Back to Journals » Drug Design, Development and Therapy » Volume 16
Comparative Dose–Response Study on the Infusion of Norepinephrine Combined with Crystalloid Coload versus Colloid Coload for Preventing Hypotension During Spinal Anesthesia for Cesarean Delivery
Authors Jin WD, Mao JQ, Liu J, Liang G, Jiang C, Sheng ZM
Received 15 June 2022
Accepted for publication 2 August 2022
Published 6 August 2022 Volume 2022:16 Pages 2617—2626
DOI https://doi.org/10.2147/DDDT.S378453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Wei-dong Jin,1 Jun-qin Mao,2 Jie Liu,2 Gang Liang,2 Chao Jiang,2 Zhi-min Sheng2
1Department of Anesthesiology, Jinhua Maternity and Child Health Care Hospital, Jinhua, People’s Republic of China; 2Department of Anesthesiology, Wenling Maternity and Child Health Care Hospital, Taizhou, People’s Republic of China
Correspondence: Zhi-min Sheng, Department of Anesthesiology, Wenling Maternity and Child Health Care Hospital, 102, Xiabao Road, Chengdong Street, Taizhou, 317500, People’s Republic of China, Tel +86-576-86168030, Email [email protected]
Background: Although the optimal infusion dose of norepinephrine combined with crystalloid coload for preventing spinal anesthesia-induced hypotension (SAIH) for cesarean delivery has been established, the infusion regimen of norepinephrine combined with colloid coload has not been fully quantified. The objective of this study was to compare and determine the median effective dose (ED50) and 90% effective dose (ED90) of norepinephrine infusion combined with crystalloid coload versus colloid coload for preventing SAIH during cesarean delivery.
Methods: Two hundred parturients were randomly assigned to receive norepinephrine infusion at 0.02, 0.04, 0.06, 0.08, or 0.10 μg/kg/min in combination with 10 mL/kg crystalloid coload or colloid coload to prevent SAIH. The study period was defined as the interval from the commencement of intrathecal injection to delivery of the neonate. The primary outcome was non-occurrence of hypotension, defined as systolic blood pressure (SBP) less than 80% of the baseline before delivery. The ED50 and ED90 of norepinephrine infusion dose were determined using probit regression analysis. By calculating the 95% confidence intervals (CIs) of relative median potency to determine whether the prophylactic infusion of norepinephrine requirement was different between the two groups.
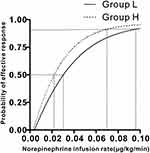
Results: The derived ED50 and ED90 of norepinephrine infusion combined with crystalloid coload were 0.030 (95% CIs 0.020 to 0.038) and 0.097 (95% CIs 0.072 to 0.157) μg/kg/min, respectively. The ED50 and ED90 of norepinephrine infusion combined with colloid coload were 0.021 (95% CIs 0.013 to 0.029) and 0.070 (95% CIs 0.053 to 0.107) μg/kg/min, respectively. The estimate of relative median potency for norepinephrine between the two groups was 1.37 (95% CIs 0.94 to 2.23).
Conclusion: Under the conditions of this study, 10 mL/kg colloid coload reduced the dose of prophylactic norepinephrine infusion by approximately 30% in parturients during spinal anesthesia for cesarean delivery compared with the crystalloid coload.
Keywords: norepinephrine, hypotension, the dose–response relationship, crystalloid, colloid, ED50, ED90
Introduction
Spinal anesthesia is associated with high incidence of maternal hypotension during cesarean delivery, which results in serious adverse effects.1–3 Vasopressors and fluid therapy are considered to be the main strategies for the prevention and treatment of spinal anesthesia-induced hypotension (SAIH).4,5 Norepinephrine is associated with less bradycardia due to its mild beta-adrenergic agonistic activity and has recently been described as a promising alternative to phenylephrine for preventing and treating SAIH for cesarean delivery.6,7 A number of variables can be manipulated for the strategy of fluid therapy during spinal anesthesia for cesarean delivery, including the volume and rate of administration, the type of fluid (crystalloid or colloid) and the timing of administration (preload or coload).8,9 The effectiveness of crystalloid infused before the time of induction of spinal anesthesia (preload) for preventing SAIH for cesarean delivery has been questioned in recent years.10–12 In comparison, fluid infused at the time of induction of spinal anesthesia (coload) limits fluid redistribution and excretion, because it maximizes the effect when spinal block occurs, which may reduce the risk and severity of SAIH. Hence, it has become a common method of fluid infusion in obstetric anesthesia.13,14 Several clinical trials have reported that the ED50, ED90 and ED95 of norepinephrine infusion combined with crystalloid coload for preventing SAIH for cesarean delivery were 0.029 µg/kg/min, 0.080 µg/kg/min and 0.105 µg/kg/min, respectively.15,16 However, the dose–response relationship on the infusion of norepinephrine combined with colloid coload for preventing SAIH for cesarean delivery is still not clear. A previous study indicated that the incidence of hypotension was significantly higher in the crystalloid coload group compared with the colloid coload group.17 Hence, we hypothesized that the optimal infusion dose of norepinephrine combined with colloid coload for preventing SAIH for cesarean delivery might be lower than crystalloid coload.
Our aim was to prospectively compare five norepinephrine infusion doses (0.02, 0.04, 0.06, 0.08 and 0.10 µg/kg/min) combined with crystalloid coload versus colloid coload for preventing SAIH during cesarean delivery, and to compare the dose–response effect by using probit analysis. The relative median potency was used to determine whether the required dose of norepinephrine was different between the two groups.
Materials and Methods
Design and Study Subjects
This prospective, controlled, double-blind, dose-finding study was undertaken from October 12, 2020 to June 29, 2021 at the Wenling Maternity and Child Health Care Hospital, Taizhou, China and approval was obtained from the ethics committee (No. 2020-IRB-001), registered at the Chinese Clinical Trial Registry (www.chictr.org.cn, registration No. Chi CTR 2000038925). Informed written consents were obtained from all subjects.
Parturients aged 18–40 years with singleton pregnancies (≥37 weeks) scheduled for elective cesarean delivery in the Wenling Maternal and Child Health Care Hospital were recruited into the study. Exclusion criteria were as follows: American Society of Anesthesiologists physical status ≥III, height (<150 or >170 cm), obesity (BMI ≥35kg/m2), hypertension, preeclampsia, diabetes mellitus, cardiovascular diseases, significant coexisting maternal disease, fetal congenital abnormalities and any contraindications to spinal anesthesia.
Study Protocol
MedCalc (Version 18.2.1 BV, Ostend, Belgium) was used to randomly divide parturients into Group L (Lactated Ringer’s solution coload group) and Group H (Hydroxyethyl Starch 130/0.4 Sodium Chloride Injection coload group), then created randomization code sequences for both groups. Each parturient’s code was placed in a sequentially numbered, sealed, opaque envelope, and the codes randomly assigned parturients evenly (20 per group) to 1 of 5 different infusion rates of norepinephrine (Hubei Yuanda Co., Ltd.; 2mg/1mL) (0.02, 0.04, 0.06, 0.08 or 0.10 µg/kg/min). The dose range of norepinephrine infusion was based on recent studies.15,16
Randomization was performed by an anesthesia resident not involved in case management who also prepared the medications according to the randomization codes. The dose of norepinephrine was calculated as follows: group 0.02, weight (kg) × 0.02 µg/kg/min× 60min; group 0.04, weight (kg) × 0.04 µg/kg/min× 60min; group 0.06, weight (kg) × 0.06 µg/kg/min× 60min; group 0.08, weight (kg) × 0.08 µg/kg/min× 60min; and group 0.10, weight (kg) × 0.10 µg/kg/min× 60min. On the day of the operation, the designated concentration of norepinephrine for each group was prepared in identical 50-mL infusion syringes by diluting with normal saline to a total volume of 50 mL, and handed over to the anesthetist who collected the data and instructed him to administer the drug at a rate of 50 mL/h. All study fluids used for coload were covered by opaque plastic bags, prepared and sealed by a pharmacist who was not involved in the study. The infusion bags of crystalloid (Lactated Ringer’s solution Zhejiang Tianrui Co., Ltd.; 500mL) adjusted for body weight (10mL/kg) were labeled as fluid A, and the infusion bags of colloid (Hydroxyethyl Starch 130/0.4 Sodium Chloride Injection Shandong Huaren Co., Ltd.; 500mL) (10mL/kg) were labeled as fluid B. The anesthetist in charge of the care of the parturients was blinded to the actual concentration of norepinephrine and the type of fluid used.
No preoperative medication was given before anesthesia. Routine monitoring including noninvasive blood pressure (NIBP) measurement, pulse oximetry, and electrocardiography were conducted. After a brief calm period, NIBP measurement was performed continuously every 2 minutes in the supine position. The mean value of three successive systolic blood pressure (SBP) with a difference of less than 10% was taken as the baseline SBP. A 16-gauge intravenous catheter was inserted in the left forearm vein. No intravenous infusion preload was given.
The parturients were placed in the left lateral decubitus position, the combined spinal–epidural puncture was performed at the L3-4 interspace after skin disinfection and infiltration anesthesia. Epidural space was confirmed using the loss-of-resistance-to-air technique with an 18-gauge Tuohy needle. Spinal anesthesia was performed using a 27-gauge pencil-point spinal needle via the Tuohy needle. After confirming flow of clear cerebrospinal fluid (CSF), 3 mL (16 mg) of hyperbaric ropivacaine (Naropin; AstraZeneca Co., Ltd.; 100 mg/10mL) (1.6 mL ropivacaine 1% + 1 mL dextrose 10% + 0.9% saline for dilution to 3 mL) were injected towards the ceiling direction at a rate of 1 mL per 10s. The spinal needle was then withdrawn and an epidural catheter was inserted into the epidural space, and gentle aspiration with the syringe to ensure there was no blood or CSF. No drug was given through the epidural catheter.
Immediately after intrathecal injection, parturients were returned to the supine with left uterine displacement. Concurrent with the intrathecal injection, co-hydration with 10 mL/kg of crystalloid or colloid using a pressurized infusion system pressurized at 200 mmHg to administer the fluid at the maximum possible rate. Each infusion was completed within 10 minutes, then the solution was infused slowly just to keep the vein open. At the time of intrathecal injection, the infusion of the study drug was initiated at a rate of 50 mL/h using a syringe pump that was connected to the parturient’s intravenous cannula via a three-way stopcock. NIBP measurement was commenced immediately after intrathecal injection and then cycled at every minute until the time of delivery, subsequently cycled at three-minute intervals until the completion of surgery. An 18-gauge blunt epidural needle was used to evaluate sensory block at 10 minutes after spinal injection (T6 or above was considered successful). If unsuccessful, the parturient was excluded from the study, and the random code was used for the next eligible enrolled parturient.
We defined hypotension after spinal anesthesia as SBP less than 80% of the baseline. When hypotension occurred, it was managed by intravenous (IV) norepinephrine 6 µg. Additional bolus of norepinephrine was given if SBP did not respond to the first dose within 1 min, until the SBP ≥90% of the baseline SBP. Reactive hypertension was defined as SBP higher than 120% of the baseline. It was managed by stopping the infusion of norepinephrine, and the infusion was restarted when SBP ≤ 90% of the baseline. Bradycardia was defined as HR <50 beats/min, If accompanied by hypotension, 0.5 mg atropine was given. If not accompanied by hypotension stopped the norepinephrine infusion and restarted the infusion when HR returns to >50 beats/min.
The study period was defined as the interval from the commencement of intrathecal injection to delivery of the neonate. Effective norepinephrine infusion dose was defined as that when no hypotension occurred during the study period.
The primary outcome of this study was the incidence of hypotension after spinal anesthesia at different fixed-rate. Secondary outcomes included the frequency of reactive hypertension, bradycardia, nausea or vomiting and neonatal outcomes.
Sample Size Estimation
We calculated the sample size with the Cochran–Armitage Test for trend in proportions using PASS® (Version 11.0.1; NCSS, LLC, Kaysville, Utah). According to a pilot study, the infusion rate of norepinephrine was 0.02, 0.04, 0.06, 0.08, and 0.10 µg/kg/min corresponding to the probability of success for preventing hypotension of 0.5, 0.6, 0.75, 0.9, and 0.95, respectively. In order to provide 90% power with a significance level of 0.05 to detect a linear trend among groups in the proportion of maternal hypotension using a Z test with continuity correction, a sample size of 65 (13 per group) parturients in each group was required. Considering possible dropouts and narrowing confidence intervals, we planned to increase the sample size to 100 per group (20 per dosage).
Statistical Analysis
Data were analyzed using IBM SPSS version 22.0 for Windows (IBM Corp, Armonk, NY) and GraphPad Prism version 8.0.2 (GraphPad Software Inc, San Diego, CA, USA). Statistical significance was determined at P < 0.05. The Kolmogorov–Smirnov test was used to evaluate continuous data for normal distribution, and the values were expressed as mean ± SD and median [interquartile range] where appropriate. Normally distributed data were analyzed by the independent-samples Student’s t-test, and non-normally distributed data were analyzed by the Mann–Whitney U-test. χ2 test was used to analyze the categorical variables such as incidence of hypotension and reactive hypertension, and the values were presented as number (%).
Probit regression was used to determine dose–response analysis. The primary endpoint was the effective prophylactic norepinephrine infusion dose. The proportion of successes at each dose level was converted to probits, and regression analysis was performed. Interpolation was used to derive the ED50 and ED90 with 95% confidence intervals (CIs) for norepinephrine infusion dose to prevent hypotension in each group. The relative median potency and 95% CIs were obtained by comparing the estimated values of ED50, to determine whether the required dose of norepinephrine was different between the two groups.
Results
A total of 226 parturients were involved and assessed in this study, and a total of 100 parturients in each group were enrolled for final analysis. The study process is shown in Figure 1. Parturient demographic data, sensory block level, spinal anesthesia to delivery interval, total norepinephrine consumption (before delivery) and intravenous fluid volume (before delivery) were comparable between the two groups (Table 1).
 |
Table 1 Demographic Data, Sensory Block Level, Spinal Anesthesia to Delivery Interval, Norepinephrine Consumption and Fluid Volume Given |
 |
Figure 1 Consolidated standards of reporting trials diagram showing patient recruitment and flow. |
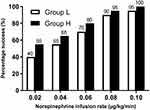
The success rate of hypotension prevention in the two groups at different norepinephrine infusion rates is shown in Figure 2. The dose–response curves of norepinephrine infusion to prevent hypotension in the two groups were derived by using probit regression analysis are presented in Figure 3. The ED50 and ED90 of prophylactic infusion of norepinephrine in Group L were 0.030 (95% CIs 0.020 to 0.038) and 0.097 (95% CIs 0.072 to 0.157) µg/kg/min, respectively. The ED50 and ED90 in Group H were 0.021 (95% CIs 0.013 to 0.029) and 0.070 (95% CIs 0.053 to 0.107) µg/kg/min, respectively. The estimate of relative median potency of prophylactic infusion of norepinephrine between the two groups was 1.37 (95% CIs 0.94 to 2.23).
The hemodynamic changes and side effects were similar among groups (Table 2).
 |
Table 2 Hemodynamic Changes, Side Effects |
Neonatal outcomes are shown in Table 3. The number of neonates in Group L with Apgar score <7 at 1 minute was 2 versus 1 in Group H (P = 0.56). All neonates had Apgar score ≥7 at 5 minutes in both groups. No difference in fetal umbilical artery blood gas analysis and birth weight between the two groups.
 |
Table 3 Neonatal Outcomes |
Discussion
In this study, we determined the dose–response properties on the infusion of norepinephrine combined with crystalloid coload versus colloid coload for preventing SAIH for cesarean delivery. The derived ED50 values were 0.030 (95% CIs 0.020 to 0.038) and 0.021 (95% CIs 0.013 to 0.029) µg/kg/min, respectively. The ED90 values were 0.097 (95% CIs 0.072 to 0.157) and 0.070 (95% CIs 0.053 to 0.107) µg/kg/min, respectively. The estimate of relative median potency of prophylactic infusion of norepinephrine between the two groups was 1.37 (95% CIs 0.94 to 2.23). Our results demonstrated that although no significant difference in the incidence of maternal hypotension between the crystalloid coload group and the colloid coload group, colloid coload reduced the dose of prophylactic norepinephrine infusion by approximately 30% in parturients during spinal anesthesia for cesarean delivery compared with the crystalloid coload.
Numerous studies have confirmed that crystalloid preload was relatively ineffective in the prevention of SAIH, despite the volume of crystalloid was as high as 30 mL/kg, so it was no longer recommended.10–12 This may be due to stimulation of atrial natriuretic peptide secretion and rapid redistribution, resulting in peripheral vasodilation and diuresis.18 Hence, it was suggested that starting fluid coload concurrent with the intrathecal injection of local anesthetic is a more reasonable approach as sympathetic blockade-related vasodilation coincides with intravascular volume expansion.13,19,20 In addition, coload saved the time spent by infusion preload before anesthesia and unnecessary to delay the surgery, especially for emergency cesarean delivery.17 Thus, the fluid coload regimen can be recommended to improve the hemodynamic stability provided by vasopressor prophylaxis.21
The comparison of colloid coload versus crystalloid coload without prophylactic vasopressor infusion in obstetric patients has been described previously.17,22 According to the current international consensus statement, it was recommended to use vasopressors prophylactically.21 We believe that in clinical practice, it is more appropriate to use fluid coload combined with prophylactic vasopressor infusion to prevent SAIH during cesarean delivery. However, few data exist on the comparative efficacy of crystalloid versus colloid combined with prophylactic norepinephrine infusion.
Many authors found that the colloid coload was superior to the crystalloid coload in reducing the incidence of SAIH, nausea and vomiting in combination with prophylactic phenylephrine infusion, but no statistical difference was found between the two groups,23–25 which was consistent with our findings. Different from previous research methods, the strength of our study was that prospectively compared quantifiable effect of crystalloid coload versus colloid coload on norepinephrine requirement for preventing SAIH for cesarean delivery, and derived ED50 and ED90 values of norepinephrine infusion dose by probit regression, so as to provide guidance for the appropriate initial infusion dose of norepinephrine combined with crystalloid coload or colloid coload.
In contrast to our findings, Kaufner L et al reported that compared with colloid coload, crystalloid coload was associated with a higher incidence of hypotension and a greater drop in mean blood pressure.17 Several factors may explain the inconsistencies, including the differences in the definitions of maternal hypotension, doses of intrathecal local anesthetics or adjuvants, different study protocols and total fluid volumes in the studies. Moreover, in the current study, we used prophylactic continuous infusion of norepinephrine, while they used intermittent phenylephrine boluses whenever hypotension occurred and without prophylactic vasopressor infusion. Hence, the incidence of SAIH in both the crystalloid group and the colloid group was much lower than their findings.
A recent study reported that the ED90 of norepinephrine infusion dose combined with crystalloid coload for preventing SIAH was 0.080 µg/kg/min.15 The value is lower than the ED90 we determined. This difference is uncertain, but could be attributed to differences in grouping dose and infusion volume of crystalloid. In particular, in our study, the volume of crystalloid coload was adjusted according to body weight (10mL/kg), while Fu et al adopted coload with Lactated Ringer’s solution continued up to a maximum of 1.5 L. Although larger infusion volume may reduce the requirement for norepinephrine, it has recently been suggested that larger infusion volume brings no additional benefits.26,27 Considering that the weight of parturients varies widely among different populations, and since obesity is increasingly recognized as a challenge to obstetric anesthesia,28,29 we believe that it is appropriate to adjust the volume of fluid infusion according to the weight, despite the debate on the optimal fluid volume has been going on for a long time.
Due to the recent controversy regarding the safety of hydroxyethyl starch (HES) in intensive care patients and the impact on renal function,30 the European Medicines Agency has decided to restrict the use of HES in critically ill patients and only for hypovolemia caused by acute blood loss.31 In addition, colloids also bear the risk of anaphylaxis and high costs, it is not clear whether colloids are used in obstetric patients with the same risks. We believe that further studies on safety in this population should be conducted before it can be fully recommended for routine clinical use. However, our results showed that compared with crystalloid coload, colloid coload did not show any adverse consequences, and reduced the requirement for prophylactic norepinephrine infusion. This information is potentially useful as a guide for the use of colloid in obstetric patients.
Our study has certain limitations. Firstly, we used fixed rate infusion of norepinephrine to prevent SIAH for cesarean delivery in our institution. Compared with fixed rate infusion regimen, variable rate infusion regimen tightly controls SBP within a narrow, individualized range to ensure optimal hemodynamics control.21 Hence, variable rate infusion regimen may be superior to fixed rate regimen in preventing hypotension. Secondly, we did not monitor maternal cardiac output (CO) and continuous invasive blood pressure. Although SBP was collected at 1-minute intervals before delivery, which can better indicate the trend of hemodynamic changes and provide information for our interventions during SAIH. However, the accuracy of NIBP monitoring and the precise timing of rescue bolus may be affected by a variety of factors, particularly in parturients with obvious shivering and nervousness, which may result in some data loss. Moreover, though HR has a good correlation with CO, non-invasive CO monitoring may provide accurate measurement of fluid responsiveness. Finally, in our study, all parturients scheduled for elective cesarean delivery have strict inclusion criteria, and our results may not be applicable to parturients whose weight and height were outside the range of the study subjects, significant coexisting maternal disease, or those who need emergency cesarean delivery.
Conclusion
In summary, we have investigated five weight-adjusted infusion doses of norepinephrine combined with crystalloid coload versus colloid coload to prevent spinal anesthesia-induced hypotension during cesarean delivery. The results of our study revealed that although the incidence of maternal hypotension was comparable between the crystalloid coload group and the colloid coload group, colloid coload reduced the dose of prophylactic norepinephrine infusion by approximately 30% in parturients during spinal anesthesia for cesarean delivery compared with the crystalloid coload.
Abbreviations
SAIH, spinal anesthesia-induced hypotension; ED50, the 50% effective dose; ED90, the 90% effective dose; CIs, confidence intervals; NIBP, noninvasive blood pressure; SBP, systolic blood pressure; HR, heart rate; IV, intravenous; CO, cardiac output; HES, hydroxyethyl starch; CSF, clear cerebrospinal fluid.
Article Highlights
● The optimal dose of norepinephrine infusion combined with colloid coload has not yet been investigated.
● Under the conditions of this study, the derived ED50 and ED90 values for norepinephrine infusion combined with crystalloid coload were 0.030 (95% CIs 0.020 to 0.038) and 0.097 (95% CIs 0.072 to 0.157) µg/kg/min, respectively. The ED50 and ED90 values for norepinephrine infusion combined with colloid coload were 0.021 (95% CIs 0.013 to 0.029) and 0.070 (95% CIs 0.053 to 0.107) µg/kg/min, respectively.
● 10 mL/kg colloid coload reduced the dose of prophylactic norepinephrine infusion by approximately 30% in parturients during spinal anesthesia for cesarean delivery compared with the crystalloid coload.
Data Sharing Statement
The data that support the findings of the study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of Wenling Maternity and Child Health Care Hospital, Taizhou, China, and approval was obtained from the ethics committee (No. 2020-IRB-001), registered at the Chinese Clinical Trial Registry (www.chictr.org.cn, registration No. Chi CTR 2000038925). Informed written consents were obtained from all subjects. We confirm our study complies with the Declaration of Helsinki.
Consent for Publication
All authors have read and approved the manuscript, and agreed to submit to your journal.
Acknowledgments
The authors thank the teachers and colleagues in the Department of Anesthesiology, Wenling Maternity and Child Health Care Hospital, Taizhou, China.
Funding
This work was supported by funding from Wenling Science and Technology Bureau (No. 2020S0180060).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Klohr S, Roth R, Hofmann T, et al. Definitions of hypotension after spinal anaesthesia for caesarean section: literature search and application to parturients. Acta Anaesthesiol Scand. 2010;54(8):909–921. doi:10.1111/j.1399-6576.2010.02239.x
2. Sanjay Nag D, Prasad Samaddar D, Chatterjee A, et al. Vasopressors in obstetric anesthesia: a current perspective. World J Clin Cases. 2015;3:58–64. doi:10.12998/wjcc.v3.i1.58
3. Stewart A, Fernando R, McDonald S, et al. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–1237. doi:10.1213/ANE.0b013e3181f2eae1
4. Lirk P, Haller I, Wong C. Management of spinal anaesthesia-induced hypotension for caesarean delivery: a European survey. Eur J Anaesthesiol. 2012;29(9):452–453. doi:10.1097/EJA.0b013e328352ab10
5. Mercier FJ. Fluid loading for cesarean delivery under spinal anesthesia: have we studied all the options? Anesth Analog. 2011;113:677–680. doi:10.1213/ANE.0b013e3182245af4
6. Onwochei DN, Ngan Kee WD, Fung L, et al. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: a sequential allocation dose-finding study. Anesth Analg. 2017;125:212–218. doi:10.1213/ANE.0000000000001846
7. Ngan Kee W, Lee S, Ng F, et al. Randomized double blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–745. doi:10.1097/ALN.0000000000000601
8. Banerjee A, Stocche RM, Angle P, Halpern SH. Preload or coload for spinal anesthesia for elective cesarean delivery: a meta-analysis. Can J Anesth. 2010;57:24–31. doi:10.1007/s12630-009-9206-7
9. Ripollés Melchor J, Espinosa Á, Martínez Hurtado E, et al. Colloids versus crystalloids in the prevention of hypotension induced by spinal anesthesia in elective cesarean section. A systematic review and meta-analysis. Minerva Anestesiol. 2015;81:1019–1030.
10. Park GE, Hauch MA, Curlin F, Datta S, Bader AM. The effects of varying volumes of crystalloid administration before cesarean delivery on maternal hemodynamics and colloid osmotic pressure. Anesth Analg. 1996;83(2):299–303. doi:10.1097/00000539-199608000-00017
11. Arora P, Singh RM, Kundra S, Gautam PL. Fluid administration before caesarean delivery: does type and timing matter? J Clin Diagn Res. 2015;9:UC01–4. doi:10.7860/JCDR/2015/12083.6008
12. Tercanli S, Schneider M, Visca E, et al. Influence of volume preloading on uteroplacental and fetal circulation during spinal anaesthesia for caesarean section in uncomplicated singleton pregnancies. Fetal Diagn Ther. 2002;17:142–146. doi:10.1159/000048027
13. Dyer RA, Farina Z, Joubert IA, Du Toit P. Crystalloid preload versus rapid crystalloid administration after induction of spinal anaesthesia (coload) for elective caesarean section. Anaesth Intensive Care. 2004;32:351–357. doi:10.1177/0310057X0403200308
14. Mercier FJ. Prevention and treatment of hypotension during spinal anesthesia for elective caesarean section: any progress in clinical practice? Ann Fr Anesth Reanim. 2011;30:622–624. doi:10.1016/j.annfar.2011.07.002
15. Feng F, Xiao F, Chen W, et al. A randomised double-blind dose-response study of weight-adjusted infusions of norepinephrine for preventing hypotension during combined spinal-epidural anaesthesia for Caesarean delivery. Br J Anaesth. 2020;124:108–114. doi:10.1016/j.bja.2019.12.019
16. Wei C, Qian J, Zhang Y, et al. Prospective, randomised, double-blind, dose-finding study of norepinephrine for preventing spinal-induced hypotension during caesarean delivery under combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2020;37:1–7. doi:10.1097/EJA.0000000000001152
17. Kaufner L, Karekla A, Henkelmann A, et al. Crystalloid coloading vs. colloid coloading in elective Caesarean section: postspinal hypotension and vasopressor consumption, a prospective, observational clinical trial. J Anesth. 2019;33:40–49. doi:10.1007/s00540-018-2581-x
18. Pouta A, Karinen J, Vuolteenaho O, Laatikainen T. Effect of intravenous fluid preload on vasoactive peptide secretion during Caesarean section under spinal anaesthesia. Anaesthesia. 1996;51:128–132. doi:10.1111/j.1365-2044.1996.tb07698.x
19. Mojica J, Melendez H, Bautista L. The timing of intravenous crystalloid administration and incidence of cardiovascular side effects during spinal anesthesia: the results from a randomized controlled trial. Anesth Analg. 2002;94:432–437. doi:10.1097/00000539-200202000-00039
20. Siddik-Sayyid SM, Nasr VG, Taha SK, et al. A randomized trial comparing colloid preload to coload during spinal anesthesia for elective cesarean delivery. Anesth Analg. 2009;109:1219–1224. doi:10.1213/ane.0b013e3181b2bd6b
21. Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92. doi:10.1111/anae.14080
22. Ali Wani S, Hussain Pandit B, Ud Din M, et al. Comparative study to evaluate the effect of colloid coloading versus crystalloid coloading for prevention of spinal anaesthesia induced hypotension and effect on fetal Apgar score in patients undergoing elective lower segment caesarean section: a prospective observational study. Int J Reprod Contracept Obstet Gynecol. 2018;7:1868–1875. doi:10.18203/2320-1770.ijrcog20181920
23. McDonald S, Fernando R, Ashpole K, et al. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective delivery: a randomized controlled trial. Anesth Analg. 2011;113:803–810.
24. Park SK, Park DN, Kim YW, et al. Colloid coload versus crystalloid coload to prevent maternal hypotension in women receiving prophylactic phenylephrine infusion during caesarean delivery: a randomised controlled trial. Int J Obstet Anesth. 2022;49:103246. doi:10.1016/j.ijoa.2021.103246
25. Lotfy ME, Moustafa AM, El Feky EM, Mowafy IA. Colloid versus crystalloid co-load with spinal anesthesia during emergent cesarean section and their effect on hemodynamic changes. J Am Sci. 2014;10:158–163.
26. Rijs K, Mercier FJ, Lucas DN, Rossaint R, Klimek M, Heesen M. Fluid loading therapy to prevent spinal hypotension in women undergoing elective caesarean section: network meta-analysis, trial sequential analysis and meta-regression. Eur J Anaesthesiol. 2020;37:1126–1142. doi:10.1097/EJA.0000000000001371
27. Chooi C, Cox JJ, Lumb RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2020;7:CD002251. doi:10.1002/14651858.CD002251.pub4
28. George RB, McKeen DM, Dominguez JE, Allen TK, Doyle PA, Habib AS. A randomized trial of phenylephrine infusion versus bolus dosing for nausea and vomiting during cesarean delivery in obese women. Can J Anaesth. 2018;65:254–262. doi:10.1007/s12630-017-1034-6
29. Ngan Kee WD. Preventing hypotension-induced nausea and vomiting during spinal anesthesia for cesarean delivery in obese parturients: a small solution for a big problem? Can J Anaesth. 2018;65:235–238. doi:10.1007/s12630-017-1035-5
30. Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S. Fluid resuscitation with 6% hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013;39:558–568. doi:10.1007/s00134-013-2840-0
31. Van Der Linden P, James M, Mythen M, Weiskopf RB. Safety of modern starches used during surgery. Anesth Analg. 2013;116:35–48. doi:10.1213/ANE.0b013e31827175da
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.