Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Comparative Study of Cognitive Function Between Treatment-Resistant Depressive Patients and First-Episode Depressive Patients
Authors Rao D , Xu G, Lu Z , Liang H, Lin K, Tang M
Received 7 August 2019
Accepted for publication 13 November 2019
Published 9 December 2019 Volume 2019:15 Pages 3411—3417
DOI https://doi.org/10.2147/NDT.S226405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Dongping Rao,1 Guiyun Xu,1 Zenghong Lu,2 Huiwei Liang,1 Kangguang Lin,1 Muni Tang1
1Department of Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, People’s Republic of China; 2The First Affiliated Hospital of Gannan Medical University, Jiangxi, People’s Republic of China
Correspondence: Muni Tang
Department of Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Mingxin Road #36, Liwan District, Guangzhou 510370, People’s Republic of China
Tel +86-20-81268720
Fax +86-20-81891391
Email [email protected]
Objective: Despite reports of cognitive dysfunction during the acute phase of depression, there is a lack of studies in patients with treatment-resistant depression (TRD). The aim of this study was to investigate the cognitive function profile of TRD and compare cognitive dysfunction between subjects with TRD and first-episode depression.
Patients and methods: The study included 31 patients with TRD and 53 with first-episode depression. Cognitive function was assessed by a series of neuropsychological tools such as the verbal fluency test, Modified Wisconsin Card Sorting Test (M-WCST), Tower of Hanoi test, Chinese-revision of the Wechsler Adult Intelligence Scale (WAIS-RC), and Trail Making Test A and B.
Results: There were no significant demographic differences between the TRD, first-episode depression, and normal control groups (gender, age, years of education). The full-scale, verbal, and performance intelligence quotients measured with the WAIS-RC were also not significantly different (p>0.05). The normal group scores were all significantly better than TRD and first-episode depression, and the TRD group performed significantly worse than subjects with first-episode depression on Trail Making Test B, two WCST subscales, and the profile score of the Tower of Hanoi test (all p<0.05).
Conclusion: Patients with depression exhibited global impairments in cognitive function, and these were more common in TRD. Poor executive function may play an important role in TRD.
Keywords: treatment-resistant depression, first-episode depression, cognitive dysfunction
Introduction
Depression is one of the most prevalent mental disorders and one of the leading causes of disease burden worldwide. It has a greater impact on health status than chronic systemic diseases such as diabetes or heart disease.1 Treatment effectiveness is suboptimal for a significant percentage of patients.2 About 20% of depressive patients do not respond satisfactorily, and roughly half will experience a chronic or recurrent course of illness.3,4 In the absence of remission, depression is associated with impairments in work, social, and family life, as well as increased mortality.5,6
Cognitive function impairment is a prominent characteristic in some chronic psychiatric disorders such as schizophrenia, obsessive-compulsive disorders, and bipolar disorders.7 Poor concentration, executive function and memory dysfunction, and slowed processing speed are central features of depression.8,9 Cognitive impairment might contribute to social and occupational impairments in different phases of depression,10,11 and cognitive performance is likely associated with mood state. However, the relationship between depression severity and cognitive function was inconsistent in a previous meta-analysis.8 Moreover, depressive individuals in remission may continue to experience cognitive dysfunction.12 Cognitive deficits persist in euthymic patients with bipolar disorder, and these are likely related to structural and functional brain abnormalities associated with that condition.13,14
While depression is a more heterogeneous condition than bipolar disorder, they may share certain cognitive trait features that reflect underlying pathophysiological changes, implicating the frontal brain system. Such cognitive deficits in euthymic patients might help characterize different subtypes of depression and provide prognostic information. Cognitive impairment is correlated with both earlier onset of depressive symptoms and longer duration of episodes, which may contribute to the ineffectiveness of antidepressant therapy.15 According to Talarowska et al,16 subjects with first-episode depression show better results than patients with recurrent depression in terms of memory, verbal fluency, and frontal functions. Empirical studies on neurocognition in depression have provided some evidence that cognitive impairment may be associated with poorer response to treatment.17
Many definitions of treatment-resistant depression (TRD) have been proposed. Depression is typically considered resistant when a depressive disorder fails to show a satisfactory response to at least two appropriate antidepressant trials from two different pharmacologic classes, at appropriate doses and durations that are sufficient to produce a robust therapeutic effect.18,19 In the past two decades, TRD has attracted growing research interest. Patients with TRD have higher rates of suicide attempts, low treatment response, relapse, and health care utilization. A large proportion of disease burden caused by depression is due to TRD.20
Many groups have searched for predictive factors or characteristics in an attempt to improve TRD diagnosis and treatment. However, data are sparse, and the cognitive function profile of TRD is not well understood. Most patients with TRD experience impairment in many areas of psychosocial functioning such as independent living, community participation, interpersonal relationships, and occupational achievement.21 The purpose of the present study was to investigate the cognitive function profile of TRD and compare the degree of cognitive dysfunction in subjects with TRD and first-episode depression.
Patients and Methods
Subjects
Eighty-four patients (aged 18–65) treated as outpatients or inpatients at the department of psychiatry in Guangzhou Psychiatric Hospital of China were recruited. All participants provided written informed consent in accordance with the guideline of the Declaration of Helsinki of the World Medical Association Assembly. The investigation was approved by the Ethics Committee of Guangzhou Huai Hospital. All patients suffered from unipolar depression diagnosed based on criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Fifty-three patients (30 males and 23 females) meeting the DSM-IV criteria for a unipolar first-episode depression diagnosis were included in the study and had not been treated with any antidepressants. Thirty-one individuals (14 males and 17 females) with TRD were defined by failure to respond to at least two prior antidepressant treatments at adequate doses and durations. The 17-item Hamilton Depression Rating Scale (HAMD) was administered to assess depression severity.
Subjects were excluded if they suffered from brain damage, alcohol or substance abuse, any other severe medical illness, were psychotic, had experienced psychosis earlier in life, or had been treated with electroconvulsive therapy within the previous 6 months.
In present study, the patients with TRD had been treated with vortioxetine, paroxetine, citalopram, nortriptyline, sertraline, and amitriptyline. Twenty-eight patients with TRD were taking benzodiazepines for insomnia. Among them, 8, 11, and 9 were taking alprazolam, clonazepam, and estazolam, respectively. Benzodiazepines were prohibited for 24 hrs before cognitive function assessment.
The normal control group included 50 individuals (28 males and 22 females) aged 18–65, who were recruited from the care staff and volunteers. All normal controls gave written informed consent before entering the study. Exclusion criteria for the control group were any severe somatic disorder, any psychosis, alcohol or substance abuse, or a history of brain damage.
Prior to study initiation, the interviewers were trained by a qualified trainer to administer the study questionnaires and neuropsychological tests. A calibration session was performed to ensure a high standard of assessment.
Depressive Symptom Measurement
The HAMD is the most commonly used severity scale in clinical practice and research and was administered to assess depressive symptoms and severity. It reflects symptom characteristics based on four factors. The anxiety/somatization factor includes mental anxiety, somatic anxiety, gastrointestinal symptoms, hypochondria, and insight. The weight factor is weight loss. The retardation factor includes depression, work and interest, retardation, and sex symptoms. The sleep disturbance factor includes difficulty falling asleep, casual sleeping, and early awakening.
Neuropsychological Assessment
We assessed cognitive domains that have proven to be sensitive to dysfunction in unipolar depression.22 All participants completed a cognitive function assessment that included executive function, psychomotor speed, and verbal fluency. The individual intelligence of all patients was measured by the Chinese revised Wechsler adult intelligence scale (WAIS-RC).
Executive function was accessed by the modified Wisconsin card sorting test (M-WCST) (48-card version),23 Tower of Hanoi test,24 and Trail Making Test B.25 Psychomotor speed was tested with Trail Making Test A,25 and verbal fluency was measured with the verbal fluency test.26
The M-WCST was designed to measure concept formation, abstraction, set shifting, and ability to utilize feedback. Four measures were included in the M-WCST, but only two were used: the number of categories completed and the number of perseverative errors.
The most common planning task used to test depressive patients is the Tower of Hanoi test. This task requires participants to solve a series of problems by moving stacked disks from a given starting position to a given final position in as few moves as possible. The test comprises 12 problems with different complexity levels, and a total profile performance was computed to reflect the ability to solve the whole series of 12 problems.
The Trail Making Test was designed to measure psychomotor speed and set shifting. Part A involves drawing lines to connect numbers in ascending order, while part B requires the participants to alternate between numbers and letters in ascending order. The time to complete parts A and B was the dependent variable.
In the verbal fluency test, participants were instructed to list as many animals as possible within one minute. The dependent variable was the number of correct animals.
The WAIS-RC includes 11 subtests including similarities, arithmetic, information, vocabulary, comprehension, digit span, digit symbol, picture completion, block design, picture arrangement, and object assembly. Three scores are produced: full-scale intelligence quotient (FIQ), verbal intelligence quotient (VIQ), and performance intelligence quotient (PIQ). VIQ was assessed by the first six subtests, and PIQ was assessed by the last five subtests.
Statistical Analyses
Analyses were conducted using the Statistical Package for Social Sciences (SPSS) 17.0 for Windows (SPSS Inc., Chicago, IL, USA). A two-tailed statistical significance level was set at p<0.05 for all analyses. One-way analyses of variance or Chi-square tests were computed to test characteristics among groups. Independent sample t-tests were used to compare HAMD scores and factor scores, VIQ, PIQ, FIQ, age at onset, and duration of illness between the TRD and first-episode depression groups. Two independent sample nonparametric tests were used to compare the numbers of episodes between the TRD and first-episode depression groups. Multivariate analysis of variance (MANOVA) was used to compare neuropsychological performance among the three groups with number of episodes, age, years of education, and duration of illness as covariates, followed by post-hoc comparisons with Bonferroni correction provided by MANOVA.
Results
Participants’ Demographic and Characteristics
The three groups did not differ significantly with regard to gender, age, or years of education (Table 1).
 |
Table 1 Comparison of Characteristics Among the TRD, First-Episode Depression, and Normal Control Groups |
Comparison of Clinical Characteristics Between First-Episode Depression and TRD
The clinical characteristics for TRD and first-episode depression are presented in Table 2. The HAMD scores and four factor scores were not significantly different (p>0.05) between groups. Patients with TRD had longer cumulative duration and more numbers of episodes than the group with first-episode depression (p<0.05).
 |
Table 2 Comparison of Clinical Characteristics Between the First-Episode Depression and TRD Groups |
Comparison of WAIS-RC Scores Between First-Episode Depression and TRD
The WAIS-RC test results for TRD and first-episode depression are presented in Table 3. The FIQ, VIQ, and PIQ were not significantly different between groups (p>0.05).
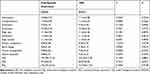 |
Table 3 Comparison of WAIS-RC Between the First-Episode Depression and TRD Groups |
Comparison of Cognitive Function
As shown in Table 4, MANOVA analysis revealed that all neuropsychological scores were significantly different between the three groups. Scores in the normal control group were all significantly better than the TRD and first-episode depression groups. Considering that number of episodes, age, years of education, and duration of illness are related to neuropsychological performance, we performed MANOVA analysis with the four variables as covariates. This revealed differences between TRD and first-episode depression, with three out of five neuropsychological test scores reaching significant differences (Pillai’s F=2.415, p<0.05). For those domains with significant main effects, we applied post hoc comparisons using Bonferroni correction and found that subjects with TRD performed significantly worse than those with first-episode depression on Trail Making Test B, the classification number and continuing error number in the M-WCST, and the profile score of Tower of Hanoi test. (p<0.05)
 |
Table 4 Comparison of Cognitive Function Among TRD, First-Episode Depression, and Normal Control Groups |
Discussion
Deficits have been identified in the areas of attention, memory, executive function, and psychomotor speed in patients with recurrent major depression and first-episode depression.27,28 In the present study, subjects with TRD performed significantly worse than those with first-episode depression on Trail Making Test B, the M-WCST, and Tower of Hanoi test, all of which reflect executive function. The M-WCST mainly reflects frontal executive function by testing the abilities of color perception, abstraction, and conceptual transformation.29 Trail Making Test B was designed to evaluate the speed of visual perception, conceptual transformation, and attention conversion. The Tower of Hanoi test reflects plan adjustment ability and suppression capacity; it can evaluate logic reasoning and problem solving.30
Executive functions are higher-level functions that can control and regulate lower cognitive operations; they are most likely linked to the prefrontal cortex and its associated networks.31,32 Specifically, two circuits are considered critical for modulating or inhibiting emotional behavior. The limbic-thalamic-cortical circuit includes the amygdalae, medial thalamus, and orbital and medial prefrontal cortices. The limbic-cortical-striatal-pallidal-thalamic circuit includes components of the previous circuit with the addition of the striatum and pallidum. Impairment in the two circuits may directly or indirectly influence emotion, motivation, and the cognitive and behavioral manifestations of mood disorders.33
In the present study, patients with TRD had longer cumulative duration and more episodes than the group with first-episode depression (p<0.05). Whether the numbers of depressive episodes or cumulative duration affect cognitive function is unknown. Two studies did not detect evidence of an association between neuropsychological test performance and the number of previous episodes.34,35 In contrast to this, others found a statistically significant association between the cumulative duration of depressive episodes and executive function.12,36 Numerous reviews of studies in currently depressed individuals reported small-to-moderate impairments in several neurocognitive domains, and these were associated with a relapsing/multi-episode or chronic course of illness.37 So, it is quite likely cognitive dysfunction that develops in the course of depression is highly variable. Considering that the number of episodes and illness duration are related to neuropsychological performance, we performed MANOVA analysis with these variables as covariates and found differences between the TRD and first-episode depression groups.
A meta-analysis reported that deficits in executive function persist beyond acute episodes of depression; between one-third and one-half of remitted patients are affected by these deficits.38 Executive function performance was likely associated with response to antidepressant medication,39 while impairment in this domain was linked with a poor response to the treatment.40,41 Therefore, executive dysfunction impairment is a core feature of TRD and may be an important trait-marker for major depression.
Limitations
This study was limited by a small sample size and its cross-sectional design. Given that this is the first investigation of cognitive function profiles in patients with TRD and first-episode depression, replication of these results in independent and large samples is needed to develop a fuller understanding of the cognitive function characteristics of TRD. In addition, when comparing cognitive function between TRD and first-episode depression, one main issue warrants comment. The diagnosis of TRD requires failed treatment with at least two classes of antidepressant treatments. In the present study, patients with TRD had been treated with vortioxetine, paroxetine, citalopram, nortriptyline, sertraline, and amitriptyline. A meta-analysis showed that antidepressants had a significant positive effect on psychomotor speed, but the effect on executive function did not reach statistical significance in head-to-head trials of tricyclic antidepressants, selective serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and norepinephrine and dopamine reuptake inhibitors.42 However, significant limitations of that meta-analysis were result heterogeneity, small sample sizes, and a limited number of studies. Since antidepressants affect neurotransmitters levels in the executive region of the brain, antidepressants may confound the predictive value of neuropsychological profiles.
Conclusions
Patients with depression exhibited global impairments in cognitive function, and these were more common in TRD. Poor executive function may play an important role in TRD. This suggested that treatments that target this cognitive domain may be effective. Clinicians should be aware of neurocognitive dysfunction in patients with depression, especially TRD.
Abbreviations
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; FIQ, full intelligence quotient; HAMD, Hamilton Depression Rating Scale; MANOVA, multivariate analysis of variance; PIQ, performance intelligence quotient; TRD, treatment-resistant depression; VIQ, verbal intelligence quotient; WAIS-RC, Chinese revised Wechsler adult intelligence scale; M-WCST, modified Wisconsin card sorting test.
Ethics Statement
All participants signed a written informed consent form in accordance with the guidelines of the Declaration of Helsinki of the World Medical Association Assembly. The investigation was approved by the Ethics Committee of Guangzhou Huiai Hospital.
Acknowledgments
We thank all psychiatrists and staff who were involved in the diagnosis and recruitment of research participants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi:10.1016/S0140-6736(07)61415-9
2. Rush AJ, Thase M, Dubé S. Research issues in study of difficult-to-treat depression. Biol Psychiatry. 2003;53(8):743–753. doi:10.1016/S0006-3223(03)00088-X
3. Crown WH, Finkelstein S, Berndt ER, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63(11):963–971. doi:10.4088/JCP.v63n1102
4. Sackeim HA. The definition and meaning of treatment resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17.
5. Ansseau M, Demyttenaere K, Heyman J, Migeotte A, Leyman S, Mignon A. Objective: remission of depression in primary care the Oreon Study. Eur Neuropsychopharmacol. 2009;19(3):169–176. doi:10.1016/j.euroneuro.2008.10.003
6. Fekadu A, Wooderson S, Donaldson C, et al. A multidimensional tool to quantify treatment resistance in depression: the Maudsley staying method. J Clin Psychiatry. 2009;70(2):177–184. doi:10.4088/JCP.08m04309
7. Bowie CR, Depp C, McGrath JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167(9):1116–1124. doi:10.1176/appi.ajp.2010.09101406
8. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1–3):1–8. doi:10.1016/j.jad.2009.04.022
9. Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2011;11(2):141–168. doi:10.1038/nrd3628
10. Fenning S, Mottes A, Richer-Levin G, Treves I, Levkoviz Y. Everyday memory and laboratory memory tests: general function predictors in schizophrenia and remitted depression. J Nerv Ment Dis. 2002;190(10):677–682. doi:10.1097/00005053-200210000-00004
11. Yen YC, Rebok GW, Gallo JJ, Jones RN, Tennstedt SL. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry. 2011;19(2):142–150. doi:10.1097/JGP.0b013e3181e89894
12. Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. The cumulative load of depressive illness is associated with cognitive function in the remitted state of unipolar depressive disorder. Eur Psychiatry. 2013;28(6):349–355. doi:10.1016/j.eurpsy.2012.03.004
13. Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1–2): 1–20. doi:10.1016/j.jad.2008.06.009
14. Hartberg CB, Sundet K, Rimol LM, et al. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1122–1130. doi:10.1016/j.pnpbp.2011.03.014
15. Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8–14. doi:10.4088/JCP.13r08710
16. Talarowska M, Zajączkowska M, Gałecki P. Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatr Danub. 2015;27(1):38–43.
17. Bruder GE, Alvarenga JE, Alschuler D, et al. Neurocognitive predictors of antidepressant clinical response. J Affect Disord. 2014;166:108–114. doi:10.1016/j.jad.2014.04.057
18. Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcomes studies. J Affective Disord. 2009;116(1–2):4–11. doi:10.1016/j.jad.2008.10.014
19. Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52(1):46–54. doi:10.1177/070674370705200108
20. Souery D, Papalostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(Suppl 6):16–22.
21. Petersen T, Papalostas GI, Mahal Y, et al. Psychosocial functioning in patients with treatment resistant depression. Eur Psychiatry. 2004;19(4):196–201. doi:10.1016/j.eurpsy.2003.11.006
22. Rund BR, Sundet K, Asbjørnsen A, et al. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatr Scand. 2006;113(4):350–359. doi:10.1111/acp.2006.113.issue-4
23. Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12(4):313–324. doi:10.1016/S0010-9452(76)80035-4
24. Goel V, Grafman J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33(5):623–642. doi:10.1016/0028-3932(95)90866-P
25. Christidi F, Kararizou E, Triantafyllou N, Anagnostouli M, Zalonis I. Derived Trail Making Test indices: demographics and cognitive background variables across the adult life span. Aging Neuropsychol Cognition. 2015;22(6):667–678. doi:10.1080/13825585.2015.1027650
26. Lezak MD. Neuropsychological assessment in behavioral toxicology-developing techniques and interpretative issues. Scand J Work Environ Health. 1984;10(Suppl 1):25–29.
27. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. doi:10.1037/a0028727
28. Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140(2):113–124. doi:10.1016/j.jad.2011.10.023
29. Fossati P, Ergis AM, Allilaire JF. Problem-solving abilities in unipolar depression patients comparison of performance on the modified version of the Wisconsin and the California sorting tests. Psychiatry Res. 2001;104(2):145–156. doi:10.1016/S0165-1781(01)00307-9
30. Arefnasab Z, Zare H, Babamahmoodi A. Emotional intelligence and problem solving strategy: comparative study based on “tower of hanoi” test. Iran J Psychiatry Behav Sci. 2012;6(2):62–68.
31. Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63(3–4):289–298. doi:10.1007/s004269900007
32. Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi:10.1146/annurev.psych.53.100901.135220
33. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi:10.1016/S0006-3223(03)00168-9
34. Li CT, Lin CP, Chou KH, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50(1):347–356. doi:10.1016/j.neuroimage.2009.11.021
35. Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–2026. doi:10.1017/S0033291712002085
36. Bhardwaj A, Wilkinson P, Srivastava C, Sharma M. Cognitive deficits in euthymic patients with recurrent depression. J Nerv Ment Dis. 2010;198(7):513–515. doi:10.1097/NMD.0b013e3181e4c5ba
37. Allott K, Fisher CA, Amminger GP, Goodall J, Hetrick S. Characterizing neurocognitive impairment in young people with major depression: state, trait, or scar? Brain Behav. 2016;6(10):e00527. doi:10.1002/brb3.2016.6.issue-10
38. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi:10.1017/S0033291713002535
39. McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25(10):933–944. doi:10.1002/gps.2431
40. Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29(12):2266–2271. doi:10.1038/sj.npp.1300551
41. Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16(9):752–759. doi:10.1097/JGP.0b013e31817e739a
42. Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):1–13.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
