Methods
Patients
We retrospectively reviewed the medical records of patients aged <45 years with T1D or T2D who underwent surgical treatment of severe PDR between January 2018 and July 2022 at a large ophthalmology hospital in Shanghai, China. The indications for PPV include prolonged VH without absorption, tractional retinal detachment (TRD) involving the macula, concurrent tractional rhegmatogenous retinal detachment (RRD), and severe FVP, all of which are considered severe PDR. 25-gauge vitrectomy was performed by a single senior vitreoretinal surgeon using the Constellation vitrectomy machine (Alcon Laboratories, Fort Worth, TX) with a Resight fundus viewing system (Carl Zeiss Meditec Co. Ltd., Tokyo, Japan). Patients with a follow-up period of <6 months, history of previous vitreoretinal surgery, pre-existing severe ocular disease (uveitis, ocular trauma, ocular tumors or other severe ocular diseases), or severe systemic disease were excluded. The Institutional Review Board of the Eye and ENT Hospital of Fudan University approved this study, which adhered to the ethical principles of the Declaration of Helsinki.
Data Collection
Detailed preoperative, intraoperative, and postoperative data were extracted from the medical records. The demographic and systemic data included age, sex, height, weight, type and duration of diabetes, the treatment of diabetes, history of other systemic diseases, blood glucose and hemoglobin A1c (HbA1c) levels, and systolic and diastolic blood pressures. The preoperative ophthalmologic data included best-corrected visual acuity (BCVA), ocular examinations, history of pan-retinal photocoagulation (PRP), and the use of anti-VEGF drugs. The intraoperative findings and surgical procedures were also retrieved from the medical records. The postoperative data included BCVA, ocular examinations, macula status assessed by optical coherence tomography (OCT), and complications. BCVA was measured using a decimal visual acuity chart and then converted to logarithms of the minimum angle of resolution (logMAR) for statistical analysis.
Surgical Procedures
Intravitreal injection of anti-VEGF drug was performed 3–5 days before PPV in patients with active FVP who were considered to be at high risk for intraoperative and postoperative bleeding.
Phacoemulsification was performed prior to PPV if there was a significant cataract that affected the surgeon’s view of the fundus during surgery.
Core vitrectomy was done first and posterior vitreous detachment was then performed with the assistance of triamcinolone acetonide (TA). The vitreoretinal traction was released as much as possible. Intraoperative bleeding could be controlled by temporary elevation of the intraocular pressure or intraocular endodiathermy. The fibrovascular membrane and proliferative epiretinal membrane were mostly removed using a cutter, microscissors, and forceps. Bimanual dissection was performed if necessary. Retinotomy was performed in patients with extensive unreleasable subretinal proliferation. Peripheral vitrectomy was performed using scleral indentation. PRP was routinely performed. Fluid–air exchange was performed in eyes with a retinal break or RRD, and C3F8 or silicone oil tamponade was used if necessary.
Statistical Analysis
All data analyses were performed using SPSS Statistics V22.0 (IBM Corp., Armonk, NY, USA). Continuous data are presented as the mean ± standard deviation and were compared between the two groups using t-tests. Categorical variables are expressed as proportions and then compared between the two groups using the χ2 test or Fisher’s exact test. Risk factors associated with the final visual acuity after surgery were analyzed by multivariable linear regression. P values of <0.05 were considered to be statically significant.
Results
Patient Characteristics
Overall, 99 patients were enrolled in this study comprising 140 eyes with severe PDR. There were 24 eyes (18 patients) in the T1D group and 116 eyes (81 patients) in the T2D group. The proportion of males in the T1D and T2D groups (77.8% and 65.4%, respectively) was much greater than that of females. The mean duration of diabetes at vitrectomy in the T1D group was significantly longer than that in the T2D group (10.07 ± 5.62 and 6.30 ± 5.22 years, respectively, P = 0.008). The mean age was significantly younger in the T1D group than that in the T2D group (33.78 ± 5.53 and 36.54 ± 5.29 years, respectively, P = 0.049). The proportion of patients requiring binocular surgery was slightly greater in the T2D group than in the T1D group, although this was not statistically significant. The T2D group had a much greater body mass index (BMI) than the T1D group (21.58 ± 2.78 and 25.00 ± 3.69 kg/m2, respectively, P < 0.001).
Due to the differences in the pathogenesis between T1D and T2D, there are significant differences in their treatment. In the T1D group, 21 (87.5%) patients were treated with insulin only, 3 (12.5%) were treated with insulin combined with oral drugs, and none received oral drugs only. By comparison, these three treatment methods each accounted for about one-third of the patients with T2D. Because all of the patients underwent adjustment of their antidiabetic therapies to control glycemia for a period of time before surgery, there were no significant differences in the blood glucose (7.06 ± 2.73 and 7.11 ± 2.18 mmol/L, respectively, P = 0.923) or HbA1c levels (8.14% ± 3.13% and 7.33% ±1.36%, respectively, P = 0.093) between the T1D and T2D groups.
The most common related systemic disease was hypertension, which was present in 11 (45.8%) patients with T1D and 56 (48.3%) patients with T2D (P = 0.827). The frequencies of other common systemic diseases did not differ significantly between the two groups (cardiovascular disease, P = 0.167; cerebrovascular disease, P = 0.648; renal disease, P = 0.544). Details of the patient characteristics are listed in Table 1.
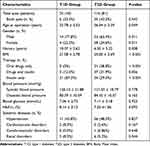 |
Table 1 The Clinical Demographic Data in Young Adults with Type 1 Diabetes versus Type 2 Diabetes |
Pars Plana Vitrectomy
Preoperative PRP was performed in 13 eyes (54.2%) in the T1D group and 70 eyes (60.3%) in the T2D group, which was not significantly different (P = 0.575). The percentage of eyes that received intravitreal injection of anti-VEGF drug was also similar in the T1D and T2D groups (66.7% and 57.8%, respectively, P = 0.419; Table 2).
 |
Table 2 The Surgical Indications and Ocular Findings in Young Adults with Type 1 Diabetes versus Type 2 Diabetes |
The surgical records showed that the main indication of PPV in the T1D group was tractional RRD (11 eyes, 45.8%), whereas VH without other indications was more prevalent in the T2D group (31 eyes, 26.7%). The T1D had a greater proportion of eyes with RRD (P = 0.040) but a smaller proportion of eyes with TRD (P = 0.027) compared with the T2D group. Active FVP was slightly more common in the T2D group than in the T1D group, although not significantly (6 eyes, 25.0% and 35 eyes, 30.2%, respectively, P = 0.612).
Cataract was found before PPV in 18 eyes (75.0%) in the T1D group and 97 eyes (83.6%) in the T2D group, three eyes had previously undergone cataract extraction and intraocular lens implantation. PPV combined with cataract surgery was performed in 8 eyes (33.3%) in the T1D group and in 32 eyes (27.6%) in the T2D group (P = 0.571). Retinectomy combined with subretinal surgery was performed in four eyes (16.7%) in the T1D group and nine eyes (7.8%) of the T2D group, which was not statistically significant (P = 0.171).
The choice of intraoperative tamponade depends on the severity of preoperative PDR and the intraoperative findings. Intraocular silicone oil tamponade was performed in 12 eyes (50.0%) in the T1D group and in 56 eyes (48.3%) in the T2D group (P = 0.878). The use of gas tamponade or balanced salt solution (BSS) tamponade was similar in both groups (P = 0.601 and P = 0.855, respectively). At the final follow-up, the retention of silicone oil tamponade was observed in 1 eye (1 patient) in the T1D group and 5 eyes (5 patients) in the T2D group.
Patients treated with silicone oil had worse preoperative vision and greater TRD involvement in the macula. Preoperative BCVA was ≥20/400 in 44 eyes (64.7%) in patients treated with silicone oil compared with 58 eyes (80.6%) in patients treated with gas or BSS tamponade (P = 0.035). Among eyes treated with silicone oil, 48 eyes (70.6%) had macular involvement with TRD, and 19 eyes (26.4%) that received gas or BSS tamponade had macular detachment (P < 0.001).
Postoperative Visual Acuity and Macular Thickness
Overall, patients with T1D generally had worse preoperative BCVA but better postoperative BCVA than patients with T2D. At the end of the follow-up period, BCVA had improved significantly compared with the preoperative BCVA in both groups. The mean BCVA improved from 1.66 ± 0.45 before surgery to 0.94 ± 0.62 after surgery in the T1D group (P < 0.001) and from 1.59 ± 0.46 before surgery to 0.96 ± 0.72 after surgery in the T2D group (P < 0.001).
The percentage of eyes in which the postoperative visual acuity improved or remained stable was greater in the T1D group than that in the T2D group, which was statistically significant (P = 0.045). The final BCVA was improved or remained stable in 100% and 85.3% of eyes in the T1D and T2D groups, respectively, and decreased in 0% and 14.7% of eyes, respectively.
The OCT examinations at 3 months after surgery showed that the average macular thickness was 245.77 ± 109.53 μm in eyes in the T1D group and 249.49 ± 89.27 μm in eyes in the T2D group, which was not statistically significant (P = 0.887).
Postoperative Complications
The postoperative complications are shown in Table 3. The main complications after primary PPV for severe PDR were NVG, recurrent VH, recurrent retinal detachment (RD), and epiretinal membrane (ERM) in this study. The overall incidence of these postoperative complications was significantly greater in the T2D group than in the T1D group (P = 0.045). No postoperative complications were found in the T1D group. In the T2D group, the most common complication was NVG, which occurred after primary PPV in 7 of 116 eyes (6.0%). Recurrent VH was also a common postoperative complication, occurring in 5 (4.3%) of 116 eyes and required vitreous washout. Regarding other postoperative complications, 3 eyes (2.6%) developed recurrent RD and 2 eyes (1.7%) developed ERM. All eyes with recurrent RD had TRD involving the macular area before surgery. Retinal reattachment was finally achieved after reoperation. Postoperative endophthalmitis was not observed in either group.
 |
Table 3 The Postoperative BCVA and Complications in Young Adults with Type 1 Diabetes versus Type 2 Diabetes |
Assessment of Risk Factors Associated with Poor Visual Acuity
Linear regression analysis was performed to identify possible risk factors associated with poor visual acuity. In the T2D group, preoperative BCVA (P = 0.010), preoperative RRD (P < 0.001), and postoperative NVG (P < 0.001) were significantly associated with worse visual acuity. The following factors were not significantly associated with visual acuity in the T2D group: age and duration of DM at surgery, gender, BMI, hypertension, renal diseases, preoperative PRP. intravitreal injection of anti-VEGF drugs, preoperative TRD, TRD involving the macular, VH, FVP, the use of silicone oil, and postoperative VH, RD and ERM.
In the T1D group, preoperative BCVA (P = 0.004), duration of DM (P = 0.031), and preoperative FVP (P = 0.004) were significantly associated with worse visual acuity. The other factors were not significantly associated with visual acuity (Table 4).
 |
Table 4 Assessment of Factors Influencing the Visual Acuity in the Young Adults with Type 1 Diabetes versus Type 2 Diabetes |
Discussion
The increasing prevalence of DM among young adults is a major public health challenge worldwide. The high incidence of diabetes-related complications in young adults with DM places a heavy burden on the patients, their families, and society.11 PDR is one of the most severe and vision-threatening diabetes-related complication.12–14 In our previous study, we compared the clinical features of PDR and the surgical outcomes between young adults and older patients with T2D.15 Although PPV is a well-established surgical procedure for severe PDR, its outcomes in young adults still need further research and few studies have compared its outcomes between young adults with T1D and T2D. Therefore, in this study, we sought to evaluate the clinical characteristics and surgical outcomes of PDR in young adults with T1D versus T2D.
This study suggests that there are some demographic and clinical differences between T1D and T2D young adults with severe PDR. These differences may have implications for the pathogenesis and treatment of the disease. The mean duration of DM at vitrectomy was significantly longer in the T1D group than in the T2D group. In addition, the mean age of the T1D group was significantly younger than that of the T2D group. These findings suggest that young adults with T1D may be at greater risk of developing severe PDR earlier in life. Previous studies have consistently shown that PDR tends to occur sooner and visual acuity is often worse in young adults with T2D than in young adults with T1D.16,17 In line with these findings, our study also observed that PDR occurred after a shorter duration of diabetes in young adults with T2D, highlighting the urgent need for early screening and effective management strategies for these patients.18 We also found that the proportion of males was greater than that of females in the T1D and T2D groups. Young adults with T2D had a higher BMI than those with T1D, which is consistent with previous studies.19 Basic studies have demonstrated that obesity amplifies hyperglycemia-induced epigenetic modifications and accelerates mitochondrial damage and diabetic retinopathy.20 These features may indicate a need for more aggressive lifestyle interventions and weight management in young T2D patients, especially males.21
In this study, we investigated the differences in indications of PPV between the two groups. We found that the main indication for PPV in the T1D group was tractional RRD, whereas VH was the most common indication in the T2D group. The frequency of RRD was slightly higher, and that of TRD was slightly lower in the T1D group than in the T2D group. Active FVP was slightly more common in the T2D group than in the T1D group, although the difference was not statistically significant. The frequencies of preoperative PRP and intravitreal injection of anti-VEGF drugs were similar in both groups. Previous studies have confirmed that pretreatment with anti-VEGF drugs is a safe and effective adjuvant to vitrectomy for reducing intraoperative bleeding and shortening operative time in young adults with PDR.22 The choice of intraoperative tamponade is largely dependent on the severity of preoperative PDR combined with the intraoperative findings.23,24 There were no significant differences in the use of silicone oil, gas, or BSS tamponade between the two groups. However, patients treated with silicone oil had worse preoperative vision and a higher frequency of macular TRD than patients treated with gas or BSS tamponade.
Interestingly, patients with T1D generally had worse preoperative BCVA but better postoperative BCVA than patients with T2D. Both groups showed significant improvements in BCVA compared with preoperative BCVA, and the percentage of patients in whom the postoperative visual acuity improved or remained stable was greater in the T1D group than that in the T2D group. The final BCVA improved or remained stable in all eyes in the T1D group but decreased in 14.7% of eyes in the T2D group. These results suggest that PPV is effective in patients with severe PDR, with either T1D and T2D, and it can achieve significant visual improvement. However, patients with T1D may have a better chance of visual improvement after PPV than patients with T2D.
Postoperative complications such as NVG, recurrent VH, and RD are more common after PPV in young adults with PDR.25,26 In our study, the overall incidence of these complications was significantly greater in the T2D group than in the T1D group. In the T2D group, the most common complication was NVG, followed by recurrent VH, recurrent RD, and ERM. The higher incidence of complications in the T2D group may be due to the more severe retinopathy in patients with T2D than in patients with T1D.
Many studies have sought to identify potential risk factors for the visual prognosis of PDR.27–30 The results of the linear regression analysis conducted in our study revealed that risk factors associated with visual acuity differed between the T1D and T2D groups. In the T2D group, preoperative BCVA, preoperative existing RRD, and postoperative NVG was significantly associated with poor visual outcomes. By comparison, preoperative BCVA, duration of DM, and preoperative FVP were significant risk factors in the T1D group. These findings may provide valuable information to clinicians assessing and managing patients with T1D or T2D undergoing PPV for severe PDR. It is important to note that other factors, such as patient age, duration of DM, gender, BMI, hypertension, preoperative PRP, and intravitreal injection of anti-VEGF drugs were not significant risk factors for visual acuity in either group in this study.
The study has some limitations, including its retrospective nature, small sample size, and lack of long-term follow-up, which may limit the generalizability of the findings. In addition, the study did not investigate the impact of other systemic data. Further studies are needed to confirm these findings. Therefore, further studies are warranted to validate our results. Nonetheless, our study provides important insights into the management of severe PDR in young adults undergoing PPV. Moreover, it highlights the differences in clinical characteristics between young adults with T1D and T2D who underwent vitrectomy. Our findings underscore the significance of early detection and intervention in managing PDR in young adults, particularly those with T2D. Furthermore, they suggest that patients with T2D require more vigilant postoperative monitoring to detect and manage complications effectively. In future research, we plan to continue following up with both groups of patients and supplementing our sample size in order to conduct more robust and long-term research on this topic.
Conclusions
The study found that both patients with T1D or T2D presenting with severe PDR benefit from PPV in terms of a significant improvement in visual outcomes. However, patients with T1D may have a better chance of visual improvement after PPV than patients with T2D. The study also identified different risk factors associated with visual acuity in the T1D and T2D groups, which may provide valuable information for clinicians when assessing and managing patients with severe PDR.
Data Sharing Statement
The data used in this study are available from the corresponding author.
Ethics Approval and Informed Consent
Our study was conducted in compliance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University.
Consent for Publication
Written consent was obtained from all patients, allowing for scientific analysis and publication of the results.
Acknowledgments
Meng Zhang and Juan Zhang are co-first authors. We would like to express our gratitude to the Retina and Vitreous Department of the Eye and ENT Hospital of Fudan University for their invaluable collaboration in providing clinical follow-up and offering their valuable input on our study.
Funding
This study was funded by National Natural Science Foundation for Young Scholar of China (81400410) and National Science Foundation of China (82070975).
Disclosure
The authors declare no conflicts of interest.
References
1. Patricia B, Andrew JB, David OH, Brian GM. Ocular sequelae in a population-based cohort of youth diagnosed with diabetes during a 50-year period. JAMA Ophthalmol. 2022;140(1):51–57. doi:10.1001/jamaophthalmol.2021.5052
2. Xuexin Y, Fan X, Wei Z. Trends and geographic variations in self-reported diabetes incidence: a prospective open cohort study of Chinese men and women, 1997–2015. Diabet Med. 2021;38(7):e14447. doi:10.1111/dme.14447
3. Joanna YLT, Elaine YWK, Betty WMB, et al. Incidence and clinical characteristics of pediatric-onset type 2 diabetes in Hong Kong: the Hong Kong childhood diabetes registry 2008 to 2017. Pediatr Diabetes. 2022;23(5):556–561. doi:10.1111/pedi.13231
4. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi:10.2337/dc18-S002
5. Fu J, Prasad HC. Changing epidemiology of metabolic syndrome and type 2 diabetes in Chinese youth. Curr Diab Rep. 2014;14(1):447. doi:10.1007/s11892-013-0447-z
6. Wei JN, Sung FC, Lin CC, Lin RS, Chiang CC, Chuang LM. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA. 2003;290(10):1345–1350. doi:10.1001/jama.290.10.1345
7. Urakami T, Suzuki J, Mugishima H, et al. Screening and treatment of childhood type 1 and type 2 diabetes mellitus in Japan. Pediatric Endocrinol Rev. 2012;10(Suppl 1):51–61.
8. Hui JC, Chang GW, Hong LD, Xue FF, Yi MX, Zhi ZM. Effect of intravitreal ranibizumab pretreatment on vitrectomy in young adults with proliferative diabetic retinopathy. Ann Palliat Med. 2020;9(1):82–89. doi:10.21037/apm.2020.01.10
9. Simunovic MP, Maberley DA. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: a systematic review and meta-analysis. Retina. 2015;35(10):1931–1942. doi:10.1097/IAE.0000000000000723
10. Liao M, Wang X, Yu J, et al. Characteristics and outcomes of vitrectomy for proliferative diabetic retinopathy in young versus senior patients. BMC Ophthalmol. 2020;20(1):416. doi:10.1186/s12886-020-01688-3
11. Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi:10.1001/jama.2014.3201
12. Wykoff CC, Khurana RN, Nguyen QD, et al. Risk of blindness among patients with diabetes and newly diagnosed diabetic retinopathy. Diabetes Care. 2021;44(3):748–756. doi:10.2337/dc20-0413
13. Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–970. doi:10.2337/dc17-1962
14. Lueder GT, Silverstein J. American Academy of Pediatrics section on ophthalmology and section on endocrinology. Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics. 2005;116(1):270–273. doi:10.1542/peds.2005-0875
15. Zhang M, Gezhi X, Ruan L, Huang X, Zhang T. Clinical characteristics and surgical outcomes of complications of proliferative diabetic retinopathy in young versus older patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2023;11(16):37–45. doi:10.2147/DMSO.S382603
16. Porter M, Channa R, Wagner J, Prichett L, Liu TYA, Wolf RM. Prevalence of diabetic retinopathy in children and adolescents at an urban tertiary eye care center. Pediatr Diabetes. 2020;21(5):856–862. doi:10.1111/pedi.13037
17. Tapley JL, G M Jr, Ashraf AP, et al. Feasibility and efficacy of diabetic retinopathy screening among youth with diabetes in a pediatric endocrinology clinic: a cross-sectional study. Diabetol Metab Syndr. 2015;24(7):56. doi:10.1186/s13098-015-0054-z
18. Anna EE, Ulf S, Annika J, Annelie C, Amira E, Claude M. Microalbuminuria and retinopathy in adolescents and young adults with type 1 and type 2 diabetes. Pediatr Diabetes. 2020;21(7):1310–1321. doi:10.1111/pedi.13074
19. Wang TL, Xia G, Wei W, et al. Association of different kinds of obesity with diabetic retinopathy in patients with type 2 diabetes. BMJ Open. 2022;12(5):e056332. doi:10.1136/bmjopen-2021-056332
20. Renu AK. Retinopathy in a diet-induced type 2 diabetic rat model and role of epigenetic modifications. Diabetes. 2020;69(4):689–698. doi:10.2337/db19-1009
21. Anastasios S, Vasileios G, Eleni PK, Assimina GT, Ekaterini S. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2021;12(4):344–365. doi:10.4239/wjd.v12.i4.344
22. Li S, Tang J, Han X, et al. Prospective comparison of surgery outcome between preoperative and intraoperative intravitreal injection of ranibizumab for vitrectomy in proliferative diabetic retinopathy patients. Ophthalmol Ther. 2022;11(5):1833–1845. doi:10.1007/s40123-022-00550-7
23. Yun DS, Chung MY. Extended silicone oil tamponade in primary vitrectomy for complex retinal detachment in proliferative diabetic retinopathy: a long-term follow-up study. Eur J Ophthalmol. 2007;17(6):954–960. doi:10.1177/112067210701700614
24. Brourman ND, Blumenkranz MS, Cox MS, Trese MT. Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology. 1989;96(6):759–764. doi:10.1016/S0161-6420(89)32828-4
25. Huang CH, Hsieh YT, Yang CM. Vitrectomy for complications of proliferative diabetic retinopathy in young adults: clinical features and surgical outcomes. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):
26. Sun D, Lin Y, Zeng R, Yang Z, Deng X, Lan Y. The incidence and risk factors of neovascular glaucoma secondary to proliferative diabetic retinopathy after vitrectomy. Eur J Ophthalmol. 2021;31(6):3057–3067. doi:10.1177/1120672120980686
27. Katsuhiro N, Koichi N, Teiko Y, Hidetoshi Y. Factors correlated with visual outcomes at two and four years after vitreous surgery for proliferative diabetic retinopathy. PLoS One. 2021;16(1):e0244281. doi:10.1371/journal.pone.0244281
28. Karthik K, Girish B, Naresh B, et al. Clinical features and surgical outcomes of complications of proliferative diabetic retinopathy in young adults with type 1 diabetes mellitus versus type 2 diabetes mellitus - A comparative observational study. Indian J Ophthalmol. 2021;69(11):3289–3295. doi:10.4103/ijo.IJO_1293_21
29. Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(3):365–368. doi:10.1136/bjo.2007.124495
30. Lin T, Gubitosi KRA, Channa R, Wolf RM. Pediatric diabetic retinopathy: updates in prevalence, risk factors, screening, and management. Curr Diab Rep. 2021;21(12):56. doi:10.1007/s11892-021-01436-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
