Back to Journals » Risk Management and Healthcare Policy » Volume 17
Comparison of Chromosomal Microarray Analysis and Noninvasive Prenatal Testing in Pregnant Women with Fetal Ultrasonic Soft Markers
Authors Hu X, Hu Y , Wang H, Yu C, Zheng J, Zhang H, Zheng J
Received 27 August 2023
Accepted for publication 20 December 2023
Published 4 January 2024 Volume 2024:17 Pages 29—40
DOI https://doi.org/10.2147/RMHP.S437441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gulsum Kubra Kaya
Xianqing Hu,1 Yanjun Hu,1 Hai Wang,2 Caicha Yu,3 Jiayong Zheng,2 Hongping Zhang,1 Jianqiong Zheng1
1Department of Obstetrics and Gynecology, The Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou People’s Hospital, Wenzhou Maternal and Child Health Care Hospital, Wenzhou, People’s Republic of China; 2Department of Fetal Medicine and Prenatal Diagnosis, The Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou People’s Hospital, Wenzhou Maternal and Child Health Care Hospital, Wenzhou, People’s Republic of China; 3Department of Ultrasonography, The Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou People’s Hospital, Wenzhou Maternal and Child Health Care Hospital, Wenzhou, People’s Republic of China
Correspondence: Jianqiong Zheng; Hongping Zhang, Email [email protected]; [email protected]
Objective: This study aimed to assess the utility of chromosomal microarray analysis (CMA) and noninvasive prenatal testing (NIPT) in detecting clinically significant chromosomal abnormalities among fetuses presenting ultrasonic soft markers (USMs).
Methods: A retrospective observational study, spanning from January 1, 2019, to September 30, 2022, enrolled 539 singleton pregnant women with fetal USMs at our center. Of these, 418 cases (77.6%) underwent NIPT, while 121 cases (22.4%) opted for invasive prenatal diagnosis post-appropriate genetic counseling. Cases with high-risk NIPT results proceeded to invasive prenatal diagnosis, where conventional karyotyping and CMA were concurrently performed. Further stratification was done based on the number of USMs, classifying cases into single-USM and multiple-USM groups.
Results: Of the 24 cases (4.5%) exhibiting abnormal findings, 17 presented numerical chromosomal abnormalities, 2 featured clinically significant copy number variations (CNVs), 3 showed variants of unknown significance (VOUS), 1 displayed LOH, and 1 exhibited chromosome nine inversion. Notably, 18 cases (75%) theoretically detectable by karyotyping (eg, sizes above 10Mb) and 16 cases (66.7%) detectable by NIPT for five common aneuploidies were identified. Six submicroscopic findings (25%) were exclusively detectable by CMA. The predominant clinically relevant aberrations were observed in the thickened nuchal-translucency (TNT) group (9/35, 25.7%), followed by the multiple soft markers group (3/32, 9.3%). In the NIPT group, the false positive rate was 1.22%, and the false negative rate was 0%.
Conclusion: The prevalence of chromosome aneuploidy exceeded that of submicroscopic chromosomal imbalance in pregnant women with fetal USMs. NIPT demonstrated efficacy, particularly for soft markers like echogenic intracardiac focus. However, for those with TNT and multiple soft markers, invasive prenatal diagnosis, including CMA testing, is recommended as the primary investigative approach.
Keywords: chromosomal microarray analysis, karyotype, noninvasive prenatal testing, ultrasonic soft markers, prenatal diagnosis
Introduction
Birth defects constitute a primary contributor to infant mortality and an elevated occurrence of childhood-related diseases. Chromosomal abnormalities, a pivotal determinant in global birth defects, pertain to structural and functional abnormalities in fetal development. Notably, it emerges as a substantial public health concern, exhibiting an approximate incidence of 1 in 150 individuals in the Chinese population.1 Over the decades, advancements in ultrasound examination technology, coupled with its widespread integration into fetal medicine, have led to the identification of an increasing number of ultrasonic soft markers (USMs). The prevalence of isolated USMs has been reported to reach nearly 10% during prenatal ultrasound examinations.2 Extensive research has underscored that fetuses exhibiting USMs face an augmented risk of chromosomal abnormalities.3 Nevertheless, the precise risk associated with abnormal chromosome aneuploidy and submicroscopic chromosomal imbalance remains unclear, compounded by the existence of diverse USM types with varying clinical outcomes. As it stands, a universally accepted consensus on the treatment of fetal USMs is lacking.
Karyotyping, a traditional cytogenetic test in use since the 1960s, has witnessed a gradual decline owing to its limited resolution (5–10 Mb), prolonged cell culture requirements, and susceptibility to culture failure. In contrast, chromosomal microarray analysis (CMA) has emerged as a superior alternative, boasting heightened resolution and detection rates for chromosomal imbalances. In recent years, CMA has gained widespread acceptance for prenatal diagnosis of fetal structural malformations globally.4 Concurrently, noninvasive prenatal testing (NIPT) became commercially available in 2011, rapidly integrating into antenatal screening protocols due to its efficiency and accuracy in detecting fetal chromosomal aneuploidies.5,6 However, the selection between CMA and NIPT for cases involving fetal USMs remains a matter of contention.
Hence, the objective of this study was to conduct a retrospective analysis of the chromosomal results obtained through CMA and NIPT for fetuses with USMs at our center. Subsequent follow-up assessments will extend up to six months post-birth. The study aimed to estimate the detection rates of CMA and NIPT among pregnant women with fetal USMs, thereby contributing additional empirical evidence to inform clinical genetic counseling practices.
Materials and Methods
Patients and Samples
Data for this study were derived from a retrospective observational study including singleton fetuses with USMs who underwent prenatal diagnosis or testing subsequent to comprehensive genetic counseling at the Fetal Medical Center of Wenzhou People’s Hospital between January 1, 2019, and September 30, 2022. Throughout the prenatal consultation process, detailed information regarding the risks associated with amniocentesis or cordocentesis-induced miscarriage, the limitations inherent in NIPT, and the pros and cons of CMA, including the incidence of identifying variants of unknown significance (VOUS), was conveyed by the genetic counselor to all expectant couples. The mean maternal age at delivery was 29.83±5.17 years, and the mean gestational week was 17.24±4.04 weeks for those undergoing NIPT, whereas for those undergoing invasive prenatal diagnosis (comprising amniocentesis and cordocentesis), the mean maternal age at delivery was 31.24±5.18 years, and the mean gestational week was 20.12±3.33 weeks (Table 1). Maternal blood samples were collected in the NIPT group (n=418), while fetal samples were acquired in the invasive prenatal diagnosis group through amniocentesis and cord blood sampling, respectively, contingent on gestational age. Diagnosis of USMs adhered to Li SL’s diagnostic criteria.7 The selection criteria for USMs, based on input from pregnant women in our center, encompassed choroid plexus cysts (CPCs), echogenic intracardiac focus (EICF), thickened nuchal translucency (TNT), absent/hypoplastic nasal bone (A/HNB), fetal echogenic bowel (FEB), ventriculomegaly (VM), and single umbilical artery (SUA). Fetuses exhibiting ultrasonographic structural abnormalities were excluded from the study. In alignment with the number of USMs present, cases were categorized into single USMs and multiple USMs. A comprehensive clinical follow-up assessment of pregnant women and their newborns was conducted, extending beyond six months post-birth, facilitated through telephonic communication or examination of medical records.
 |
Table 1 The Demographic Characteristics of 539 Patients |
NIPT
NIPT procedures were conducted in the gestational window of 12–28 weeks. Peripheral blood, exceeding 5 mL in volume, was collected from each pregnant woman, and then placed into ethylenediaminetetraacetic acid anticoagulant tubes, and centrifuged. Fetal-free DNA extraction and purification were executed using the MGISP960 instrument (Shenzhen BGI Intelligent Manufacturing Technology Co., Ltd., Shenzhen, China). Sequencing analysis was performed in accordance with the experimental guidelines, utilizing the BGISEQ-500 platform (Shenzhen BGI Intelligent Manufacturing Technology Co., Ltd., Shenzhen, China). Post-sequencing data underwent evaluation through bioinformatics alignment analysis. The assessment of fetal autosomal trisomy risk was predicated upon Z-scores. A Z-score exceeding 3 for chromosomes 21, 18, or 13 was indicative of a heightened risk in NIPT. Regarding the evaluation of sex chromosome risk, the computation involved determining the ratios of standard X and Y chromosome read counts to the total autosomal read counts.8 According to the screening results, clinical geneticists provided appropriate clinical counseling to high-risk NIPT pregnant women and recommend further invasive prenatal diagnosis to establish a definitive diagnosis. The test was considered unsuccessful if an inadequately qualified total cell-free DNA (cfDNA) concentration was obtained twice or if the fetal fraction was calculated to be less than 4% in two successive assessments.
Invasive Prenatal Diagnosis
Amniocentesis was performed within the gestational range of 17 to 24 weeks, while cordocentesis was conducted between the 25th and 32nd gestational weeks. Simultaneous application of CMA and karyotyping aimed to identify fetal aneuploidy, chromosome microdeletions, microduplications, and additional fetal chromosomal abnormalities.
Conventional karyotyping procedures involved the collection of fetal sample cells through amniocentesis or cordocentesis, and then these cells were cultured and analyzed utilizing G-banding at a resolution of 450 bands in our laboratory.
In the case of CMA, genomic DNA extraction from uncultured amniotic fluid or fetal cord blood was accomplished using a DNA extraction kit (Tiangen Biochemical Technology Co., Ltd, Beijing, China) in adherence to the manufacturer’s protocol. CMA was then performed utilizing a comprehensive Affymetrix CytoScan 750 K array (Affymetrix Inc., Santa Clara, CA, USA) to analyze the sample. Analysis of results was conducted using the Chromosome Analysis Suite software (Affymetrix) and human genome version GRCh37 (hg19). Parameters were set to recognize a 200 kb duplication or 100 kb deletion, and a 10 Mb loss of heterozygosity (LOH).
Data Interpretation
In accordance with the American College of Medical Genetics (ACMG) guidelines,9 copy number variations (CNVs) were categorized into the following classes: Benign, likely benign, VOUS, likely pathogenic, and pathogenic. CNVs designated as pathogenic or likely pathogenic were considered clinically significant.
Statistical Analysis
Statistical analysis of the data was performed using SPSS software version 23.0. Normality testing was applied to measurement data, with normally distributed data presented as mean with standard deviation. Between-group differences were assessed using t-test. Non-normally distributed data were conveyed as the median with the range from minimum to maximum, and intergroup differences were evaluated through the rank sum test. P <0.05 was considered statistically significant.
Ethical Statement
Approval for this study was obtained from the Ethics Review Committee of Wenzhou People’s Hospital. Prior to undergoing CMA, NIPT, or karyotyping in accordance with the Declaration of Helsinki, all participants provided written informed consent. The data underwent anonymization.
Results
Fetal Profile
From January 1, 2019 to September 30, 2022, our center encountered 1330 pregnant women marked by fetal USMs. In this cohort, 549 cases underwent NIPT or invasive prenatal diagnosis. Notably, subsequent examinations revealed seven cases with additional structural malformations. In addition, one instance initially categorized in the SUA group was reclassified as a double umbilical artery following subsequent ultrasound assessments. Furthermore, two cases in the VM group were later identified as ependymal cysts through subsequent ultrasound and magnetic resonance imaging (MRI) examinations. These cases were then excluded from the analysis (Figure 1). The remaining 539 cases formed the basis of this retrospective study. This group comprised 507 cases featuring single USMs and 32 cases with double USMs. The cohort with single USMs encompassed diverse manifestations: 35 cases of TNT, 11 cases of VM, 10 cases of A/HNB, 12 cases of EB, 183 cases of CPC, 242 cases of EICF, and 14 cases of SUA (Table 2). To facilitate a comprehensive analysis, the 539 cases were divided into two groups: the NIPT group (n=418) and the invasive prenatal diagnosis group (n=121). The latter group included cases subjected to amniocentesis (n=119) and cordocentesis (n=2) procedures (Table 3). Crucial demographic information is presented in Table 1. It is noteworthy that none of the pregnant women in this study experienced a miscarriage attributable to either amniocentesis or cordocentesis procedures. This observation underscores the safety of these diagnostic interventions in the studied population.
 |
Figure 1 Flow diagram illustrating the study population. Abbreviation: NIPT, Non-invasive Prenatal Testing. |
 |
Table 2 Phenotypic Characteristics of 539 Pregnancies with Ultrasonic Soft Markers |
 |
Table 3 Summary of Chromosomal Aberrations in 539 Fetuses with Soft Markers |
Identification of High-Risk Cases in NIPT and Validation
All cases identified as high-risk through NIPT underwent subsequent verification via amniocentesis or cordocentesis, with comprehensive follow-up. Importantly, no cases of false-negative outcomes were observed among the cohort characterized as NIPT negative. Among the 14 cases deemed high-risk by NIPT and subsequently subjected to amniocentesis, nine exhibited chromosome abnormalities. Specifically, these abnormalities comprised one case of 47XXY, one case of 45,X[10]/46,XY[40], one case of 45,X, two cases of Trisomy 18, and four cases of Trisomy 21. Remarkably, the remaining five cases exhibited normal karyotyping and CMA results (Figure 2). Of the five cases, three manifested high-risk sex chromosome abnormalities, one exhibited a partial deletion of chromosome 18, and another presented with a partial duplication of chromosome 17. The study’s rigorous analysis revealed a 0% false-negative rate, indicating a robust sensitivity of NIPT in detecting cases with chromosomal abnormalities. Conversely, the false-positive rate was determined to be 1.22%, underscoring the importance of further confirmatory diagnostic procedures to validate positive NIPT findings within the scope of this study.
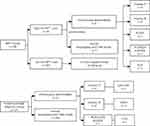 |
Figure 2 Comparative flow diagram of the NIPT Group and the invasive prenatal diagnosis Group. Abbreviation: NIPT, Non-invasive Prenatal Testing. |
Karyotyping Analysis of Chromosome Abnormalities
Conventional karyotyping analysis identified chromosome abnormalities in 18 fetuses, including 16 cases of aneuploidy, one case of mosaic chromosomal number abnormality, and one case of chromosomal structural abnormality. Specifically, among the aneuploidies, there were eight cases of trisomy-21, six cases of trisomy-18, one case of 47,XXY, and one case of 45,X. The singular chromosomal structural abnormality identified was 46,XN, inv(9)(p12q13). Additionally, a mosaic chromosomal number abnormality was observed, denoted as 45,X[10]/46,XY[40], as detailed in Table 4.
 |
Table 4 Abnormalities Detected and Clinically Relevant Characteristics in Fetuses with Ultrasonic Soft Markers |
CMA of Chromosomal Abnormalities
In addition to the 18 fetuses exhibiting chromosomal abnormalities as identified by conventional karyotyping analysis, CMA detected an additional 1.11% (6/539) CNVs. These CNVs, as detailed in Table 4, comprised one pathogenic, one likely pathogenic, three VOUS, and one LOH. The prevalence of clinically relevant CNVs varied across different clinical subgroups. Notably, the TNT group exhibited the highest clinical aberration rate (9/35, 25.7%), followed by the multiple soft markers group (3/32, 9.3%), and the CPCs group (12/183, 6.6%).
Among the identified pathogenic CNVs, Case 20 displayed a 585 kb deletion associated with the 16p11.2 microdeletion syndrome. This syndrome is linked to developmental delay, cognitive deficits, language developmental delay, autism spectrum disorders, and cardiac malformations. Importantly, inheritance analysis revealed this CNV to be de novo. Case 10, featuring a 702 kb deletion in the 16p12.2 region, implicated four OMIM genes and was deemed likely pathogenic, with variable clinical phenotypes including developmental delay, mental retardation, and behavioral abnormalities. The patient chose to terminate the pregnancy without parental validation. Case 9 revealed a 411 kb deletion in the 16p12.2 region, classified as VOUS, with potential consequences such as deafness, autism, facial fissure, and developmental delay. Given that the CNV was inherited from unaffected parents, the patient chose to proceed with the pregnancy. However, at 26 weeks of gestation, relative to the same gestational age, ultrasound indicated that the fetal biparietal diameter and head circumference increased for 3 weeks, abdominal circumference bigger for 5 weeks, umbilical blood flow diastolic deficiency, and amniotic fluid index of 300mm. Concerning fetal parameters, prompting the patient to opt for pregnancy termination. Case 24 exhibited a 1.02 Mb duplication in the 4q35.2 region, categorized as a VOUS. The patient declined parental verification and chose pregnancy termination. Case 21, featuring a 106 kb deletion in the 2q13 region with OMIM genes MALL and NPHP1, was classified as VOUS with potential implications for renal insufficiency and cerebellar vermis hypoplasia. Considering the fragment’s relatively small size and the CNV’s inheritance from unaffected parents, the patient decided to continue the pregnancy, resulting in a live birth.
Incorporating maternal age into the analysis revealed a correlation between advanced maternal age and an increased rate of chromosomal aberrations. Specifically, among pregnancies with USMs and maternal age over 35, the rate of chromosomal aberrations was 6/118 (5.08%). This rate increased to 5/47 (10.64%) for maternal age over 38 and 3/26 (11.54%) for maternal age over 40, as detailed in Table 5.
 |
Table 5 Chromosomal Aberrations with Ultrasonic Soft Markers in Advanced Maternal Age |
Pregnancy Outcomes of Cases with Chromosomal Abnormalities
Among the 24 cases identified with chromosomal abnormalities, 20 pregnant women (83.33%, 20/24) opted for pregnancy termination in response to the chromosomal abnormalities. In contrast, the remaining four pregnant women (16.66%, 4/24) chose to continue the pregnancies, culminating in successful deliveries. A singular case resulted in a stillbirth at 22 weeks, with chromosomal genetic results determined as normal; however, no further autopsy examination was performed. The newborns from the study underwent follow-up for a duration exceeding six months post-birth, and reassuringly, the outcomes revealed robust health.
Discussion
The reported incidence of chromosomal abnormalities in pregnancies is approximately 2%. While fetal USMs are typically transient findings, they may indicate an elevated risk of fetal chromosomal imbalance.10 The identification of such markers can induce anxiety in expectant couples, and this stress may persist until delivery.11 Moreover, there is a lack of consensus regarding the management of pregnancies with USMs. Therefore, we designed this study to explore the potential applications of NIPT and CMA in pregnancies featuring fetal USMs. In our study, 539 patients with fetal USMs were recruited, resulting in the identification of abnormal results in 24 cases (4.45%). The detection rate of fetal submicroscopic CNVs, detectable solely through CMA, was 1.13% (6/539), while the detection rate of fetal aneuploidy abnormalities was 2.97% (16/539). This aligns with previous research,12 underscoring the higher likelihood of chromosome number abnormalities in cases of ultrasound soft markers compared to copy number variations.
Moreover, our study revealed heterogeneous CMA results among different USMs. The incidence of chromosome abnormalities in the nuchal translucency (NT) group and multiple soft markers group was 25.71% (9/35) and 9.38% (3/32), respectively, exceeding the rates observed in other groups. The nine cases in the NT group encompassed six cases of trisomy 21, one case of 45,X (Turner syndrome), one case of a pathogenic microdeletion, and one case of a VOUS microdeletion. Consistent with our findings, Maya’s study13 also identified CNVs in fetuses with TNT., highlighting the high incidence of chromosome abnormalities in such cases. Therefore, we propose that NIPT may not be suitable for pregnancies with TNT,14 and CMA, with its high resolution, should be the preferred first-line technique in these instances. Canadian practice guidelines15 recommend CMA examination for pregnancies with TNT, and some researchers16 suggest CMA for prenatal diagnosis when NT exceeds 3.5 mm. Additionally, Su17 advocates for CMA in cases with NT ranging from 2.5 to 3.4 mm, as it enhances the detection rate of normal chromosomal karyotype aberrations. In conclusion, our study supports the notion that CMA should be the first-line technique for pregnancies characterized by TNT due to its heightened sensitivity to chromosome abnormalities.
In our study, all 242 cases of EICF exhibited normal results upon chromosome examination. Despite the elevated incidence of EICF, its predictive value for chromosome abnormalities remains notably low, aligning with findings from previous studies.18 Initially, EICF was found to be associated with a heightened risk of Down Syndrome.19 However, contemporary research has indicated that isolated EICF does not increase the risk of aneuploidy.20 The Society for Maternal-Fetal Medicine (SMFM)21 advocates for NIPT as a prudent choice for cases of EICF, particularly in instances where aneuploidy screening has not been performed previously. Li S et al22 suggest that when NIPT results are negative, EICF should be considered a normal variant.
Due to the heightened accuracy and positive predictive value of NIPT in screening for trisomy 21, 18, and 13, the widespread adoption of NIPT in prenatal screening has become increasingly prevalent.23–25 Notably, invasive prenatal diagnostic procedures pose a risk of miscarriage and incur higher costs. Therefore, based on current knowledge, NIPT emerges as a more suitable option for pregnancies involving EICF than invasive testing. In our study cohort of 418 cases subjected to NIPT, the false positive rate was 1.22%. Over a follow-up period exceeding six months post-birth, no false negative cases were identified, aligning with the result of a previous study.26 Subsequent prenatal diagnosis revealed 14 positive cases, and the false positive five cases were three cases of high-risk sex chromosome anomalies, one case with partial deletion of chromosome 18, and one case with partial duplication of chromosome 17. As expected, NIPT exhibited high screening accuracy for aneuploidies on chromosomes 21, 18, and 13, while displaying comparatively lower accuracy for sex chromosomes and other chromosomal regions.27 Studies28,29 underscored that the accuracy of NIPT for sex chromosome detection was approximately 45.0%. Currently, NIPT has rapidly gained traction for screening a limited number of chromosomal diseases, including T21, T18, T13, and sex chromosome abnormalities. Although the feasibility of expanded NIPT panels for certain microdeletion syndromes,30 such as 22q11.21 microdeletion (DiGeorge syndrome) and 5p deletion (cri du chat syndrome), has been explored, it is crucial to acknowledge that these expanded panels may miss almost half of abnormal findings.31 Notably, for soft markers exhibiting a high incidence of chromosomal abnormalities, invasive testing is currently recommended over NIPT, as the latter lacks clinical validation for submicroscopic CNVs.32 While traditional invasive examinations predominantly involve karyotyping, CMA has gained traction due to its heightened resolution and time efficiency, obviating the need for cell culture. A consensus has been established regarding CMA’s applicability in cases of ultrasound structural abnormalities.33 However, the use of CMA in fetuses presenting soft markers remains a subject of contention. Scholars have proposed its application in advanced pregnancies,34 and guidelines suggest its use in low-risk, voluntary pregnancies.25,32,35 Some scholars36,37 has proposed that CMA could enhance the diagnostic scope of chromosomal imbalances in fetuses identified through isolated USMs and advanced maternal age. Nevertheless, due to the low abortion rate and elevated costs associated with invasive implantation for common soft markers, the domestic application of CMA in China is deemed impractical. It is imperative to note that VOUS results contribute to parental anxiety,38 with reports indicating an incidence rate of up to 1.5% in normal karyotypes.39 As CMA becomes more prevalent and databases expand, the incidence of VOUS is expected to decrease. In the context of this study and existing literature, aneuploidy among soft markers in fetuses surpasses that of copy number variants. Consequently, considering the current landscape, it is posited that NIPT is more fitting for pregnancies featuring soft markers, with the exception of cases involving TNT and multiple soft markers. Clinical counseling should diligently consider the testing range and limitations of NIPT.
Our study’s strength lies in the meticulous classification of USMs, providing clinicians with nuanced and targeted genetic counseling when confronted with specific fetal USM presentations. All cases in the positive NIPT group underwent further validation through karyotyping and CMA. Nevertheless, the study is not without its limitations. First, certain categories of fetal USMs were underrepresented, introducing the potential for selection bias due to their limited occurrence. Second, future endeavors in the form of multi-center studies would benefit from a more expansive sample size to enhance statistical robustness.
In summary, in our country and based on the present data, CMA is recommended as a primary choice for fetuses exhibiting TNT and multiple soft markers. Conversely, for other ultrasound soft markers, NIPT emerges as a more suitable option for initial screening, particularly in cases involving fetuses with echogenic intracardiac lesions.
Data Sharing Statement
The data that support the findings of this study are publicly available. DOI 10.57760/sciencedb.08841.
Acknowledgments
We thank all the patients and researchers for their participation.
Funding
This research was funded by the Zhejiang Provincial Science and Technology Bureau Foundation (LGF22H040010), Fundamental Scientific Research Project of Wenzhou (Y20220409, Y20210018), and Science and Technology Planning Project of Wenzhou (ZY2021025, ZY2023032).
Disclosure
The authors declare that there was no conflict of interest.
References
1. Neufeld-Kaiser WA, Cheng EY, Liu YJ. Positive predictive value of noninvasive prenatal screening for fetal chromosome disorders using cell-free DNA in maternal serum: independent clinical experience of a tertiary referral center. BMC Med. 2015;13(1):129. doi:10.1186/s12916-015-0374-8
2. Ginsberg Y, Khatib N, Weiner Z, et al. The recurrence of sonographic ‘soft markers’: ominous sign or ‘just’ genetics? Prenat Diagn. 2017;37(5):469–472. doi:10.1002/pd.5034
3. Cai M, Lin N, Chen X, et al. Evaluation of chromosomal abnormalities and copy number variations in fetuses with ultrasonic soft markers. BMC Med Genomics. 2021;14(1):19. doi:10.1186/s12920-021-00870-w
4. American College of Obstetricians and Gynecologists. Practice Bulletin No. 162 summary: prenatal diagnostic testing for genetic disorders. Obstet Gynecol. 2016;127(5):976–978. doi:10.1097/AOG.0000000000001438
5. Borth H, Teubert A, Glaubitz R, et al. Analysis of cell-free DNA in a consecutive series of 13,607 routine cases for the detection of fetal chromosomal aneuploidies in a single center in Germany. Arch Gynecol Obstet. 2021;303(6):1407–1414. doi:10.1007/s00404-020-05856-0
6. La Verde M, De Falco L, Torella A, et al. Performance of cell-free DNA sequencing-based non-invasive prenatal testing: experience on 36,456 singleton and multiple pregnancies. BMC Med Genomics. 2021;14(1):93. doi:10.1186/s12920-021-00941-y
7. Li SL, Zhu J. Prenatal Ultrasound Diagnosis of Fetal Malformation. Beijing: People’s Military Medical Publishing House; 2015.
8. Mazloom AR, Džakula Ž, Oeth P, et al. Noninvasive prenatal detection of sex chromosomal aneuploidies by sequencing circulating cell-free DNA from maternal plasma. Prenat Diagn. 2013;33(6):591–597. doi:10.1002/pd.4127
9. Kearney HM, Thorland EC, Brown KK, et al.; Working Group of the American College of Medical Genetics Laboratory Quality Assurance Committee. American college of medical genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med. 2011;13(7):680–685. doi:10.1097/GIM.0b013e3182217a3a
10. Getz L, Kirkengen AL. Ultrasound screening in pregnancy: advancing technology, soft markers for fetal chromosomal aberrations, and unacknowledged ethical dilemmas. Soc Sci Med. 2003;56(10):2045–2057. doi:10.1016/S0277-9536(02)00200-9
11. Bernhardt BA, Soucier D, Hanson K, et al. Women’s experiences receiving abnormal prenatal chromosomal microarray testing results. Genet Med. 2013;15(2):139–145. doi:10.1038/gim.2012.113
12. Wang J, Chen L, Zhou C, et al. Identification of copy number variations among fetuses with ultrasound soft markers using next-generation sequencing. Sci Rep. 2018;8(1):8134. doi:10.1038/s41598-018-26555-6
13. Maya I, Yacobson S, Kahana S, et al. Cut-off value of nuchal translucency as indication for chromosomal microarray analysis. Ultrasound Obstet Gynecol. 2017;50(3):332–335. doi:10.1002/uog.17421
14. Sagi-Dain L, Cohen Vig L, Kahana S, et al. Chromosomal microarray vs. NIPS: analysis of 5541 low-risk pregnancies. Genet Med. 2019;21(11):2462–2467. doi:10.1038/s41436-019-0550-x
15. Armour CM, Dougan SD, Brock JA, et al; On-behalf-of the canadian college of medical geneticists. Practice guideline: joint CCMG-SOGC recommendations for the use of chromosomal microarray analysis for prenatal diagnosis and assessment of fetal loss in Canada. J Med Genet. 2018;55(4):215–221. doi:10.1136/jmedgenet-2017-105013
16. Bunnell ME, Adams S, Pelletier A, et al. Increased use of diagnostic testing after increased nuchal translucency: the influence of non-invasive prenatal testing and chromosomal microarray. Prenat Diagn. 2022;42(13):1606–1611. doi:10.1002/pd.6255
17. Su L, Huang H, An G, et al. Clinical application of chromosomal microarray analysis in fetuses with increased nuchal translucency and normal karyotype. Mol Genet Genomic Med. 2019;7(8):e811. doi:10.1002/mgg3.811
18. Liu Y, Jing X, Xing L, et al. Noninvasive prenatal screening based on second-trimester ultrasonographic soft markers in low-risk pregnant women. Front Genet. 2021;12:793894. doi:10.3389/fgene.2021.793894
19. Bromley B, Lieberman E, Laboda L, et al. Echogenic intracardiac focus: a sonographic sign for fetal Down syndrome. Obstet Gynecol. 1995;86(6):998–1001. doi:10.1016/0029-7844(95)00323-J
20. Wang J, Chen L, Wang L, et al. Segmental aneuploidies in fetuses with isolated echogenic intracardiac focus among women younger than 35 years. Sci Rep. 2020;10(1):10496. doi:10.1038/s41598-020-67501-9
21. Prabhu M, Kuller JA, Biggio JR; Society for Maternal-Fetal Medicine (SMFM). Society for maternal-fetal medicine consult series #57: evaluation and management of isolated soft ultrasound markers for aneuploidy in the second trimester. Am J Obstet Gynecol. 2021;225(4):B2–B15. doi:10.1016/j.ajog.2021.06.079
22. Li S, Han X, Ye M, et al. Should chromosomal microarray be offered to fetuses with ultrasonographic soft markers in second trimester: a prospective cohort study and meta-analysis. Prenat Diagn. 2020;40(12):1569–1577. doi:10.1002/pd.5815
23. Gil MM, Accurti V, Santacruz B, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):302–314. doi:10.1002/uog.17484
24. van Schendel RV, van ECG, Pajkrt E, et al. Implementing non-invasive prenatal testing for aneuploidy in a national healthcare system: global challenges and national solutions. BMC Health Serv Res. 2017;17(1):670. doi:10.1186/s12913-017-2618-0
25. Rose NC, Kaimal AJ, Dugoff L. Screening for fetal chromosomal abnormalities: ACOG Practice bulletin summary, number 226. Obstet Gynecol. 2020;136(4):859–867. doi:10.1097/AOG.0000000000004107
26. Guy GP, Hargrave J, Dunn R, et al. Secondary non-invasive prenatal screening for fetal trisomy: an effectiveness study in a public health setting. BJOG. 2021;128(2):440–446. doi:10.1111/1471-0528.16464
27. Abel DE, Alagh A. Benefits and limitations of noninvasive prenatal aneuploidy screening. JAAPA. 2020;33(4):49–53. doi:10.1097/01.JAA.0000654208.03441.23
28. Zheng Y, Wan S, Dang Y, et al. Clinical experience regarding the accuracy of NIPT in the detection of sex chromosome abnormality. J Gene Med. 2020;22(8):e3199. doi:10.1002/jgm.3199
29. Deng C, Zhu Q, Liu S, et al. Clinical application of noninvasive prenatal screening for sex chromosome aneuploidies in 50,301 pregnancies: initial experience in a Chinese hospital. Sci Rep. 2019;9(1):7767. doi:10.1038/s41598-019-44018-4
30. Yang J, Chen M, Shen W, et al. Knowledge, attitudes, and practices of healthcare professionals working in prenatal diagnosis toward expanded non-invasive prenatal testing in China. Prenat Diagn. 2022;42(1):3–14. doi:10.1002/pd.6075
31. Hu Y, Liu W, He G, et al. Clinical utility of expanded NIPT for chromosomal abnormalities and etiology analysis of cytogenetic discrepancies cases. J Assist Reprod Genet. 2022;39(1):267–279. doi:10.1007/s10815-021-02351-6
32. Practice Bulletin No. 163 summary: screening for fetal aneuploidy. Obstet Gynecol. 2016;127(5):979–981. doi:10.1097/AOG.0000000000001439
33. Dugoff L, Norton ME, Kuller JA, et al.; Society for Maternal-Fetal Medicine (SMFM). The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol. 2016;215(4):B2–9. doi:10.1016/j.ajog.2016.07.016
34. Wu X, An G, Xie X, et al. Chromosomal microarray analysis for pregnancies with or without ultrasound abnormalities in women of advanced maternal age. J Clin Lab Anal. 2020;34(4):e23117. doi:10.1002/jcla.23117
35. Srebniak MI, Joosten M, Knapen MFCM, et al. Frequency of submicroscopic chromosomal aberrations in pregnancies without increased risk for structural chromosomal aberrations: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(4):445–452. doi:10.1002/uog.17533
36. Hu ZM, Li LL, Zhang H, et al. Clinical application of chromosomal microarray analysis in pregnant women with advanced maternal age and fetuses with ultrasonographic soft markers. Med Sci Monit. 2021;27:e929074. doi:10.12659/MSM.929074
37. Fan X, Huang H, Lin X, et al. Performance of chromosomal microarray analysis for detection of copy number variations in fetal echogenic bowel. Risk Manag Healthc Policy. 2021;14:1431–1438. doi:10.2147/RMHP.S299806
38. Durham L, Papanna R, Stevens B, et al. The utilization of prenatal microarray: a survey of current genetic counseling practices and barriers. Prenat Diagn. 2019;39(5):351–360. doi:10.1002/pd.5435
39. Rj W, Cl M, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184. doi:10.1056/NEJMoa1203382
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
