Back to Journals » Journal of Pain Research » Volume 13
Comparison of Analgesic and Adverse Effects of Oxycodone- and Fentanyl-Based Patient-Controlled Analgesia in Patients Undergoing Robot-Assisted Laparoscopic Gastrectomy Using a 55:1 Potency Ratio of Oxycodone to Fentanyl: A Retrospective Study
Authors Koh JC , Kong HJ, Kim MH, Hong JH, Seong H, Kim NY , Bai SJ
Received 4 June 2020
Accepted for publication 31 July 2020
Published 4 September 2020 Volume 2020:13 Pages 2197—2204
DOI https://doi.org/10.2147/JPR.S264764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Jae Chul Koh,1,* Hee Jung Kong,2,* Myoung Hwa Kim,3 Jung Hwa Hong,4 Hyunyoung Seong,1 Na Young Kim,2 Sun Joon Bai2
1Department of Anesthesiology and Pain Medicine, Korea University Anam Hospital, Seoul, Republic of Korea; 2Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Republic of Korea; 3Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; 4Department of Policy Research Affairs, National Health Insurance Service Ilsan Hospital, Goyang, Gyeonggi-do, Republic of Korea
*These authors contributed equally to this work
Correspondence: Na Young Kim; Sun Joon Bai
Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Republic of Korea
Tel +82 2 2228 4435
; +82 2 2228 4432
Email [email protected]; [email protected]
Purpose: Oxycodone has affinities for both kappa- and mu-opioid receptors. Therefore, it has been used for postoperative analgesia of surgeries in which visceral pain is expected to be the main cause of pain. However, there are few studies of the 55:1 potency ratio of oxycodone to fentanyl when using it as intravenous patient-controlled analgesia (IV-PCA). Thus, we compared the analgesic and adverse effects of IV-PCA using the 55:1 potency ratio of oxycodone to fentanyl in patients who underwent robot-assisted laparoscopic gastrectomy.
Patients and Methods: This retrospective study included 100 patients using an automatic PCA pump with oxycodone or fentanyl who underwent robot-assisted laparoscopic gastrectomy between January and November 2017. All patients were provided with an IV-PCA consisting of 20 μg/kg of fentanyl or 1.1 mg/kg of oxycodone mixed with 0.9% normal saline solution to a total volume of 250 mL, which was infused basally at a rate of 0.1 mL/h with a bolus dose of 1 mL and lockout time of 6 min. The primary and secondary endpoints were to evaluate the efficacies of IV-PCA using the 55:1 potency ratio of oxycodone to fentanyl on analgesic and adverse effects.
Results: Pain intensity on arrival at the post-anesthesia care unit (PACU; 3.6± 1.4 vs 4.4± 2.0, P=0.031) and additional analgesic requirement within an hour after surgery (including the PACU period) (12% vs 37%; P=0.005) were significantly lower in the oxycodone group (n=49) than in the fentanyl group (n=51). Regarding adverse effects, the rate of postoperative nausea within 1 h after surgery (2% vs 16%; P=0.031) was also significantly lower in the oxycodone group than that in the fentanyl group.
Conclusion: Oxycodone-based IV-PCA by dose calculations with a 55:1 potency ratio may achieve better analgesia without any significant adverse effects, when using IV-PCA in patients undergoing robot-assisted laparoscopic gastrectomy.
Keywords: oxycodone, fentanyl, 55:1 potency ratio, intravenous patient-controlled analgesia, robot-assisted laparoscopic gastrectomy
Introduction
For the treatment of stomach cancer, minimally invasive gastrectomy using laparoscopic or robotic assistance has become a standard procedure. It has the advantages of reducing postoperative pain, lowering morbidity, and speeding up recovery by minimizing skin incisions compared to open surgery.1 Nevertheless, postoperative pain remains a problem for patients undergoing robot-assisted laparoscopic gastrectomy, and visceral pain is thought to be a significant contributor.2
Traditionally, opioid-based intravenous (IV) patient-controlled analgesia (PCA) has been the most commonly employed modality for relieving moderate to severe acute postoperative pain.3 Many studies have been conducted to investigate the ideal IV-PCA regimen for maximizing pain relief and minimizing side effects simultaneously.3,4 Among the opioids used to perform IV-PCA, fentanyl is widely used and considered more appropriate and suitable than morphine due to its fast onset and short duration of action.4,5 However, fentanyl is a potent agonist for mu-opioid receptors but has little effect on kappa-opioid receptors, which has been found to be one of the major opioid receptors involved in visceral pain.6 This mechanism may be one of the reasons why many patients complain of diffuse, vague, and poorly defined discomfort, even after IV-PCA has been applied following abdominal surgery.6 Oxycodone is a semisynthetic thebaine derivative opioid, which has affinity for both mu- and kappa-opioid receptors despite its relatively lower affinity for kappa-opioid receptors.7 Considering the important role of kappa-opioid receptors in the management of visceral pain, it is possible that oxycodone may provide more effective analgesia than pure mu-opioid receptor agonists in patients undergoing major abdominal surgery.8,9 So far, there have been studies of several efficacy ratios of oxycodone to fentanyl ranging from 55:1 to 100:1; however, there is still little evidence for analgesia and side effects of the 55:1 potency ratio of oxycodone to fentanyl during IV-PCA.10–12
Thus, this study aimed to compare the analgesic and adverse effects of oxycodone- and fentanyl-based IV-PCA in patients who have undergone robot-assisted laparoscopic gastrectomy using a 55:1 potency ratio of oxycodone to fentanyl.
Methods
Patients
This retrospective study received approval from the Institutional Review Board (IRB) and Hospital Research Ethics Committee (Yonsei University Health System, Seoul, Korea; IRB protocol No. 4-2017-0985) and was conducted in accordance with the ethical standards of the current version of the Declaration of Helsinki. The patient records and information were anonymized before analysis, which involves no more than minimal risk to subject, thus, the requirement for informed consent to obtain the medical records was exempted by IRB. On IRB approval, the electronic medical records of a total of 119 consecutive patients who underwent robot-assisted laparoscopic gastrectomy using an automatic PCA pump with oxycodone or fentanyl between January and November 2017 were identified at the single institution of the university health system.
Anesthesia Protocol
Standardized anesthesia was applied in all patients. After the patients arrived in the operating room, their baseline heart rate, mean arterial blood pressure, and oxygen saturation were monitored. Following the administration of 0.1 mg of IV glycopyrrolate as a premedication, anesthesia was induced with 1.0–1.5 mg/kg of propofol, 0.6–1.0 mg/kg of rocuronium, and 0.05–0.1 μg/kg/min of remifentanil. Age-adjusted minimal alveolar concentration end-tidal sevoflurane of 0.6–1.0 and remifentanil of 0.03–0.1 µg/kg/min were adjusted according to hemodynamic variables. To maintain a constant anesthetic depth, the bispectral index was monitored continuously, the target range of which was between 40 and 60. At the start of umbilical closure, fentanyl at a dose of 1.5 µg/kg or oxycodone at a dose of 0.0825 mg/kg and ramosetron at a dose of 0.3 mg were administered to prevent postoperative pain and nausea and vomiting. At the end of surgery, all patients were provided with an IV-PCA machine (Accumate 1100®; Woo Young Medical Co., Ltd., Seoul, Korea) consisting of 20 μg/kg of fentanyl or 1.1 mg/kg of oxycodone and 0.3 mg of ramosetron (Nasea, Astellas, Tokyo, Japan) mixed with 0.9% normal saline solution to a total volume of 250 mL, which was infused basally at a rate of 0.1 mL/h with a bolus dose of 1 mL and lockout time of 6 min. The maximum total volume per hour was set up to 10 mL. After their transfer to the post-anesthesia care unit (PACU) after surgery, instructions for the use of the PCA machine were provided to all patients, and patients were encouraged to push the button whenever they experienced the pain. Patients who expressed sustained pain (resting numerical rating scale [NRS] score ≥ 4) were intravenously administered 50 mg of tridol (Tramadol HCL®, Yuhan Co., Seoul, Korea) as additional rescue analgesic.
Data Collection
The data of age, body mass index (BMI), sex, American Society of Anesthesiologists (ASA) physical status, and coincident diseases (including hypertension, diabetes mellitus, hepatitis, and heart disease) were collected. The type of surgery, duration of anesthesia, duration of pneumoperitoneum, dose of administered remifentanil, intraoperative fluid input and output, and duration of hospitalization were recorded. The active and resting NRSs, number of cumulative bolus deliveries, number of cumulative bolus attempts, mean blood pressure (MBP), and heart rate (HR) were collected from the medical records at PACU, 1, 6, 12, 24, and 48 h after the surgery. The number of patients who received additional rescue analgesics and experienced side effects such as nausea, vomiting, dizziness, headache, retching, numbness, and chills were assessed during 48 hours after surgery. And level of sedation was assessed on arrival to the PACU.
Statistical Analysis
The primary endpoint was the evaluation of the analgesic efficacies of oxycodone- and fentanyl-based IV-PCA in patients who underwent robot-assisted laparoscopic gastrectomy using a 55:1 potency ratio of oxycodone to fentanyl. The secondary endpoints were the influence of the 55:1 potency ratio of oxycodone to fentanyl on the adverse effects during 48 hours after the surgery.
Comparisons of continuous variables were performed using independent two-sample t-tests and are presented as mean ± standard deviation. And the comparisons of categorical variables were performed using the chi-square test or Fisher`s exact test and are presented as number of patients (proportion). Analyses of repeatedly measured variables such as the NRS, MBP, HR, number of bolus deliveries, and the ratio of deliveries to attempts were performed using a linear mixed model. When the interaction of group, time, and group-by-time showed statistical significance, a post hoc analysis was performed with Bonferroni correction to adjust for multiple comparisons. Logistic and linear regression were performed for the administration of additional rescue analgesic and nausea as well as NRS at the PACU. A P value of <0.05 was regarded as statistically significant. All statistical analyses were performed using the SAS version 9.4 software (SAS Inc., Cary, NC, USA).
Results
Of 119 patients who underwent robot-assisted laparoscopic gastrectomy using an automatic PCA pump with oxycodone or fentanyl, 1 patient for whom the surgery type was converted to open gastrectomy and 18 patients who had incomplete data were excluded. Of the remaining 100 patients, 49 and 51 patients received oxycodone- and fentanyl-based IV-PCA, respectively (Figure 1).
 |
Figure 1 Consort diagram of patients. Abbreviation: IV-PCA, intravenous patient-controlled analgesia. |
Table 1 shows the demographics and intraoperative variables of the 100 patients who underwent robot-assisted laparoscopic gastrectomy. There were no significant differences in other variables between the two groups except sex and history of hypertension. The numbers of female patients and those with a history of hypertension were significantly higher in the fentanyl group than those in the oxycodone group. The pain intensity on arrival at the PACU was significantly lower in the oxycodone group than in the fentanyl group (3.6 ± 1.4 vs 4.4 ± 2.0, P = 0.031). However, from the discharge of the PACU until 48 h after surgery, there were no significant differences in NRSs between the two groups (Figure 2). In addition, there was no significant difference between the numbers of patients who reported NRS≥4 and NRS<4 at any time points.
 |
Table 1 Demographics and Intraoperative Parameters |
 |
Figure 2 Changes in resting (A) and active (B) pain intensity until 48 hours after surgery.Abbreviation: NRS, numeric rating score. |
Significant intergroup differences in the cumulative bolus deliveries were observed over time in the linear mixed model analysis (P Group × Time < 0.001) (Figure 3A). Patients in the oxycodone group had a significantly lower number of bolus deliveries than those in the fentanyl group at 24 and 48 h after surgery (Bonferroni corrected P = 0.009 and 0.003, respectively). However, no significant intergroup differences in the cumulative bolus attempts and deliveries/attempts ratio were noted throughout all time points after surgery (Figure 3B and C).
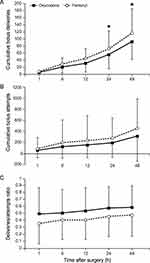 |
Figure 3 Number of cumulative bolus deliveries (A), cumulative bolus attempts (B), and ratio of deliveries to attempts (C). *Bonferroni-corrected P < 0.05 compared with the fentanyl group. |
Furthermore, the number of patients who required additional rescue analgesics and experienced postoperative nausea at the PACU was significantly lower in the oxycodone group than that in the fentanyl group (rescue analgesics, 12% vs 37%; P = 0.005, nausea, 2% vs 16%; P = 0.031, respectively). However, after discharge from the PACU, no significant differences were observed between the two groups (Table 2). In multivariate regression, the fentanyl group had a significantly higher risk of additional rescue analgesic requirements at the PACU compared to the oxycodone group (odds ratio [OR] = 4.68; 95% confidence interval [CI], 1.53–14.37, P = 0.007). However, the risk of nausea in the PACU was no longer significant (Table 3). In addition, the NRS in the PACU remained significantly higher in the fentanyl group than that in the oxycodone group even after multivariate analysis (Beta, 1.017; Standard error, 0.374, P = 0.008).
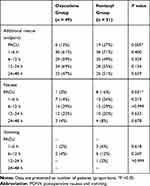 |
Table 2 Number of Patients Who Required Additional Rescue Analgesics and Expressed PONV During 48 Hours After Surgery |
 |
Table 3 Multivariate Regression Analysis of the Number of Patients Requiring Additional Rescue Analgesics and Who Expressed Postoperative Nausea in the PACU |
Oher postoperative adverse effects such as dizziness, headache, retching, numbness, and chills were not statistically different between the two groups. Also, there were no significant differences in sedation level on arrival to the PACU (Table 4). No differences in MBP and HR were observed between the two groups, as shown in Figure 4.
 |
Table 4 Other Adverse Effects During 48 Hours After Surgery |
 |
Figure 4 Mean blood pressure (A) and heart rate during 48 h after surgery (B). Abbreviation: PACU, post-anesthesia care unit. |
Discussion
We conducted this retrospective study based on a review of medical records to compare the analgesic and adverse effects of oxycodone- and fentanyl-based IV-PCA in patients who underwent robot-assisted laparoscopic gastrectomy using a 55:1 potency ratio of oxycodone to fentanyl. According to the results of this study, IV-PCA based on oxycodone showed better analgesia and lower postoperative nausea than that based on fentanyl during early postoperative period.
Several opioids have been used for IV-PCA. Among the opioids, morphine is well known for its side effects such as sedation, nausea/vomiting, pruritis, and respiratory depression. Therefore, fentanyl has been more widely used for IV-PCA.13 However, another hydrophilic opioid, oxycodone, has been introduced. This opioid showed lower sedation than morphine, and the incidence of other side effects were comparable with that of fentanyl.14 For this reason, attempts have been made to use oxycodone in IV-PCA.
Morphine and oxycodone have been reported to have analgesic potencies of 1:1–1.5 when administered intravenously.15–17 Thus, a number of studies have been conducted with potency ratios of oxycodone to fentanyl of 75–100:1.8,13,18,19 In these studies, during the postoperative period, oxycodone produced similar or better results in terms of analgesia than fentanyl while the incidences of adverse effects were higher. However, in terms of analgesic effects, opioids do not have a ceiling effect, and more effective analgesia can be achieved by higher doses. However, higher doses of opioids are associated with adverse effects such as respiratory depression, sedation, and nausea/vomiting. Therefore, an important aspect in the use of opioids is to use as low of a dose as possible to maximize analgesic effects while minimizing side effects.
Therefore, studies have been conducted using a potency ratio lower than 1:75 for the purpose of postoperative analgesia.10,12 Despite the lower doses used in these studies, oxycodone showed similar analgesic potency compared to that of fentanyl. However, in the present study with a potency ratio of 55:1, oxycodone was found to have even superior analgesic effects in the PACU than those of fentanyl. This study was performed with patients who underwent robot-assisted laparoscopic gastrectomy, which can be performed with less skin or muscle damage compared to open repair.1 In such surgeries, visceral pain may be a very important contributor to postoperative pain. Kappa-opioid receptor affinity may be associated with better analgesia in these surgeries.
Oxycodone has similar properties to those of morphine in terms of hydrophilicity and protein binding capacity.20 However, oxycodone has higher affinities for kappa and delta receptors than morphine.7,21 Kappa-opioid receptors are known to have a distribution and function that differentiate them from the most well-known mu-opioid receptors. Kappa-opioid receptors are widely distributed in the peripheral sensory neurons, as well as in the brain and spinal cord.17,22 It has been reported that they may be associated with effective control of visceral pain.17,23 This mechanism may explain the result of sufficient analgesia with a lower dose of oxycodone when visceral pain may play a major role in postoperative pain, as observed in this study. In this study, fewer patients in the oxycodone group required rescue analgesics in the early postoperative period even after multivariate analysis. In addition, patients in the oxycodone group had a significantly lower number of bolus deliveries than those in the fentanyl group at 24 and 48 h after surgery, which indicates that the usage of oxycodone at a 55:1 potency ratio may have increased the efficacy. Therefore, the results of the present study suggest that oxycodone-based IV-PCA with a 55:1 potency ratio used in these surgeries can provide sufficient analgesia both in the early and late postoperative periods. However, further investigation is required to better clarify these observations.
Oxycodone has been reported to be associated with a higher nausea incidence than fentanyl.8,19,24 Even at a 60:1 potency ratio of oxycodone to fentanyl, Park et al reported a higher incidence of nausea in the oxycodone group.12 In contrast, Jung et al reported that there is no difference in the incidence rate of nausea between groups at the 55:1 potency ratio.10 Consistent with previous reports, multivariate analysis in the current study revealed no significant difference in the incidence rate of nausea between the two groups. In addition, oxycodone has been reported to be associated with a higher incidence of other side effects than fentanyl, such as dizziness, headache, and sedation.12,14,25 In particular, Park et al reported significantly higher rates of dizziness even at a potency ratio of 60:1.8,12 However, according to the results of this study, oxycodone using a potency ratio of 55:1 would not increase the incidence of other side effects compared with fentanyl.
There are some limitations to this study. The retrospective design made it difficult to thoroughly control the variables. Although we tried to use standardized anesthesia and pain control methods in our institution, additional factors might have affected the results, such as the bolus dose administered at the end of surgery and the influence of additional rescue analgesics on the adverse effects. In addition, because some quantitative indicators were not measured, such as the degree of nausea in scales, it was difficult to perform a more in-depth analysis. The power may have been increased if the sample size had been larger. Additional, larger-scale studies with other designs are warranted for better clarification.
Conclusions
Oxycodone-based IV-PCA by dose calculations with a 55:1 potency ratio may achieve better analgesia without any significant adverse effects, when using IV-PCA in patients undergoing robot-assisted laparoscopic gastrectomy. Further, larger-scale studies with other designs are needed for better clarification.
Abbreviations
ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; HR, heart rate; IV, intravenous; IV-PCA, intravenous patient-controlled analgesia; MBP, mean blood pressure; NRS, numerical rating scale; OR, odds ratio; PACU, post-anesthesia care unit; PCA, patient-controlled analgesia.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Gholami S, Cassidy MR, Strong VE. Minimally invasive surgical approaches to gastric resection. Surg Clin North Am. 2017;97(2):249–264. doi:10.1016/j.suc.2016.11.003
2. Ding Z, Wang K, Wang B, Zhou N, Li H, Yan B. Efficacy and tolerability of oxycodone versus fentanyl for intravenous patient-controlled analgesia after gastrointestinal laparotomy: a prospective, randomized, double-blind study. Medicine (Baltimore). 2016;95(39):e4943. doi:10.1097/md.0000000000004943
3. Palmer PP, Miller RD. Current and developing methods of patient-controlled analgesia. Anesthesiol Clin. 2010;28(4):587–599. doi:10.1016/j.anclin.2010.08.010
4. Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66(18):2321–2337. doi:10.2165/00003495-200666180-00005
5. Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101(5 Suppl):S44–S61. doi:10.1213/01.ane.0000177102.11682.20
6. Han L, Su Y, Xiong H, et al. Oxycodone versus sufentanil in adult patient-controlled intravenous analgesia after abdominal surgery: a prospective, randomized, double-blinded, multiple-center clinical trial. Medicine (Baltimore). 2018;97(31):e11552. doi:10.1097/md.0000000000011552
7. Kokki H, Kokki M, Sjövall S. Oxycodone for the treatment of postoperative pain. Expert Opin Pharmacother. 2012;13(7):1045–1058. doi:10.1517/14656566.2012.677823
8. Kim NS, Lee JS, Park SY, et al. Oxycodone versus fentanyl for intravenous patient-controlled analgesia after laparoscopic supracervical hysterectomy: a prospective, randomized, double-blind study. Medicine (Baltimore). 2017;96(10):e6286. doi:10.1097/md.0000000000006286
9. Kucharz J, Filipczak-Bryniarska I, Michalowska-Kaczmarczyk A, Herman RM, Krzemieniecki K. Use of high-dose oxycodone hydrochloride in patients with visceral and neuropathic pain. Contemp Oncol (Pozn). 2015;19(3):257–259. doi:10.5114/wo.2015.52662
10. Jung KW, Kang HW, Park CH, et al. Comparison of the analgesic effect of patient-controlled oxycodone and fentanyl for pain management in patients undergoing colorectal surgery. Clin Exp Pharmacol Physiol. 2016;43(8):745–752. doi:10.1111/1440-1681.12586
11. Koch S, Ahlburg P, Spangsberg N, Brock B, Tønnesen E, Nikolajsen L. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta Anaesthesiol Scand. 2008;52(6):845–850. doi:10.1111/j.1399-6576.2008.01643.x
12. Park JH, Lee C, Shin Y, An JH, Ban JS, Lee JH. Comparison of oxycodone and fentanyl for postoperative patient-controlled analgesia after laparoscopic gynecological surgery. Korean J Anesthesiol. 2015;68(2):153–158. doi:10.4097/kjae.2015.68.2.153
13. Kim MK, Ahn SE, Shin E, Park SW, Choi JH, Kang HY. Comparison of analgesic efficacy of oxycodone and fentanyl after total hip replacement surgery: a randomized controlled trial. Medicine (Baltimore). 2018;97(49):e13385. doi:10.1097/md.0000000000013385
14. Raff M, Belbachir A, El-Tallawy S, et al. Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain Ther. 2019;8(1):19–39. doi:10.1007/s40122-019-0122-4
15. Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand. 1998;42(5):576–580. doi:10.1111/j.1399-6576.1998.tb05169.x
16. Kalso E, Pöyhiä R, Onnela P, Linko K, Tigerstedt I, Tammisto T. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand. 1991;35(7):642–646. doi:10.1111/j.1399-6576.1991.tb03364.x
17. Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123(1–2):28–36. doi:10.1016/j.pain.2006.02.006
18. Herrick IA, Ganapathy S, Komar W, et al. Postoperative cognitive impairment in the elderly. Choice of patient-controlled analgesia opioid. Anaesthesia. 1996;51(4):356–360. doi:10.1111/j.1365-2044.1996.tb07748.x
19. Hwang BY, Kwon JY, Kim E, Lee DW, Kim TK, Kim HK. Oxycodone vs. fentanyl patient-controlled analgesia after laparoscopic cholecystectomy. Int J Med Sci. 2014;11(7):658–662. doi:10.7150/ijms.8331
20. Pöyhiä R, Seppälä T. Liposolubility and protein binding of oxycodone in vitro. Pharmacol Toxicol. 1994;74(1):23–27. doi:10.1111/j.1600-0773.1994.tb01068.x
21. Garcia MM, Goicoechea C, Avellanal M, Traseira S, Martín MI, Sánchez-Robles EM. Comparison of the antinociceptive profiles of morphine and oxycodone in two models of inflammatory and osteoarthritic pain in rat. Eur J Pharmacol. 2019;854:109–118. doi:10.1016/j.ejphar.2019.04.011
22. Snyder LM, Chiang MC, Loeza-Alcocer E, et al. Kappa opioid receptor distribution and function in primary afferents. Neuron. 2018;99(6):1274–1288. doi:10.1016/j.neuron.2018.08.044
23. Olesen AE, Staahl C, Arendt-Nielsen L, Drewes AM. Different effects of morphine and oxycodone in experimentally evoked hyperalgesia: a human translational study. Br J Clin Pharmacol. 2010;70(2):189–200. doi:10.1111/j.1365-2125.2010.03700.x
24. Dinges HC, Otto S, Stay DK, et al. Side effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Analg. 2019;129(4):1153–1162. doi:10.1213/ane.0000000000003887
25. Kim NS, Kang KS, Yoo SH, et al. A comparison of oxycodone and fentanyl in intravenous patient-controlled analgesia after laparoscopic hysterectomy. Korean J Anesthesiol. 2015;68(3):261–266. doi:10.4097/kjae.2015.68.3.261
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
