Back to Journals » Cancer Management and Research » Volume 10
Comparative effectiveness of early-line nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer: a US community-based real-world analysis
Authors Mahtani RL, Parisi M, Glück S, Ni Q, Park S , Pelletier C, Faria C, Braiteh F
Received 20 September 2017
Accepted for publication 25 December 2017
Published 8 February 2018 Volume 2018:10 Pages 249—256
DOI https://doi.org/10.2147/CMAR.S150960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Reshma L Mahtani,1 Monika Parisi,2 Stefan Glück,3 Quanhong Ni,2 Siyeon Park,4 Corey Pelletier,2 Claudio Faria,2 Fadi Braiteh5,6
1Division of Hematology/Oncology, University of Miami, Miami, FL, 2Health Economics and Outcomes Research, Celgene Corporation, Summit, NJ, 3Global Medical Affairs, Celgene Corporation, Summit, NJ, 4School of Pharmacy, The Ohio State University, Columbus, OH, 5Department of Hematology/Oncology, University of Nevada School of Medicine, Las Vegas, NV, 6Department of Hematology/Oncology, Comprehensive Cancer Centers of Nevada, Las Vegas, NV, USA
Background: Real-world analyses of treatments for patients with metastatic breast cancer are limited. We evaluated the comparative effectiveness of nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer using data from an electronic medical record database from community practices across the USA.
Methods: We performed a retrospective cohort study using fully de-identified data from an independent US electronic medical record platform of patients with metastatic breast cancer initiating single-agent nab-paclitaxel or paclitaxel as a first- or second-line treatment from December 1, 2010 to October 6, 2014. The clinical efficacy objectives were time to treatment discontinuation (TTD) and time to next treatment (TTNT). Subgroup analyses were performed in patients with 2 types of metastatic breast cancer as follows: 1) hormone receptor-positive and human epidermal growth factor receptor 2 negative, and 2) triple-negative disease.
Results: This analysis included 925 patients. Patients receiving nab-paclitaxel vs. paclitaxel had significantly longer TTD (median 4.2 vs. 2.8 months, P<0.0001) and TTNT (median 6.0 vs. 4.2 months, P<0.0001); similar outcomes were observed for patients with hormone receptor-positive/human epidermal growth factor receptor 2 negative disease. Compared with paclitaxel, nab-paclitaxel was associated with significantly longer TTD in patients with triple-negative disease. nab-Paclitaxel was associated with significantly less all-grade neuropathy, anemia, pain, and diarrhea than paclitaxel. Antiemetic and antihistamine use were significantly less frequent with nab-paclitaxel vs. paclitaxel, whereas use of granulocyte colony-stimulating factor, hydrating agents, and bone-directed therapy to decrease skeletal-related events were more frequent.
Conclusion: nab-Paclitaxel demonstrated improved clinical effectiveness compared with paclitaxel when examining TTD and TTNT in patients with metastatic breast cancer in a real-world setting.
Keywords: metastatic breast cancer, nab-paclitaxel, paclitaxel, hormone receptor positive, triple negative
Introduction
Breast cancer is the second leading cause of cancer death in women in the USA.1 Five-year survival rate for breast cancer across all stages is 89%, whereas for patients with metastatic disease, the rate is only 26%.2,3 Breast cancer is a heterogeneous disease that includes different subtypes, some more aggressive than others.4 For example, patients with hormone receptor-positive (HR+) metastatic breast cancer generally have a better outcome than those with triple-negative disease. In addition, expanding treatment options have considerably improved outcomes for patients with human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer.5
Chemotherapy is recommended for patients with metastatic breast cancer and is currently the only treatment option for patients with triple-negative breast cancer.6 Chemotherapy may also be used for patients with HR+ disease who have developed resistance to hormonal therapy, or when patients are sufficiently symptomatic to warrant the use of chemotherapy.6 Taxanes, such as paclitaxel and nab-paclitaxel, are a commonly used class of chemotherapy for metastatic breast cancer, including in the setting of recurrent disease after adjuvant treatment.6 Paclitaxel is solvent-based and formulated in a mixture of polyoxyethylated castor oil (Kolliphor® EL, formerly known as Cremophor® EL; BASF, Ludwigshafen, Germany) and dehydrated alcohol, while nab-paclitaxel is an albumin-bound nanoparticle formulation of paclitaxel and is free of solvents.7,8 Both nab-paclitaxel and paclitaxel have demonstrated antitumor activity in patients with metastatic breast cancer, but differences in their efficacy and safety profiles exist, as demonstrated in a head-to-head Phase III trial.6,8,9 nab-Paclitaxel was subsequently approved in the USA for metastatic breast cancer treatment after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy, which should have included an anthracycline.7
Although there are limitations with observational research and randomized controlled trials remain the gold standard for drug approval, real-world data are useful for making treatment decisions.10,11 To date, real-world comparative-effectiveness data for early-line use of nab-paclitaxel vs. paclitaxel in metastatic breast cancer are limited. This analysis explored treatment patterns, outcomes, adverse events, premedication, and supportive-care use of nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer.
Methods
Data source
A retrospective analysis was performed using fully de-identified data from the Navigating Cancer electronic medical record (EMR) platform, an independently owned US database providing services to several community-based oncologists. This database collects and aggregates health record data from different EMR systems, and then stores the data in a cloud-based network. The data is available for a licensing fee. At the time of analysis, which included the index period (December 1, 2010, to October 6, 2014) and follow-up period (through April 6, 2015), the database use included ~1300 providers in mostly oncology/hematology practices and represented 2,500,000 patients. Due to the data being fully de-identified, institutional review board approval was not required.
Patients
Figure 1 summarizes key eligibility criteria. Patient data was collected at the start of either first- or second-line nab-paclitaxel or paclitaxel therapy. This was considered the date the patient was indexed into the study. Patients were excluded if they received other chemotherapy agents in combination with nab-paclitaxel or paclitaxel; however, patients who received targeted agents in combination with bevacizumab, HER2-targeted therapy, or hormone therapy could be included in this analysis. First-line patients received either nab-paclitaxel or paclitaxel after a diagnosis of metastatic breast cancer. For second-line patients, acceptable first-line therapies included chemotherapy (excluding taxanes), HER2-targeted therapy, and/or hormone therapy, and second-line treatment with either nab-paclitaxel or paclitaxel was required.
  | Figure 1 Patient flow. Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; qw, weekly; q3w, every 3 weeks. |
Endpoints
Clinical efficacy endpoints were time to treatment discontinuation (TTD) and time to next treatment (TTNT). TTD was defined as time between first and last dose +7 days for a weekly (qw) cycle or +21 days for an every-3-week (q3w) cycle. Treatment was considered a new treatment line if there was a gap of ≥60 days between 2 administrations of the same drug or if the patient started a new chemotherapy drug. Patients were censored if they remained on treatment during the last 30 days of available data in the database (data cutoff: April 6, 2015). TTNT was defined as the period from day 1 of index drug to day 1 of next treatment line. Subgroup analyses of clinical efficacy endpoints included patients with HR+/HER2− disease and those with triple-negative disease. Safety and utilization endpoints included EMR-documented adverse events (using International Classifications of Diseases [ICD]-9 codes and laboratory values) and use of premedication (≤48 h prior to administration of first treatment) and supportive care (number of doses/patient/100 days and percentage of patients utilizing supportive care) during treatment.
Statistical analysis
For baseline demographic characteristics, Student’s t-tests and Wilcoxon rank-sum tests were used to compare differences between treatments for continuous variables, while c2-tests and Fisher’s exact tests were used for categorical variables. Median TTD was calculated using the Kaplan–Meier method. Multivariate analyses of TTD were performed using the Cox proportional hazards model adjusted for age, number of metastases, targeted-agent use, adjuvant chemotherapy (≤1 year prior to date of diagnosis of metastatic breast cancer), HER2 status, triple-negative status, and Charlson Comorbidity Index score without age. TTNT was evaluated using the analysis of covariance model with the same covariates as the Cox proportional hazards model (log transformation used to normalize data due to skewness). Doses of supportive care use were compared using Poisson regression analysis adjusted for the same covariates described above. Logistic regression was used to assess the percentage of patients with adverse events or on supportive care between treatments, with the same adjusted covariates. The same outcomes were analyzed in patients with HR+/HER2− or triple-negative disease using the same statistical processes. For patients with triple-negative disease, treatment effect on TTD was also adjusted for secondary malignant neoplasms of bone and bone marrow and adjuvant taxane use (≤1 year prior to date of diagnosis of metastatic breast cancer).
Results
Baseline characteristics
After eligibility criteria were applied, the analysis included 925 patients (nab-paclitaxel, n=334; paclitaxel, n=591; Figure 1). Baseline characteristics were similar between groups, with few exceptions (Table 1). More patients received paclitaxel than nab-paclitaxel, and more were treated qw than q3w. Additionally, a higher proportion of patients in the nab-paclitaxel cohort received first-line index treatment vs. those in the paclitaxel cohort overall. A significantly higher percentage of patients in the nab-paclitaxel vs. the paclitaxel group had HER2− or triple-negative disease. The paclitaxel group had a higher percentage of patients with HER2+ disease than the nab-paclitaxel group. Accordingly, a significantly higher percentage of patients in the paclitaxel group received a HER2-targeted agent than in the nab-paclitaxel group; however, bevacizumab and hormone therapy use were similar. Compared with the paclitaxel group, a significantly higher proportion of patients in the nab-paclitaxel group had bone metastases. The percentage of patients using adjuvant taxane treatment within 1 year from the diagnosis of metastatic disease was significantly higher in the nab-paclitaxel group than the paclitaxel group.
TTD and TTNT
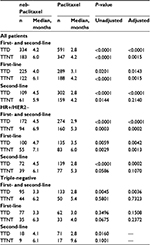
Median TTD was significantly longer in patients receiving nab-paclitaxel vs. paclitaxel (4.2 vs. 2.8 months, P<0.0001) and remained significant in the multivariate analysis, regardless of line of therapy (Figure 2A, Table 2). Median TTNT was also significantly longer in patients receiving first- or second-line nab-paclitaxel than those receiving paclitaxel (Figures 2B, C, Table 2). For patients receiving second-line therapy, treatment differences for TTNT did not remain significant after adjusting for covariates.
  | Figure 2 TTD. (A) All patients. (B) First-line. (C) Second-line. Abbreviation: TTD, time to treatment discontinuation. |
TTD and TTNT in patients with HR+/HER2− metastatic breast cancer
Overall, 446 of 925 patients (48%) had HR+/HER2− disease (nab-paclitaxel, n=172; paclitaxel, n=274). Baseline characteristics were similar between treatment groups; however, a higher percentage of patients received nab-paclitaxel than paclitaxel (Table 1). Within each treatment group, significantly more patients were treated qw vs. q3w. The nab-paclitaxel vs. paclitaxel cohort had a significantly higher percentage of bone and liver metastases. Patients with HR+/HER2− disease receiving nab-paclitaxel vs. paclitaxel had significantly longer median TTD and TTNT (Table 2).
TTD and TTNT in patients with triple-negative metastatic breast cancer
In total, 228 of 925 patients (25%) had triple-negative disease (nab-paclitaxel, n=95; paclitaxel, n=133). Baseline characteristics were similar between groups, with some exceptions. A significant majority of these patients were treated qw rather than q3w (Table 1). A significantly higher proportion of patients in the nab-paclitaxel cohort received index treatment as first-line therapy compared with those in the paclitaxel cohort. Patients receiving nab-paclitaxel vs. paclitaxel had significantly more bone metastases and were significantly more likely to receive an adjuvant taxane within the last year. Median TTD was significantly longer for patients receiving nab-paclitaxel vs. paclitaxel as first- or second-line treatment (Table 2), and median TTNT was also longer with nab-paclitaxel vs. paclitaxel, although this difference was not significant.
Adverse events, premedication, and supportive care
Overall, nab-paclitaxel was associated with significantly lower rates of any-grade anemia, diarrhea, pain, and neuropathy than paclitaxel (Table 3); no significant treatment differences were observed in the rates of neutropenia and nausea/vomiting between the treatment groups. Fewer premedication doses of antiemetics, antihistamines, and steroids were administered with nab-paclitaxel than in the paclitaxel group (Table 4). In the nab-paclitaxel group, fewer doses of antiemetics and antihistamines but more doses of granulocyte colony-stimulating factor (G-CSF) and bone-directed therapy to decrease skeletal-related events were administered as supportive care than in the paclitaxel group.
  | Table 3 Any-grade adverse events in all patientsa Notes: aReported if ≥5% of patients in either treatment group experienced an adverse event. |
  | Table 4 Premedication and supportive care in all patients Notes: aAntihistamine treatments included diphenhydramine and epinephrine. Abbreviations: G-CSF, granulocyte colony-stimulating factor. |
Discussion
In this real-world analysis of patients with metastatic breast cancer, median TTD and TTNT were significantly longer with nab-paclitaxel than paclitaxel, regardless of treatment line. Of note, 13% of patients treated with nab-paclitaxel and 7% of those treated with paclitaxel received a taxane in the 12 months prior to their metastatic breast cancer diagnosis and were retreated with a taxane after metastatic diagnosis. It is possible that some of these patients received nab-paclitaxel or paclitaxel as second-line therapy and had received another type of chemotherapy as their first-line treatment for metastatic breast cancer. Subgroup analyses demonstrated significantly longer TTD with nab-paclitaxel in patients with HR+/HER2− or triple-negative disease than with paclitaxel; TTNT was significantly longer with nab-paclitaxel than paclitaxel in patients with the HR+/HER2− disease. In the overall population, nab-paclitaxel was associated with less neuropathy, anemia, pain, and diarrhea than paclitaxel. Fewer premedication doses of antiemetics, antihistamines, and steroids were required in the nab-paclitaxel cohort than in the paclitaxel cohort. Compared with paclitaxel, nab-paclitaxel also required fewer doses of antiemetics or antihistamines, but more G-CSF and bone-directed therapy to decrease skeletal-related events as supportive care.
In some analyses, TTNT has been used as a proxy for time to progression (TTP), as one might deduce that a patient has started a new therapy because they progressed on their prior therapy.12,13 In a randomized, prospective Phase III trial of patients with metastatic breast cancer, patients receiving nab-paclitaxel vs. paclitaxel had a longer TTP (median, 5.3 vs. 3.9 months; P=0.006).9 In this analysis, TTNT was also significantly longer with nab-paclitaxel than paclitaxel (median, 6.0 vs. 4.2 months; unadjusted P<0.0001; adjusted P=0.0015). This concordance between the clinical trial and the real-world analysis confirms the clinical benefit of nab-paclitaxel compared with paclitaxel in patients with metastatic breast cancer. However, clinical studies and real-world analyses should not be directly compared due to differences in their populations and lack of randomization in the latter. In this study, only patients receiving a subsequent treatment line were included in the TTNT analysis, which may help in explaining the slightly longer TTNT observed here compared with the TTP in the Phase III study.9 Additionally, 56% of patients in this study received either nab-paclitaxel or paclitaxel as first-line therapy, including 67% of the nab-paclitaxel group, while only 14% of patients in the Phase III study received these agents as first-line therapy.
In the aforementioned Phase III trial, significantly less treatment-related grade 4 neutropenia but more treatment-related grade 3 sensory neuropathy and all-grade nausea and diarrhea were observed with nab-paclitaxel than with paclitaxel.9 In this real-world analysis of patients with metastatic breast cancer, nab-paclitaxel was associated with less any-grade neuropathy, anemia, pain, and diarrhea than paclitaxel, and no significant treatment difference in any-grade neutropenia was observed. These differences in the safety profiles between the 2 studies may be partly attributable to differences in the nab-paclitaxel schedule used (q3w in the Phase III trial vs. qw in 89% of patients in this study).9 Additionally, adverse event reporting was limited in the EMR database to ICD-9 codes and laboratory values only and could have been underreported. Antihistamine use, both as supportive care and premedication, was higher with paclitaxel than nab-paclitaxel; however, this is likely due to their common co-administration with paclitaxel to prevent hypersensitivity reactions.14 Bone-directed therapy to decrease skeletal-related events was used more frequently in patients receiving nab-paclitaxel compared with paclitaxel, though significantly more patients receiving nab-paclitaxel had bone metastases.
In patients with hormone-resistant/refractory HR+/HER2− or triple-negative disease, chemotherapy is a standard treatment option. nab-Paclitaxel has shown clinical activity in HER2− and triple-negative metastatic breast cancer.15,16 In this analysis, patients with HR+/HER2− subtype receiving nab-paclitaxel instead of paclitaxel had a longer TTD and TTNT, while patients with triple-negative subtype who received nab-paclitaxel instead of paclitaxel had significantly improved TTD. TTNT was longer in patients with triple-negative metastatic breast cancer receiving second-line paclitaxel instead of nab-paclitaxel; however, this trend should be analyzed cautiously given the small size of the triple-negative subgroup. Additionally, because a limited number of patients received second-line nab-paclitaxel, particularly patients with triple-negative disease, the observed numerical difference between first- and second-line TTD may be an overestimation.
This being a real-world analysis, there are several limitations to this study.11 This retrospective analysis using EMR data included some differences in baseline characteristics between groups; however, we adjusted for these differences with multivariate or logistic regression analyses. The EMR database lacked information on the actual dose administered due to missing body surface area data; therefore, the influence of dose intensity, delays, and modifications could not be evaluated. Additionally, because information on response and/or progression was unavailable from the EMR database, we could not calculate objective response rates and TTP for comparison with the Phase III study. Finally, adverse events and supportive care use were restricted to those reported in the EMR system via ICD-9 codes, laboratory values, and the medication field, which limit collection of adverse events by grade and may result in underreporting.
Conclusion
This analysis confirms the improved clinical benefit of nab-paclitaxel compared with paclitaxel in a real-world setting and supports the Phase III trial results.9 Patients receiving nab-paclitaxel had a significantly longer TTD and TTNT compared with patients receiving paclitaxel. The clinical benefit of nab-paclitaxel was also confirmed in patients with HR+/HER2− and triple-negative subtypes. Additionally, nab-paclitaxel was associated with fewer adverse events and doses of premedication and supportive care. This real-world study supports nab-paclitaxel as a preferred treatment option for patients with metastatic breast cancer.
Acknowledgments
Writing assistance was provided by Tara Wabbersen, PhD, MediTech Media, through funding by Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript.
The real-world analysis data of nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer, as well as the sub-analysis by HR+/HER2− and triple negative disease, were presented at the 33rd Annual Miami Breast Cancer Conference (2016). A second-line treatment only subset of these data was presented at the European Society for Medical Oncology Congress (2016).
Disclosure
RLM served on advisory boards for Genentech, Amgen, Sandoz, Celgene, and Pfizer, received research support from Genentech, and served as a consultant for Celgene and Pfizer. MP, SG, QN, CP, and CF are all employees of Celgene Corporation. FB is a speaker and consultant for Celgene Corporation. The authors report no other conflicts of interest in this work.
References
American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society, Inc; 2016. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 14, 2016. | ||
American Cancer Society. Breast Cancer Facts and Figures 2015-2016. Atlanta, GA: American Cancer Society, Inc; 2015. | ||
Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute. Available from: https://seer.cancer.gov/archive/csr/1975_2014/#contents. Updated September 12, 2016. Accessed August 29, 2017. | ||
Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121(10):3786–3788. | ||
Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–514. | ||
NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. V2.2017. Available from: https://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Accessed March 6, 2017. | ||
Abraxane [package insert]. Summit, NJ: Celgene Corporation; 2015. | ||
Taxol [package insert]. North Wales, PA: Teva Parenteral Medicines, Inc; 2015. | ||
Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. | ||
Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30(34):4215–4222. | ||
Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. | ||
Arikian SR, Milentijevic D, Binder G, et al. Patterns of total cost and economic consequences of progression for patients with newly diagnosed multiple myeloma. Curr Med Res Opin. 2015;31(6):1105–1115. | ||
Chen CC, Parikh K, Abouzaid S, et al. Real-world treatment patterns, time to next treatment, and economic outcomes in relapsed or refractory multiple myeloma patients treated with pomalidomide or carfilzomib. J Manag Care Spec Pharm. 2017;23(2):236–246. | ||
Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–1268. | ||
Palumbo R, Sottotetti F, Trifiro G, et al. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as second-line chemotherapy in HER2-negative, taxane-pretreated metastatic breast cancer patients: prospective evaluation of activity, safety, and quality of life. Drug Des Devel Ther. 2015;9:2189–2199. | ||
Forero-Torres A, Varley K, Abramson V, et al. TBCRC 019: a Phase II trial of nanoparticle albumin-bound paclitaxel with or without the anti-death receptor 5 monoclonal antibody tigatuzumab in patients with triple-negative breast cancer. Clin Cancer Res. 2015;21(12):2722–2729. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.