Back to Journals » OncoTargets and Therapy » Volume 9
Comparative effects of different enteral feeding methods in head and neck cancer patients receiving radiotherapy or chemoradiotherapy: a network meta-analysis
Authors Zhang Z, Zhu Y, Ling Y, Zhang L, Wan H
Received 7 December 2015
Accepted for publication 8 March 2016
Published 18 May 2016 Volume 2016:9 Pages 2897—2909
DOI https://doi.org/10.2147/OTT.S101983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Zhihong Zhang,1,2 Yu Zhu,1 Yun Ling,3 Lijuan Zhang,1 Hongwei Wan1
1Department of Nursing, Shanghai Proton and Heavy Ion Center, 2Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, 3Department of Human Resource, Shanghai Proton and Heavy Ion Center, Shanghai, People’s Republic of China
Abstract: Nasogastric tube (NGT) and percutaneous endoscopic gastrostomy were frequently used in the head and neck cancer patients when malnutrition was present. Nevertheless, the evidence was inclusive in terms of the choice and the time of tube placement. The aim of this network meta-analysis was to evaluate the comparative effects of prophylactic percutaneous endoscopic gastrostomy (pPEG), reactive percutaneous endoscopic gastrostomy (rPEG), and NGT in the head and neck cancer patients receiving radiotherapy or chemoradiotherapy. Databases of PubMed, Web of Science, and Elsevier were searched from inception to October 2015. Thirteen studies enrolling 1,631 participants were included in this network meta-analysis. The results indicated that both pPEG and NGT were superior to rPEG in the management of weight loss. pPEG was associated with the least rate of treatment interruption and nutrition-related hospital admission among pPEG, rPEG, and NGT. Meanwhile, there was no difference in tube-related complications. Our study suggested that pPEG might be a better choice in malnutrition management in the head and neck cancer patients undergoing radiotherapy or chemoradiotherapy. However, its effects need to be further investigated in more randomized controlled trials.
Keywords: malnutrition, tube feeding, weight loss, treatment interruption, readmission, complication
Introduction
Head and neck cancer is one of the most common malignancies in the world, including a variety of cancers, such as cancers of oral cavity, tongue, salivary glands, pharynx, larynx, and nasal cavity. There are ~45,780 new cases of oral cavity and pharynx cancers reported in the USA.1 In these patients, malnutrition occurs frequently as a consequence of local tumor invasion,2–4 obstructing the function of swallowing and chewing, which lead to malnutrition prior to the therapy. In addition, mucositis, anorexia, dysphagia, mouth sores, and other acute and late toxic effects of radiotherapy (RT) and chemotherapy may worsen the nutritional status of patients.5–9
Poor nutritional status was associated with less resistance to chemotherapy or RT-toxicity, leading to treatment interruption.9–11 Malnutrition was also associated with higher risk of infection and hospital admission, worse survival outcomes, and deterioration in the quality of life (QoL).4,12,13 Therefore, nutrition intervention is essential to maintain nutritional status and improve outcomes in head and neck cancer patients undergoing RT and chemotherapy.
Dietary counseling and oral supplements showed positive influences on nutritional outcomes and QoL in the head and neck cancer patients receiving RT or chemoradiotherapy.14–16 However, their role is limited when it comes to obstruction or mucositis.17 Thus, enteral feeding may be a choice either through nasogastric tube (NGT) or percutaneous endoscopic gastrostomy (PEG). Studies investigated the effects of PEG on patients’ outcomes compared with NGT, but the conclusion was inconsistent due to the lack of adequate evidence.18,19 Research also indicated that prophylactic percutaneous endoscopic gastrostomy (pPEG), usually early PEG before the initiation of therapy, was able to meet nutrition needs during chemoradiotherapy20 and increase the completeness rate of concurrent chemotherapy.21 However, when compared with reactive percutaneous endoscopic gastrostomy (rPEG), in which situation PEG was used when necessary, pPEG was associated with less complications, but higher dependence, with no difference in weight loss.22 Until now, the optical method and timing of placement are still the topic of debate in recent researches.23,24
Traditional head-to-head meta-analyses can only compare the effects of two individual interventions. A network analysis allows the combination of direct and indirect evidence simultaneously to compare the effects of more than two interventions and establish the optimum intervention.25 Therefore, we intended to perform a network analysis to assess the relative effectiveness of NGT, pPEG, and rPEG on nutritional status, treatment interruption, nutrition-related hospital admission, and tube-related complications in the head and neck cancer patients receiving RT or chemoradiotherapy.
Materials and methods
Search strategy and selection criteria
Publications were identified through PubMed, Web of Science (Web of Science™ Core Collection, Medline, Chinese Science Citation DatabaseSM, Derwent Innovations IndexSM, KCI Korean Journal Database), and Elsevier (ScienceDirect) from inception up to October 2015. The search terms used were as follows: “percutaneous gastrostomy” or “gastrostomy” or “nasogastric tubes” or “enteral nutrition”, “head and neck neoplasm” or “head and neck cancer”, and “radiation” or “radiotherapy” or “chemoradiotherapy”. Reference lists of relevant reviews and studies were also examined for additional studies.
Inclusion criteria
Full-text articles published in English were included if they met the following criteria:
- Population: Head and neck cancer patients receiving RT or radiochemotherapy.
- Intervention and comparison: Study compared one or more tube-feeding methods with the control group, including pPEG used before the beginning of the therapeutic sequence or when the presence of malnutrition needed intervention, rPEG used only when necessary during and after the therapy, and NGT.
- Outcomes of interest: Change in body weight, rates of treatment interruption, nutrition-related hospital admission, and tube-related complications. Trials included must report complete data for at least one of the aforementioned outcomes.
Data extraction and methodological quality assessment
Two investigators independently extracted the details of each included study, including the first author, year, study design, intervention method, sample size, and outcomes of interest. The methodological quality of each study was assessed by two independent investigators, and any discrepancies were resolved by discussion and consultation with the third person. Observational studies were appraised against the Newcastle–Ottawa scale,26,27 including the selection of the study groups, the comparability between groups, and the ascertainment of exposure or outcome. If the study met one of the items, a star was awarded. The risks of bias of randomized controlled trials were appraised using the Cochrane Handbook for Systematic Reviews of Interventions. The following sources of bias were checked: randomization sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and others. Each criterion was categorized as high risk, low risk, and unclear.
Statistical analysis
All analyses were performed using STATA version 13 (StataCorp LP, College Station, TX, USA). P-values <0.05 were considered statistically significant. For each outcome, both traditional pairwise meta-analysis and network meta-analysis were performed to compare the effect of different interventions. Pooled relative risk (RR) and standardized mean difference (SMD) were estimated for dichotomous and continuous data, respectively. For pairwise meta-analysis, the heterogeneity between studies was assessed with Q test and I2 value. If heterogeneity was present, a random-effects model was applied; otherwise, a fixed-effects model was used.
Network meta-analysis is a generalization of meta-analysis methods that allows comparisons of interventions both directly and indirectly. We conducted this network meta-analysis using mvmeta in STATA.28,29 The small-study effect was assessed using a comparison-adjusted funnel plot. We also calculated the inconsistency factor to assess the consistency of results between direct and indirect comparisons. Finally, the surface under the cumulative ranking curve (SUCRA) was calculated, and a higher SUCRA value was regarded as a better result for individual intervention.
Results
Characteristics of included studies
The literature search identified 253 potentially relevant studies, based on the search strategy. After screening the titles and abstracts, 230 studies were excluded. The full-texts of 43 articles, including 20 articles from reference lists, were assessed for eligibility. Overall, 13 studies met the inclusion criteria.22,30–41 The flowchart of literature selection is illustrated in Figure 1. There were ten two-arm studies,22,31–34,36–40 two three-arm studies,35,41 and one four-arm study.30 Table 1 describes the characteristics of these studies.
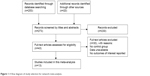  | Figure 1 A flow diagram of study selection for network meta-analysis. |
Six of the included studies were retrospective studies,30,31,35–37,41 five were prospective studies,22,33,38–40 and the remaining two randomized controlled trials.32,34 The qualities of eleven retrospective and prospective studies were assessed according to the Newcastle–Ottawa scale. Most of the studies were of poor quality and failed to reach a baseline balanced among groups or reported incomplete follow-up information, while the risk of blinding, incomplete outcome data, and selective reporting were high in another two randomized controlled trials, based on the Cochrane Handbook for Systematic Reviews of Interventions. The quality assessment of included studies is described in Tables 2 and 3.
  | Table 2 Quality assessment of the eleven retrospective or prospective studies |
  | Table 3 Quality assessment of the two randomized controlled trials |
Weight change during therapy
Five studies reported the effect of different enteral feeding methods on body weight (n=215).30–32,36,37 Three studies directly compared the effect of pPEG vs no-pPEG (NGT, rPEG, or oral feeding),31,36,37 one PEG vs NGT,32 and one pPEG vs rPEG vs NGT vs oral feeding.30 A network plot is shown in Figure 2A. A funnel plot indicated that the pooled results might be negatively influenced by small-study effect (Figure 3A).
pPEG showed significant benefits on the management of body weight when compared with rPEG (I2=79%, SMD =1.38, 95% CI: 0.91–1.84) and no-pPEG (I2=86.9, SMD =0.45, 95% CI: 0.14–0.76; Table 4). No significant differences were detected during comparisons of PEG vs NGT, pPEG vs NGT, or rPEG vs NGT.
The SUCRA probabilities of different enteral feeding methods on the control of weight loss are shown in Figure 4A. The corresponding values were 42.7%, 2.6%, 85.6%, 68.7%, 64.3%, and 36% for pPEG, rPEG, NGT, oral feeding, PEG, and no-pPEG, respectively, which may indicate that both pPEG and NGT were preferable to rPEG.
Interruption of treatment
Seven eligible studies,22,31,34,35,37,38,41 including 1,066 patients, evaluated the effect of different enteral feeding methods on the rate of treatment interruption. Four studies compared pPEG vs no-pPEG,31,34,35,37 two pPEG vs rPEG,22,38 and one pPEG vs rPEG vs oral.41 A network plot is shown in Figure 2B. No asymmetry was observed in a funnel plot (Figure 3B). We also tested the inconsistency of results between direct and indirect comparisons. The inconsistency plot suggested that no statistically significant inconsistency was detected (Figure 5A).
All pooled results did not reach significance in traditional pairwise meta-analysis, except for pPEG vs NGT (RR =0.33, 95% CI: 0.16–0.63; Table 4). The results from the network meta-analysis that combined both direct and indirect comparisons indicated that both pPEG and rPEG were superior to NGT. The rates of treatment interruption were higher in the NGT group compared with the pPEG (RR =15.09, 95% CI: 3.45–66.10; Figure 6A) and rPEG (RR =9.18, 95% CI: 1.87–45.17; Figure 6A) groups. However, there was no significant difference between pPEG and rPEG (Table 5).
The SUCRA probabilities of different intervention methods on the effect of treatment interruption were estimated. Figure 4B shows the ranking of different enteral feeding methods. The corresponding values were 85.2%, 53.4%, 0.9%, 63.3%, and 47.1% for pPEG, rPEG, NGT, oral feeding, and no-pPEG, respectively, which may indicate that either pPEG or rPEG was more effective than NGT in reducing the rate of treatment interruption and pPEG may be the optimal method to insure the completion of treatment.
Tube-related complications
Five studies,22,33,38–40 involving 1,562 patients, investigated the effect of different enteral feeding methods on the rate of tube-related complications. Three studies compared pPEG vs rPEG,22,38,40 one PEG vs NGT,33 and one pPEG vs NGT.39 Figure 2C describes the network plot of different interventions. Slight asymmetry was shown in the comparison-adjusted funnel plot (Figure 3C), suggesting the presence of small-study effect. No closed loop was formed among the intervention methods. Thus, the inconsistency of outcomes between direct and indirect comparisons could not be tested.
The pairwise meta-analysis indicated that no statistical significant difference was detected in the comparison of either pPEG vs rPEG or PEG vs NGT. Higher rate of complications was only found in the pPEG group compared with the NGT group in the study by Magné et al39 (RR =0.42, 95% CI: 0.28–0.68; Table 4). The results generated from network meta-analysis implied that no significant difference existed among these enteral feeding methods in terms of tube-related complications (Figure 6B and Table 5).
In addition, we calculated SUCRA probabilities of enteral feeding methods for the incidence of tube-related complications. The SUCRA probabilities were 44.5%, 57.5%, 45.7%, and 52.2% for pPEG, rPEG, NGT, and PEG, respectively (Figure 4C).
Nutrition-related hospital admission
Five studies investigated the correlation between different enteral feeding methods and nutrition-related hospital admission (n=565).31,35,37,38,41 There were two studies comparing the effect of pPEG vs no-pPEG,31,37 two pPEG vs rPEG vs oral feeding,35,38 and one pPEG vs rPEG vs NGT.41 Figure 2D describes the network plot of four intervention methods. No asymmetry was shown in the comparison-adjusted funnel plot (Figure 3D). We then evaluated the inconsistency between direct and indirect comparisons, the results of which indicated that there was some inconsistency in the loop of oral–pPEG–rPEG (Figure 5B). However, no inconsistency was found in the loop of NGT–pPEG–rPEG.
No heterogeneity existed among the direct comparisons. The results of pairwise meta-analysis suggested that pPEG was effective in reducing the nutritional problems and nutrition-related hospital admission than rPEG (I2=52.5%, RR =0.58, 95% CI: 0.42–0.80; Table 4). There was no evidence of difference in either pPEG vs NGT or rPEG vs NGT. A similar trend was observed in the results of network meta-analysis, in which rPEG (RR =3.22, 95% CI: 1.31–7.89), NGT (RR =4.68, 95% CI: 1.07–20.47), and no-pPEG (RR =3.07, 95% CI: 1.05–9.01) all increased the rate of nutritional problems and related hospital admission, compared with pPEG (Figure 6C). No difference was detected in the comparison of rPEG vs NGT (Table 5).
The SUCRA probabilities of different feeding methods on the effect of nutrition-related hospital admission were calculated. The ranking of different enteral feeding methods is shown in Figure 4D. The corresponding values were 98.8%, 36.1%, 23.5%, 48.2%, and 43.4% for pPEG, rPEG, NGT, oral feeding, and no-pPEG, respectively. Thus, the pPEG is likely to be the most effective method in reducing nutritional problems and related hospital admission in the head and neck cancer patients receiving RT or radiochemotherapy.
Discussion
We performed this network meta-analysis to compare the effects of pPEG, rPEG, and NGT on the control of weight loss, interruption of therapy, tube-related complications, and nutrition-related hospital admission. Overall, the results of this network meta-analysis indicated that both pPEG and NGT were superior to rPEG in the management of malnutritional status. Besides, there were no pronounced differences on tube-related complications among the three tube-feeding methods. Meanwhile, when compared with NGT and rPEG, pPEG may be the optimal method in reducing the rate of treatment interruption and nutrition-related hospital admission.
Studies that compared the effect of PEG on the weight loss with NGT had concluded that the use of PEG and NGT had a similar effect on the weight loss during RT or chemoradiotherapy.32,39 Magné et al39 found no significant difference in weight changes between NGT and PEG groups.39 The weight loss also did not reach significance in a randomized control trial32 between two groups. However, the conclusions about the effect of pPEG were inconclusive, compared with rPEG or NGT. Some studies found that pPEG did not show pronounced benefit in the control of malnutrition and weight loss, compared with rPEG or NGT.30,34,42 Other studies suggested that pPEG was associated with significant advantage than rPEG and NGT in the control of weight loss.31,37,43 We conducted a network meta-analysis using the evidence from both direct and indirect comparisons and found that both pPEG and NGT were preferable to rPEG on weight loss management. Although the value of SUCRA probabilities of NGT was higher than pPEG in the change of body weight, there is great possibility that this better nutritional status may be the result of better performance status and clinical condition in patients with NGT.
Early nutrition intervention was correlated with better nutritional status, improved treatment tolerance, and fewer hospital admission.44 Unfortunately, several systematic reviews failed to discuss the influences of different enteral feeding methods on the incidence of treatment break and nutrition-related admission.23,27,45 This network meta-analysis evaluated the effect of different enteral feeding methods in head and neck cancer patients who received RT or chemoradiotherapy and found that pPEG was the optimal enteral method to increase the completion of treatment and reduce hospital admission for nutrition problems compared with rPEG and NGT.
Koyfman et al23 had only described the tube-related complications of several studies in a recent review but failed to synthesize the current data. Meanwhile, Wang et al27 assessed three kinds of complications, which indicated that the total effect of PEG was associated with fewer complications than NGT. Contrary to this, no difference was detected in complication rates among pPEG, rPEG, and NGT, according to the current network meta-analysis. The possible explanation of this discrepancy may be the difference in included complications. All the reported complications, including pharyngoesophageal dilation, gastrocutaneous fistula, infection, leakage, pain, dislodgement, and malfunction, were included in the present study, while only three complications were included in the study of Wang et al.27
Previous meta-analyses could only evaluate the comparative effects of two different enteral feeding methods on patients’ outcomes. We performed a network meta-analysis combining both direct and indirect comparisons, which made it possible to evaluate the comparative effects of pPEG, rPEG, NGT, and oral feeding (no tube) simultaneously. Another advantage of this network meta-analysis was that it provided probability statements and alternative rankings for pPEG, rPEG, NGT, and oral feeding, which can be used to identify the optimal intervention.
NGT was used only when necessary in trials, and some included studies did not distinguish pPEG from rPEG. Thus, we classified the interventions into pPEG, rPEG, NGT, PEG, no-pPEG (NGT, rPEG, or no tube), and oral feeding (no tube). However, this meta-analysis aimed to investigate the comparative effect of pPEG, rPEG, and NGT, determining the best enteral feeding method in managing malnutrition and related outcomes in the head and neck patients undergoing RT or chemoradiotherapy. Besides, the potential difference of baseline characteristics among groups inhibited our interpretation for the comparative effects of oral feeding, pPEG, rPEG, and NGT, in which patients with oral feeding usually were in better clinical conditions than those in the PEG or NGT group. We, therefore, only interpreted the relative efficiency of pPEG, rPEG, and NGT.
Furthermore, most of the included studies were retrospective or prospective reviews, with only two randomized controlled trials. The lack of evidence from randomized controlled trials is likely to weaken the reliability of our conclusions. In addition, there might be false positive to some extent, owing to the effect of some small number of studies. A final limitation is that we only focused on weight loss, treatment interruption, nutrition-related hospital admission, and tube-related complications and did not attempt to assess the tube dependency, cost, and QoL related to tube use, which are also indispensable factors for a comprehensive assessment of different tube-feeding methods.
Conclusion
As malnutrition is associated with poorer outcomes in head and neck cancer patients undergoing RT or chemoradiotherapy, enteral feeding is available to meet their nutritional needs. Based on the evidence of this network meta-analysis, pPEG may be the optimal method in reducing the rate of treatment interruption and nutrition-related hospital admission, with no pronounced differences in terms of tube-related complications, compared with rPEG and NGT. Nevertheless, given the poor quality of included studies, more research is required to further confirm the current evidence in large randomized controlled trials.
Acknowledgment
This work was financially supported by the Shanghai Science and Technology Committee under grants 14495810900 and 13ZR1432800.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
van Bokhorst-de van der Schueren, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86(3):519–527. | ||
De Luis DA, Izaola O, Aller R. Nutritional status in head and neck cancer patients. Eur Rev Med Pharmacol Sci. 2007;11(4):91–94. | ||
van Wayenburg CA, Rasmussen-Conrad EL, van den Berg MG, et al. Weight loss in head and neck cancer patients little noticed in general practice. J Prim Health Care. 2010;2(1):16–21. | ||
Larsson M. Eating problems in patients with head and neck cancer treated with radiotherapy: needs, problems and support during the trajectory of care. Fakulteten för samhälls-och livsvetenskaper, 2006. | ||
Kubrak C, Olson K, Jha N, et al. Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck. 2010;32(3):290–300. | ||
Langius JA, Doornaert P, Spreeuwenberg MD, et al. Radiotherapy on the neck nodes predicts severe weight loss in patients with early stage laryngeal cancer. Radiother Oncol. 2010;97(1):80–85. | ||
Ng K, Leung SF, Johnson PJ, Woo J. Nutritional consequences of radiotherapy in nasopharynx cancer patients. Nutr Cancer. 2004;49(2):156–161. | ||
Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–262. | ||
Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007;5(9 Suppl 4):23–31. | ||
Montserrat VL, Gerry O, May H, Stephen S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106(2):329–336. | ||
Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 2000;34(3):137–168. | ||
Langius JA, van Dijk AM, Doornaert P, et al. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr Cancer. 2013;65(1):76–83. | ||
Paula R, Isabel MG, Pedro MV, Ermelinda CM. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27(8):659–668. | ||
van den Berg MG, Rasmussen-Conrad EL, Wei KH, Lintz-Luidens H, Kaanders JH, Merkx MA. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104(6):872–877. | ||
Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91(3):447–452. | ||
Cheng SS, Terrell JE, Bradford CR, et al. Variables associated with feeding tube placement in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132(6):655–661. | ||
Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91(9):1785–1790. | ||
Nugent B, Lewis S, O’Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev. 2010;1(1):1399–1400. | ||
Wiggenraad RG, Flierman L, Goossens A, et al. Prophylactic gastrostomy placement and early tube feeding may limit loss of weight during chemoradiotherapy for advanced head and neck cancer, a preliminary study. Clin Otolaryngol. 2007;32(5):384–390. | ||
Atasoy BM, Yonal O, Demirel B, et al. The impact of early percutaneous endoscopic gastrostomy placement on treatment completeness and nutritional status in locally advanced head and neck cancer patients receiving chemoradiotherapy. Eur Arch Otorhinolaryngol. 2012;269(1):275–282. | ||
Olson R, Karam I, Wilson G, Bowman A, Lee C, Wong F. Population-based comparison of two feeding tube approaches for head and neck cancer patients receiving concurrent systemic–radiation therapy: is a prophylactic feeding tube approach harmful or helpful? Support Care Cancer. 2013;21(12):3433–3439. | ||
Koyfman SA, Adelstein DJ. Enteral feeding tubes in patients undergoing definitive chemoradiation therapy for head-and-neck cancer: a critical review. Int J Radiat Oncol Biol Phys. 2012;84(3):581–589. | ||
Paleri V, Patterson J. Use of gastrostomy in head and neck cancer: a systematic review to identify areas for future research. Clin Otolaryngol. 2010;35(3):177–189. | ||
Salanti G, Ades AE, Ioannidis JP, Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. | ||
Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Wang J, Liu M, Chao L, Yun Y, Huang G. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J Radiat Res. 2014;55(3):559–567. | ||
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. | ||
White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 2011;11(2):255–270. | ||
Nugent B, Parker MJ, McIntyre IA. Nasogastric tube feeding and percutaneous endoscopic gastrostomy tube feeding in patients with head and neck cancer. J Hum Nutr Diet. 2010;23(3):277–284. | ||
Chang JH, Gosling T, Larsen J, Powell S, Scanlon R, Chander S. Prophylactic gastrostomy tubes for patients receiving radical radiotherapy for head and neck cancers: a retrospective review. J Med Imaging Radiat Oncol. 2009;53(5):494–499. | ||
Corry J, Poon W, Mcphee N, et al. Randomized study of percutaneous endoscopic gastrostomy versus nasogastric tubes for enteral feeding in head and neck cancer patients treated with (chemo)radiation. J Med Imaging Radiat Oncol. 2008;52(5):503–510. | ||
Corry J, Poon WN, McPhee N, et al. Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo)radiation. Head Neck. 2009;31(7):867–876. | ||
Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer – a randomized study. Head Neck. 2012;34(1):1–9. | ||
Lewis SL, Rebecca B, Touger-Decker R, Parrott JS, Epstein J. Feeding tube use in patients with head and neck cancer. Head Neck. 2014;36(12):1789–1795. | ||
Langmore S, Krisciunas GP, Miloro KV, Evans SR, Cheng DM. Does PEG use cause dysphagia in head and neck cancer patients? Dysphagia. 2012;27(2):251–259. | ||
Lee JH, Machtay M, Unger LD, et al. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(8):871–875. | ||
Baschnagel AM, Yadav S, Marina O, et al. Toxicities and costs of placing prophylactic and reactive percutaneous gastrostomy tubes in patients with locally advanced head and neck cancers treated with chemoradiotherapy. Head Neck. 2014;36(8):1155–1161. | ||
Magné N, Marcy P, Foa C, et al. Comparison between nasogastric tube feeding and percutaneous fluoroscopic gastrostomy in advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2001;258(2):89–92. | ||
McLaughlin BT, Gokhale AS, Shuai Y. Management of patients treated with chemoradiotherapy for head and neck cancer without prophylactic feeding tubes: the University of Pittsburgh experience. Laryngoscope. 2010;120(1):71–75. | ||
Williams GF, Teo MT, Sen M, Dyker KE, Coyle C, Prestwich RJ. Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: a role for a prophylactic gastrostomy? Oral Oncol. 2012;48(5):434–440. | ||
Salas S, Baumstarck-Barrau K, Alfonsi M, et al. Impact of the prophylactic gastrostomy for unresectable squamous cell head and neck carcinomas treated with radio-chemotherapy on quality of life: Prospective randomized trial. Radiother Oncol. 2009;93(3):503–509. | ||
Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78(4):1026–1032. | ||
Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18(7):837–845. | ||
Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32(5):671–678. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.