Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Comparing Clinical Outcomes of Tiotropium/Olodaterol, Umeclidinium/Vilanterol, and Indacaterol/Glycopyrronium Fixed-Dose Combination Therapy in Patients with Chronic Obstructive Pulmonary Disease in Taiwan: A Multicenter Cohort Study
Authors Hsieh MJ , Chen NH, Cheng SL, Tao CW, Wei YF , Wu YK , Chan MC, Liu SF , Hsu WH, Yang TM, Lin MS, Liu CL , Kuo PH , Tsai YH
Received 13 December 2021
Accepted for publication 28 March 2022
Published 27 April 2022 Volume 2022:17 Pages 967—976
DOI https://doi.org/10.2147/COPD.S353799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Meng-Jer Hsieh,1 Ning-Hung Chen,2 Shih-Lung Cheng,3,4 Chi-Wei Tao,5 Yu-Feng Wei,6,7 Yao-Kuang Wu,8 Ming-Cheng Chan,9,10 Shih-Feng Liu,11– 13 Wu-Huei Hsu,14 Tsung-Ming Yang,15 Ming-Shian Lin,16,17 Ching-Lung Liu,18 Ping-Hung Kuo,19 Ying-Huang Tsai1,20
1Department of Pulmonary and Critical Care Medicine, Linkou Chang Gung Memorial Hospital, Chang Gung Medical Foundation and Department of Respiratory Therapy, College of Medicine, Chang Gung University, Taoyuan, Taiwan; 2Department of Pulmonary and Critical Care Medicine, Linkou Chang Gung Memorial Hospital and School of Traditional Chinese Medicine, Chang Gung University, Taoyuan, Taiwan; 3Department of Internal Medicine, Far Eastern Memorial Hospital, Taipei, Taiwan; 4Department of Chemical Engineering and Materials Science, Yuan Ze University, Zhongli, Taoyuan, Taiwan; 5Department of Internal Medicine, Cheng-Hsin General Hospital, Taipei, Taiwan; 6School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung, Taiwan; 7Department of Internal Medicine, E-Da Cancer Hospital, Kaohsiung, Taiwan; 8Division of Pulmonary Medicine, Department of Internal Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; 9Division of Critical Care and Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 10National Chung Hsing University, Taichung, Taiwan; 11Department of Respiratory Therapy, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 12Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 13College of Medicine, Chang Gung University, Taoyuan, Taiwan; 14Critical Medical Center, China Medical University Hospital, Taichung, Taiwan; 15Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan; 16Division of Pulmonary Medicine, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan; 17Department of Respiratory Care, Chang Gung University of Science and Technology, Chiayi, Taiwan; 18Division of Chest Medicine, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan; 19Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan; 20Department of Pulmonary and Critical Care Medicine, Xiamen Chang Gung Hospital, Xiamen, 361028, People’s Republic of China
Correspondence: Ying-Huang Tsai, Department of Pulmonary and Critical Care Medicine, Linkou Chang Gung Memorial Hospital, Chang Gung Medical Foundation and Department of Respiratory Therapy, College of Medicine, Chang Gung University, No. 5 Fu-Xin St, Kweishan District, Taoyuan, Taiwan, Email [email protected]
Background: Long-acting beta-agonists (LABA) and long-acting muscarinic antagonists (LAMA) combination therapy improved lung function and health-related quality-of-life and reduced exacerbation rates and dyspnea in symptomatic chronic obstructive pulmonary disease (COPD) patients. We compared the real-world effects of three fixed-dose LABA/LAMA combinations for COPD in Taiwan.
Methods: This multicenter, retrospective study evaluated 1-year outcomes after LABA/LAMA combination therapy in patients with symptomatic COPD. Exacerbations and symptoms of COPD, lung functions, and therapy escalation were compared among patients using tiotropium/olodaterol, umeclidinium/vilanterol and indacaterol/glycopyrronium. Propensity score matching (PSM) was applied to balance the baseline characteristics.
Results: Data of 1,617 patients were collected. After PSM, time to first moderate-to-severe COPD exacerbation was comparable among three groups, while the annualized rates of the exacerbation (episodes/patient/year) in patients receiving tiotropium/olodaterol (0.19) or umeclidinium/vilanterol (0.17) were significantly lower than those receiving indacaterol/glycopyrronium (0.38). COPD-related symptoms were stable over the treatment period, and there was no significant difference in the changes of symptom scores including CAT and mMRC among three groups at the end of the study period.
Conclusion: This study presented valuable real-world outcome in terms of exacerbation and treatment response of COPD patients treated with fixed-dose LABA/LAMA regimens in Taiwan. The annualized rates of moderate-to-severe exacerbation in patients receiving tiotropium/olodaterol or umeclidinium/vilanterol were significantly lower than those receiving indacaterol/glycopyrronium, though the time to first moderate-to-severe exacerbation was similar among different fixed-dose LABA/LAMA combinations.
Keywords: tiotropium/olodaterol, umeclidinium/vilanterol, indacaterol/glycopyrronium, LABA/LAMA therapy, Taiwan, cohort study, propensity score matching, chronic obstructive pulmonary disease, moderate-to-severe exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is a treatable lung disease characterized by persistent symptoms, including cough, sputum production, dyspnea, and progressive airflow obstruction that is not fully reversible.1 According to a report of the World Health Organization (WHO),2 COPD was the fifth cause of burden of disease in 2010 and became the third leading cause of death in 2019 around the world.3 In Taiwan, COPD was the seventh leading cause of death in 2016,4 with an estimated prevalence of 6.1% in 2013.5 Frequent exacerbations occurring ≥2 times/year is a predictor for a poor prognosis and increase in mortality in COPD.6,7 According to a Taiwanese population-based study, the in-hospital mortality for patients who had hospitalized exacerbations was 4.2%, and 1-year mortality after discharge was 22%.8 Therefore, prevention of the exacerbation remains one of the most important issues for COPD management.
Bronchodilator therapy is the core element in COPD management. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines claims that combination therapy (fixed-dose combination [FDC] or free combinations) with long-acting beta-agonists (LABA) and long-acting muscarinic antagonists (LAMA) can increase forced expiratory volume in 1 second (FEV1) and decrease symptoms and exacerbation of COPD in comparison with monotherapy.1 A systematic review and meta-analysis in support of the American Thoracic Society clinical practice guideline also presents similar beneficial results.9 However, the treatment outcomes following different LABA/LAMA regimens are variable and there has been no consensus about the optimal LABA/LAMA regimen. A network meta-analysis showed that tiotropium/olodaterol might have a better improvement in efficacy and safety profile compared with other LABA/LAMA therapy.10 However, another network meta-analysis found that, regardless of moderate-to-severe exacerbation and mortality, no significant difference between LABA/LAMA combinations (ie, tiotropium/salmeterol, indacaterol/glycopyrronium, umeclidinium/vilanterol, tiotropium/olodaterol, aclidinium/formoterol, and glycopyrronium/formoterol) was observed.11
Data on acute exacerbation of COPD patients treated with different LABA/LAMA therapy in Taiwan are limited. Hence, we conducted a retrospective, multicenter study to collect the clinical outcomes of COPD patients receiving different LABA/LAMA therapies for exploring 1) outcomes of COPD exacerbation in patients treated with different LABA/LAMA combinations or LAMA following the routine practice and 2) the clinical characteristics of COPD patients in Taiwan. Data of patients receiving tiotropium/olodaterol (FDC, cohort A), other LABA/LAMA combinations (FDC or free combination, cohort B), or LAMA monotherapy were collected. The current analysis reported clinical outcomes compared among three fixed-dose LABA/LAMA combinations after balancing their baseline characteristics using propensity score matching (PSM).
Methods
Study Design
This retrospective, multicenter study collected data from patients with COPD in 12 medical centers and regional hospitals in Taiwan (TOReTO; NCT04011475). Patients meeting the following criteria were included: 1) were diagnosed with COPD and prescribed with LABA/LAMA (FDC or free combinations) as a new initiation or switching from another therapy (ie, single/dual/triple), or newly receiving LAMA treatment for 3 months at least prior to June 30, 2018; and 2) aged ≥40 years. Patients who had bronchial asthma, asthma–COPD overlap syndrome, bronchiectasis, cystic fibrosis, or lung cancer were excluded. Eligible patients were categorized into three cohorts based on the prescriptions for COPD, including cohort A (tiotropium/olodaterol; Spiolto®, Boehringer Ingelheim), cohort B (other LABA/LAMA combination therapies), and cohort C (LAMA monotherapy). Patients were followed from the index date (ie, started receiving LABA/LAMA or LAMA treatment or receiving LABA/LAMA switched from other single/dual/triple treatment) until the date of death or 1 year after the index date, whichever occurred first.
The current analysis presents the comparative treatment outcomes among three fixed-dose LABA/LAMA combinations, ie, tiotropium/olodaterol (Tio/Olo), umeclidinium/vilanterol (Umec/Vi), and indacaterol/glycopyrronium (Ind/Gly). The primary endpoint was time to first moderate-to-severe exacerbation. Secondary endpoints included annualized rate of moderate-to-severe exacerbation, time to therapy escalation, and changes in lung functions (ie, FEV1 and forced volume vital capacity [FVC], COPD assessment test (CAT) score, and modified Medical Research Council dyspnea scale (mMRC).
Data Collection
Collected data included clinical diagnosis of COPD, records of exacerbations and hospitalization, spirometry data (FEV1 and FVC), questionnaire results (CAT and mMRC), blood eosinophil counts, and COPD-related treatments in the pre-treatment period (within 1 year before starting or shifting to LABA/LAMA combinations) and 1 year follow-up period.
Questionnaires Used for Evaluating the Symptoms and Dyspnea
Questionnaires of CAT and mMRC were applied for assessing the COPD-related symptoms and severity of dyspnea, respectively. For the CAT, a decreasing score represented an improvement in the symptoms, whereas an increasing score represented a worsening in the symptoms.12 The most reliable estimate of the minimum significant difference in the CAT score was 2 points.13 The minimal clinically important difference (MCID) for mMRC score was −1.14
Exacerbations of COPD
The severities of COPD exacerbations were defined according to GOLD guidelines.1 The guidelines define COPD exacerbation as an acute worsening of respiratory symptoms that results in additional therapy. Exacerbations are classified as moderate if they are treated with short-acting bronchodilators plus antibiotics and/or oral corticosteroids; or severe if the patient visits the emergency room or requires hospitalization because of an exacerbation.
Statistical Analysis
Continuous variables were compared among groups using one-way ANOVA or Kruskal Wallis tests. Categorical variables were analyzed using Chi-square test. Time-to-event were illustrated using Kaplan-Meier curves and analyzed by Log rank tests. Moreover, Cox regression with Likelihood score tests were applied for analyzing the hazard ratio of time to first exacerbation among groups. The annualized rate of moderate-to-severe exacerbation was calculated for each study group (episodes/patient/year), with differences between the groups compared by rate ratio using Poisson regression.
PSM was used to balance the patient characteristics of three fixed-dose LABA/LAMA combinations before comparison. Multivariable logistic regression was used to calculate the propensity score for three groups using the baseline covariates, including age, gender, smoking status (current smoker vs former smoker vs non-smoker), GOLD grade (grade 1/2 vs grade 3/4), and history of exacerbation (yes vs no). Following this, 1:1:1 matching was conducted by the nearest neighbor method within a caliper of 0.2 of the propensity score.
A significant difference was defined as a P-value <0.05. Data analyses were performed using statistical analysis software (SAS®) version 9.4 (SAS Institute, Cary, NC).
Ethics Statement
This study was approved by the National Taiwan University Hospital Research Ethics Committee (REC) (201908002RSA), Taichung Veterans General Hospital Institutional Review Board (IRB) (SE19299B), Chiayi Christian Hospital IRB (IRB2019066), Chang Gung Medical Foundation IRB (201901282B0), E-Da Hospital IRB (EMRP-108-105), China Medical University Hospital REC (CMUH108-REC3-119), MacKay Memorial Hospital IRB (19CT048be), Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation IRB (08-FS-090), Far Eastern Memorial Hospital Research Ethics Review Committee (108148-E), and Cheng Hsin General Hospital IRB ([727]108B-43), and conducted per the applicable local regulations and the ethical principles outlined in the Declaration of Helsinki. The informed consent was waived due to secondary use of de-identification data.
Results
This study collected data of 1,617 patients with COPD. Of these patients, 239 received tiotropium/olodaterol (cohort A), 937 received other LABA/LAMA combination therapy (cohort B), and 441 received LAMA monotherapy (cohort C). In cohort B, 447 received umeclidinium/vilanterol, 357 received indacaterol/glycopyrronium, and 133 received other free LABA/LAMA combinations. After PSM, 168 patients in each of the fixed-dose LABA/LAMA combinations (Tio/Olo, Umec/Vi and Ind/Gly) were analyzed (Figure 1).
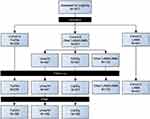 |
Figure 1 Patient distribution according to the prescribed bronchodilators. |
Table 1 summarizes the demographic data and baseline characteristics per group. Before PSM, three groups showed significant differences in age, metal dust exposure, smoking status, family history of lung cancer, GOLD grade of airway obstruction, and GOLD grouping of COPD. After PSM, the overall characteristics were comparable except for the metal dust exposure history, family history of lung cancer, and COPD groups distribution (P=0.0318). After PSM, those who received Tio/Olo had a lower percentage of patients in group A (7.4% vs 18.7% and 22.8%) and a higher percentage in group D (24.7% vs 14.4% and 12.6%). Table 2 demonstrates other baseline characteristics related to the risk of future exacerbation after PSM. The percentages of patients who experienced exacerbation within 1 year prior to index date have no significant difference among three FDC groups. There was also no significant difference in the baseline lung functions, CAT scores, mMRC scores, and eosinophil counts after PSM. Regarding inhaled medications used prior to current LABA/LAMA FDC, only a small proportion of patients were de-escalated from ICS/LABA/LAMA combination. The distribution of inhaled medications prior to the index date was similar among three groups.
 |
Table 1 Baseline Characteristics of Patients Treated with Fixed-Dose LABA/LAMA Before and After Propensity Score Matching |
 |
Table 2 Baseline Characteristics Related to Future Exacerbation of Patients Receiving Fixed-Dose LABA/LAMA After PSM |
Moderate-to-Severe Exacerbation Occurring in Patients Receiving LABA/LAMA FDC
The time to first moderate-to-severe COPD exacerbation within 1-year follow-up did not significantly differ among three groups (Figure 2A), with a similar risk of exacerbation (hazard ratio=1.485, 95% confidence interval [CI]=0.860–2.565, P=0.1564 in Ume/Vi, and hazard ratio=0.934, 95% confidence interval [CI]=0.510–1.712, P=0.8252 in Ind/Gly, with Tio/Olo as reference) observed after PSM. Up to 87% of patients experienced acute exacerbation during the study period related to infection and were treated with antibiotics. All of the patients with acute exacerbation had received a short course of oral corticosteroids.
 |
Figure 2 (A) Time to first moderate-to-severe acute exacerbation, (B) time to therapy escalation, within 1-year follow-up using Kaplan-Meier curves after propensity score matching. |
We tabulated the annualized rate of moderate-to-severe exacerbation by LABA/LAMA FDC groups in Table 3. The annualized rate of acute exacerbation was not significantly different between Tio/Olo and Umec/Vi (RR=1.10, 95% CI=0.67–1.82, P=0.7010). The annualized rates of acute exacerbation in Tio/Olo and Ume/Vi were significantly lower than the rate in Ind/Gly after PSM (0.19 and 0.17 vs 0.38), with P-values of 0.0004 and 0.0014, respectively.
 |
Table 3 Comparison of Annualized Rates of Moderate-to-Severe Exacerbation in Patients Receiving Fixed-Dose LABA/LAMA Combinations After PSM |
Therapy Escalation to Triple Therapy After Patients Receiving Tiotropium/Olodaterol, Umeclidinium/Vilanterol, or Indacaterol/Glycopyrronium
Figure 2B presents the time to therapy escalation to triple therapy within 1-year follow-up by groups after PSM. In general, there was no significant difference in therapy escalation among the three groups during the follow-up period (P=0.7620).
Changes in Lung Function, COPD-Related Symptoms, and Dyspnea by Cohorts
The summary of change from baseline in lung function, COPD-related symptoms, and dyspnea scores after PSM is presented in Tables 4 and 5. Compared with Umec/Vi and Ind/Gly after 12-month treatment, the lung function of patients under Tio/Olo became much worse, while their COPD-related symptoms remained stable. Regarding the lung function, a decreasing trend in FEV1 was found in Tio/Olo but not in Umec/Vi or Ind/Gly, with similar trends detected in FVC at 12 months (Table 4). In terms of COPD-related symptoms assessed by CAT, changes in CAT in all three groups were limited after 12 month-treatment (Table 5). However, though without among-group difference, those receiving fixed-dose LABA/LAMA therapy had 25–38.5% of patients reaching the minimal clinically important difference (MCID) for the CAT (ie, decline in CAT ≥2 points). For mMRC, there was an among-group difference in the changes of mMRC score and the percentages of patients reaching MCID for mMRC at 6 months but not at 12 months.
 |
Table 4 Changes in Lung Function in Patients Receiving Fixed-Dose LABA/LAMA Combinations After PSM |
 |
Table 5 Change of CAT and mMRC from Baseline and Proportions of Patients Achieving MCID for CAT or mMRC in Patients Receiving Fixed-Dose LABA/LAMA Combinations After PSM |
Discussion
To our knowledge, this is the first real-world, nationwide, multicenter study to evaluate the clinical outcomes of fixed-dose tiotropium/olodaterol, umeclidinium/vilanterol, and indacaterol/glycopyrronium therapy for COPD in Taiwan. Our study demonstrates that there was no significant difference in the time to first moderate-to-severe exacerbation among patients receiving three different LABA/LAMA FDC. Notably, although a previous network meta-analysis reported a comparable risk of moderate-to-severe exacerbation between different dual regimens,11 our results showed a significantly lower annualized exacerbation rates of 0.19 or 0.17 per patient per year in patients under tiotropium/olodaterol or umeclidinium/vilanterol therapy than that under indacaterol/glycopyrronium therapy (0.38 per patient per year). The exacerbation rate with tiotropium/olodaterol was also lower than those reported in the DYNAGITO study (0.90 per patient per year) and the Japanese subgroup of the DYNAGITO study (0.94 per patient per year).15,16 However, the different study design may contribute to the different outcomes among these studies.
Concerning the lung function, the FEV1 of patients receiving umeclidinium/vilanterol combinations was improved more than that of patients receiving tiotropium/olodaterol or indacaterol/glycopyrronium, which is similar to the previous results presented by Feldman et al17 (tiotropium/olodaterol vs umeclidinium/vilanterol: 128 mL vs 180 mL after a 2-month treatment). Confusingly, the FEV1 of patients treated with tiotropium/olodaterol in our study was decreased, which conflicted with the findings of other studies, where an improvement of FEV1 after patients receiving tiotropium/olodaterol was observed.18,19 The lack of data integrity for spirometry examination in the current real-world study may be one of the reasons. In a real-world setting, stable patients may not regularly return to the hospitals to take the spirometry test, and those with unstable symptoms might have more frequent follow-ups with spirometry. These could have resulted in the missing data in our study. Another possible reason for the different lung function responses among three groups could be the different GOLD distributions we had using PSM to adjust the baseline GOLD obstruction grades and previous exacerbation history. Caution is needed in interpreting the current results.
Results of CAT and mMRC also inherited with similar limitations regarding data integrity. We observed that 21.9–35.3% of patients had reached the MCID of mMRC score and 25–38.5% of patient had reached the MCID of CAT score with the LABA/LAMA therapy. The changes of mMRC and CAT scores at the end the study period were not significantly different among patients receiving tiotropium/olodaterol, umeclidinium/vilaerol, and indacaterol/glycopyrronium. On the other hand, only 10.8%, 8.9%, and 11.3% of patients receiving tiotropium/olodaterol, umeclidinium/vilaerol, or indacaterol/glycopyrronium escalated to triple therapy to manage their symptoms, and the rates of therapy escalation was not significantly different among three groups. These results might suggest that LABA/LAMA combinations could effectively improve the symptoms in a large proportion of symptomatic COPD patients. Our results echoed the treatment strategy recommended by GOLD management.1
There were several limitations to this study. First, the lack of data integrity was inherited in a retrospective study design. To reflect routine practice under the management for COPD in Taiwan, we did not deal with the missing data. Second, we collected data of COPD patients who had been treated with LABA/LAMA for 3 months at least. These patients might have relatively stable COPD symptoms and lung function and might not require regular follow-ups of pulmonary function test under the routine practice. Those patients with stable condition might also have lower willing to perform these tests. Such a real-world pattern can further impact the data integrity. Hence, our findings need to be interpreted with caution. Third, the imbalance of baseline characteristics and numbers of patients among groups are also common in a non-randomized real-world study. The choice of different inhaled devices frequently depends on the availability of medications and the preference of the patients and physicians. Consequently, we utilized the PSM to balance the baseline characteristics. Lastly, the study merely collected 1-year clinical data after patients receiving COPD treatments. The short follow-up period might limit the interpretation of time to first moderate-to-severe acute exacerbation. A long-term study may be warranted.
Conclusion
This study presented valuable real-world outcomes in terms of exacerbation and treatment response of COPD patients treated with different fixed-dose LABA/LAMA regimens in Taiwan. Although the time to first moderate-to-severe exacerbation was comparable among three fixed-dose LABA/LAMA combinations, the annualized rates of patients receiving tiotropium/olodaterol or umeclidinium/vilanterol were significantly lower than the rate in those receiving indacaterol/glycopyrronium under real-world practice. We also observed a similar effect in the improvement of COPD symptoms in patients receiving different fixed-dose LABA/LAMA combinations. However, though we have adjusted the baseline characteristics of our patients with PSM, the findings need to be interpreted with caution due to the non-randomized study design.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank all patients and investigators involved in the study. We also thank study nurses, and other clinical staff.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was fully sponsored by Boehringer Ingelheim Taiwan Ltd. The operation, data management, planned and post-hoc statistics, and writing support during the development of this manuscript in the clinical study was outsourced to Formosa Biomedical Technology Corp.
Disclosure
The authors declare no conflicts of interest for this work. The authors did not receive payment related to the development of the manuscript and publication.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease—2020 report. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
2. World Health Organization. The top 10 causes of death. WHO. Available from: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death; 2020.
3. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196.
4. Ministry of Health and Welfare, Taiwan. Taiwan’s Leading Causes of Death in 2016. Ministry of Health and Welfare; 2017. Available from: https://www.mohw.gov.tw/cp-3425-33347-2.html.
5. Cheng SL, Chan MC, Wang CC, et al. COPD in Taiwan: a National Epidemiology Survey. Int J Chron Obstruct Pulmon Dis. 2015;10:2459–2467.
6. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796.
7. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138.
8. Ho TW, Tsai YJ, Ruan SY, Huang CT, Lai F, Yu CJ. In-hospital and one-year mortality and their predictors in patients hospitalized for first-ever chronic obstructive pulmonary disease exacerbations: a nationwide population-based study. PLoS One. 2014;9(12):e114866.
9. Mammen MJ, Pai V, Aaron SD, Nici L, Alhazzani W, Alexander PE. Dual LABA/LAMA Therapy versus LABA or LAMA Monotherapy for Chronic Obstructive Pulmonary Disease. A Systematic Review and Meta-analysis in Support of the American Thoracic Society Clinical Practice Guideline. Ann Am Thorac Soc. 2020;17(9):1133–1143.
10. Rogliani P, Matera MG, Ritondo BL, et al. Efficacy and cardiovascular safety profile of dual bronchodilation therapy in chronic obstructive pulmonary disease: a bidimensional comparative analysis across fixed-dose combinations. Pulm Pharmacol Ther. 2019;59:101841.
11. Lee HW, Park J, Jang EJ, Lee CH. Comparisons of exacerbations and mortality among LAMA/LABA combinations in stable chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis. Respir Res. 2020;21(1):310.
12. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654.
13. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203.
14. Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD–revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725–738.
15. Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344.
16. Ichinose M, Nishimura M, Akimoto M, et al. Tiotropium/olodaterol versus tiotropium in Japanese patients with COPD: results from the DYNAGITO study. Int J Chron Obstruct Pulmon Dis. 2018;13:2147–2156.
17. Feldman GJ, Sousa AR, Lipson DA, et al. Comparative Efficacy of Once-Daily Umeclidinium/Vilanterol and Tiotropium/Olodaterol Therapy in Symptomatic Chronic Obstructive Pulmonary Disease: a Randomized Study. Adv Ther. 2017;34(11):2518–2533.
18. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979.
19. Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
