Back to Journals » Clinical Epidemiology » Volume 10
Comparative anti-fracture effectiveness of different oral anti-osteoporosis therapies based on “real-world” data: a meta-analysis of propensity-matched cohort findings from the UK Clinical Practice Research Database and the Catalan SIDIAP Database
Authors Khalid S, Calderon-Larrañaga S, Hawley S , Ali MS, Judge A , Arden N , van Staa T, Cooper C, Javaid MK, Prieto-Alhambra D
Received 30 January 2018
Accepted for publication 20 April 2018
Published 9 October 2018 Volume 2018:10 Pages 1417—1431
DOI https://doi.org/10.2147/CLEP.S164112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Henrik Sørensen
Sara Khalid,1,2 Sara Calderon-Larrañaga,3 Samuel Hawley,2 M Sanni Ali1,2,4 Andrew Judge,1,2,5 Nigel Arden,6 Tjeerd van Staa,7,8 Cyrus Cooper,2,9,10 Muhammad Kassim Javaid,2 Daniel Prieto-Alhambra2,11
1Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, 2Oxford NIHR Biomedical Research Centre, University of Oxford, Oxford, United Kingdom; 3Family and Community Medicine Teaching Unit of Granada. Cartuja University Health Centre. Andalusian Health Service (SAS), Granada, Spain; 4London School of Hygiene and Tropical Medicine, London, United Kingdom; 5Bristol NIHR Biomedical Research Centre, Musculoskeletal Research Unit, Southmead Hospital, University of Bristol, Bristol, United Kingdom; 6Arthritis Research UK Centre for Sport, Exercise, and Osteoarthritis, University of Oxford, Oxford, United Kingdom; 7Farr Institute, University of Manchester, Manchester, United Kingdom; 8Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, the Netherlands; 9Medical Research Council Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom; 10NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom; 11GREMPAL (Grup de Recerca en Malalties Prevalents de l’Aparell Locomotor) Research Group, Idiap Jordi Gol Primary Care Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain
Purpose: This paper aims to compare the clinical effectiveness of oral anti-osteoporosis drugs based on the observed risk of fracture while on treatment in primary care actual practice.
Materials and methods: We investigated two primary care records databases covering UK National Health Service (Clinical Practice Research Datalink, CPRD) and Catalan healthcare (Information System for Research in Primary Care, SIDIAP) patients during 1995–2014 and 2006–2014, respectivey. Treatment-naive incident users of anti-osteoporosis drugs were included and followed until treatment cessation, switching, death, transfer out, or study completion. We considered hip fracture while on treatment as main outcome and major osteoporotic fractures (hip, clinical spine, wrist, and proximal humerus) as secondary outcome. Users of alendronate (reference group) were compared to those of (1) OBP, (2) strontium ranelate (SR), and (3) selective estrogen receptor modulators (SERMs), after matching on baseline characteristics using propensity scores. Multiple imputation was used to handle missing data on confounders and competing risk modelling for the calculation of relative risk according to therapy. Country-specific data were analyzed separately and meta-analyzed.
Results: A total of 163,950 UK and 145,236 Catalan patients were identified. Hip (sub-hazard ratio [SHR] [95% CI] 1.04 [0.77–1.40]) and major osteoporotic (SHR [95% CI] 1 [0.78–1.27]) fracture risks were similar among OBP compared to alendronate users. Both hip (SHR [95% CI] 1.26 [1.14–1.39]) and major osteoporotic (SHR [95% CI] 1.06 [1.02–1.12]) fracture risk were higher in SR compared to alendronate users. SERM users had a reduced hip (SHR [95% CI] 0.75 [0.60–0.94]) and major osteoporotic (SHR [95% CI] 0.77 [0.72–0.83]) fracture risk compared to alendronate users.
Conclusion: We found a 26% excess hip fracture risk among SR compared to matched alendronate users, in line with placebo-controlled RCT findings. Conversely, in a lower risk population, SERM users had a 25% reduced hip fracture risk compared to alendronate users. Head-to-head RCTs are needed to confirm these findings.
Keywords: pharmaco-epidemiology, anti-osteoporosis medication, osteoporosis, fracture risk, electronic health records
Introduction
Osteoporosis is characterized by progressive loss of bone mass and increased fracture risk.1 It is an age-related process, being more frequent among postmenopausal women due to reduced estrogen levels during or after menopause.2,3 The aim of anti-osteoporosis treatment is prevention of fragility fractures, which are associated with substantial disability, mortality, and considerable socioeconomic consequences.4,5
Many randomized controlled trials (RCTs) have assessed the efficacy of different anti-osteoporosis drugs (AODs). However, the majority of studies used placebo or no treatment as a control, with the resulting gap of knowledge on the comparative effectiveness of different alternatives.6 Few studies have directly compared AODs, having performed their primary analysis on a per-protocol rather than an intention-to-treat set.7 Moreover, observational evidence comparing fracture rates among different AOD users appears to be scarce.8,9
The strict selection criteria used in most RCTs lead to significant differences between their participants and the users of drugs in the community.10,11 This is particularly relevant for certain subgroups of patients who, although at high fracture risk, are underrepresented or actively excluded in RCTs. In addition, several studies have reported suboptimal compliance and persistence with different anti-osteoporosis therapies in “real life” conditions,12–14 whereas some RCTs were designed to likely preselect high adherent patients.15 These distinctive conditions and characteristics might affect the external validity of the RCT findings, widening inconsistency between (RCT based) efficacy and “real-world” effectiveness.16
In this paper, we compare the anti-fracture effectiveness of available AODs based on the observed risk of fracture while on treatment using “real-world” data from the UK National Health Service (NHS) and Spanish healthcare records.
Materials and methods
Study design
A retrospective cohort study was conducted including all registered users of anti-osteoporosis medications. Data from two anonymized primary care outpatient records were used.
Data sources
Clinical Practice Research Datalink (CPRD)
The CPRD database contains anonymized, computerized primary care outpatient records for a representative sample of the UK population.43 In addition to comprehensive demographic information, data include medication prescriptions by general practitioners (GPs), clinical events, referrals, and hospital admissions with their major outcomes in a sample of >7 million patients.17 The CPRD is administered by the Medicines and Healthcare Products Regulatory Agency and has broad National Research Ethics Service Committee ethics approval for purely observational research using the primary care data and established data linkages. For this study, an extract from 1994–2014 was used.
SIDIAP
The Information System for Research in Primary Care (SIDIAP) database comprises primary care anonymized electronic medical records for >80% representative population of Catalonia.42 Similar to the UK NHS, the Catalan healthcare system is universal in coverage. Catalan GPs act as gatekeepers to the system and are responsible for long-term prescriptions. SIDIAP is linked to community pharmacy dispensations data and – for this specific study – hospital inpatient data as provided by the regional department of health. This study obtained approval from the SIDIAP Scientific Committee, responsible for reviewing protocols for scientific quality. We extracted data from SIDIAP participants from 2006 to 2014.
Patient level data from both CPRD and SIDIAP used for the current study are only available for researchers mentioned in both data access applications.
Variables
Study exposure was defined as the use (as defined by GP prescriptions in CPRD and dispensations in SIDIAP, considering that in both countries AODs are available only under prescription) of alendronate (reference group) compared to (1) other oral bisphosphonates (OBP), (2) strontium ranelate (SR), and (3) selective estrogen receptor modulators (SERMs). Regarding the mechanism of action of the AODs included in the study, bisphosphonates inhibit osteoclastic bone resorption (specially on surfaces undergoing active resorption),18 and appear to promote survival of osteocytes and osteoblasts as well.19 SR inhibits osteoclasts, which decreases bone resorption, while stimulating the formation of new bone tissue.20 SERMs, on the other hand, bind to estrogen receptors and inhibit bone resorption, decreasing bone turnover as assessed by biochemical markers.21 The OBP included in this study were risedronate and ibandronate, as these are the most commonly prescribed OBP in both countries. Among SERMs, raloxifene was the most widely prescribed drug in both datasets, but bazedoxifene users were also identified and included in SIDIAP. Male selective estrogen receptor modulator (SERM) users were excluded from both datasets as SERMs are only licensed for use in women. Users of denosumab and teriparatide were also excluded due to low numbers (n = 29 and n = 7, respectively) in the CPRD dataset. Drug use was identified using previously validated lists of British National Formulary codes for CPRD and Anatomic Therapeutic Chemical classification codes, as created by the World Health Organization, for SIDIAP participants.
Outcomes studied were the first occurrence of either (1) hip fracture (primary), (2) major fracture (hip, spine, wrist, and proximal humerus), and (3) all (except digits and skull/face) non-hip fractures (secondary outcomes) that were ascertained using READ/OXMIS (CPRD) and International Classification of Diseases (Hospital Episode Statistics and SIDIAP) codes.
Follow-up time was the duration between the start of treatment (first AOD prescription) and end of treatment, defined as the first occurrence of the following events: (a) a gap in prescription/dispensation of 90 days or more, (b) switching to another AOD treatment, (c) transfer out of the study or loss to follow up, (d) end of study period (2014), (e) death, or (f) fracture. In cases (a) and (b), a washout period of 180 days and an “on-prescription” period of 28 days was added to the last prescription date to account for carry over effect/s. Only naïve subjects to any available AOD/s were included and followed up during their first episode of treatment.
Confounders included in propensity score (PS) models were age, gender, body mass index (BMI), smoking, drinking, fracture/s history, co-morbidities (Charlson index), and concomitant medications with an effect on bone health or fracture risk (oral glucocorticoids, anti-coagulants, hormone replacement therapy and contraceptives, aromatase inhibitors, calcium, corticosteroids, heparin, anxiolytics, and sedatives).
Statistical methods
Multiple imputation22 was used to handle missing data within confounders, and PS matching22 was performed to minimize the effect of confounding. A Fine and Gray survival model23 was used to estimate the risk of fracture while taking into account the competing risk of death.
Missing data
Assuming that data were missing at random, a series of multiple imputations were performed using multiple imputation with chained equations methods. In brief, variables (confounders) associated with confounder/s (BMI, smoking, and drinking status) missingness and/or their values, as well as all variables in the PS/s, the study exposure, time to event, and outcome status were included in the multiple imputation models. Interactions as prespecified were included in the imputation equations. Multiple imputation by chained equations was performed using the Imputation by Chained Equations library implemented in the Stata software (version 13).
PS matching
For an intervention with control and treatment arms, PS is defined as the probability of a subject j being in a given arm of the intervention C, conditional on a set of covariates X (i.e., prespecified confounders as described above). Patients who are similar with respect to the set of covariates are hypothesized to have similar PS. PS are therefore commonly used to match “comparable” patients from control and treatment groups in a nonrandomized setting, thereby addressing the issue of confounding posed by the covariates. PS matching was performed for each drug comparison (i.e., alendronate vs OBP; alendronate vs SERMs; and alendronate vs SR users) to reduce the difference between the covariates/baseline characteristics of the control group (alendronate users) and each of the treatment groups.
First, logistic regression was performed to estimate the PS distributions for the control and treatment groups, by regressing treatment group assignment on baseline characteristics. Next, subjects from the control and treatment groups were matched according to their respective PSs. The package MatchIt was implemented in software package R (version 3.3.2) to perform PS matching. The matching algorithm used was k-nearest neighbors (kNN), and a caliper width of 0.2 (a caliper width of 0.2 of the SD of the logit of the PS is expected to minimize the mean squared error in the treatment effect estimate) of the SD of the logit of the PS22 was used to restrict the kNN to search for matches within the caliper distance. A subject in the treatment group could be matched to up to four subjects in the control group, without replacement.
Balance diagnostics
For a given variable, the standardized mean difference (SMD) in the distribution of the variable for the control and treatment groups was used to assess whether a good match had been obtained.22 Control and treatment groups were considered to be well-matched with respect to a variable if the absolute SMD was <0.1 after PS matching. Multivariable adjustment was performed for any confounders with a remaining SMD ≥ 0.1 after PS matching.
Survival analysis
Once a set of PS-matched subjects was obtained, the effect of treatment on the primary outcome (hip, major, or non-hip fracture) was estimated by directly comparing the outcomes and follow-up times in the treatment and control groups in the matched sample in a survival model. In order to estimate the relative risk (RR) of fracture in the presence of a competing risk of death, the proportional hazards regression model described by Fine and Gray23 was used (as implemented in the cpmrsk package in R) to calculate sub-hazard ratios (SHRs) for each of the outcomes accounting for a competing risk with death.24 Cumulative incidence fracture (CIF) curves stratified by drug use in the PS-matched sets were plotted to depict the observed differences in fracture risk in the different exposure groups over time.
Number needed to treat (NNT)/number needed to harm calculation/s
To determine the clinical effect size, we calculated the NNT to avoid one additional fracture at 3 and 5 years of follow-up (typical treatment duration), based on the registered fracture rates within each dataset.
Meta-analysis
Meta-analyses were performed to compare and combine the results obtained for the CPRD and SIDIAP datasets. It was implemented in Review Manager (RevMan version 5.3; Cochrane, London, UK). Country-specific data (i.e., results for the CPRD and SIDIAP datasets) were analyzed separately and pooled using fixed effects model in case of homogeneity and random effects model if a significant between-study heterogeneity was found. Heterogeneity was assessed using the I2 test statistic and the χ2 test (P < 0.01 indicated possible significance).
Sub-group analyses
In addition to the primary analysis described above, a secondary analysis was performed in order to test for interaction between the treatment and a given variable. To do so, an interaction term (treatment–variable) was included in the Fine and Gray model, in addition to the terms included above. Treatment–variable interaction was investigated for the following variables as prespecified per protocol: octogenarian (age 80 years), obesity (BMI > 30), gender, previous glucocorticoid use, and previous fracture history. Stratified analyses are reported in Table S1 and discussed where the p for interaction is borderline or significant (p < 0.1), consistent in both CPRD and SIDIAP analyses, and considered of clinical relevance.
Sensitivity analyses
To assess if there was potential unresolved confounding due to any factors that were not accounted for in the PS matching, a sensitivity analysis was performed using Rosenbaum’s boundaries testing.25 Rosenbaum’s “critical” γ inform on how imbalanced a strong unobserved confounder (with almost perfect prediction of the study outcome, i.e., fracture) needs to be between treatment/exposure groups in order to explain the observed association/s as reported after PS matching.25,26 The “critical” γ of such imbalance that would make the observed association/s no longer significant (“critical” γ as denominated by Rosenbaum) is reported for each of the significant differences seen between PS-matched treatment group/s.
Results
Study population
The cohort consisted of 163,950 and 145,236 patients included from the UK (CPRD) and Catalan (SIDIAP) populations, respectively, some of which were excluded when PS matching (Figure 1). Patients were followed-up for a median (interquartile range) of 1.45 (2.61) years and 5.34 (4.25) years in CPRD and SIDIAP, respectively. Baseline patient characteristics after matching are shown in Tables 1 and 2 for CPRD and SIDIAP participants, respectively. Baseline characteristics of alendronate and users of other drugs were similar after propensity matching, with an absolute SMD below 10% for all baseline characteristics. When comparing each matched sample to one another, SERM users appeared to be younger than alendronate, OBP, and SR users within both datasets. We also found a higher prior hip fracture rate among SR compared to the remaining AOD users within the CPRD.
Outcomes
Alendronate versus other bisphosphonate users
When analyzing the difference in fracture rate among alendronate and OBP users within the CPRD database, we identified 0.80 and 0.98 hip fractures per 100 person-years (PYs) at risk, 1.40 and 1.63 major osteoporotic fractures, and 1.19 and 1.28 non-hip fractures, respectively. Hip, major osteoporotic, and non-hip fracture rates within the SIDIAP turned out to be 0.51 and 0.43, 3.01 and 2.51, and 2.55 and 2.14 among alendronate and OBP users, respectively (Table 3).
When fracture rates in alendronate and OBP users within the CPRD dataset where compared, the estimated RRs showed an increased hip (SHR [95% CI] 1.21 [1.11, 1.32]) and major osteoporotic (SHR [95% CI] 1.13 [1.05, 1.21]) fracture risk among users of OBP, while no significant differences were found for non-hip fractures (SHR [95% CI] 1.05 [0.97, 1.13]). Among the patients in the SIDIAP identified as OBP users, there was an 11% decreased hip (SHR [95% CI] 0.89 [0.82, 0.97]), 12% lower major osteoporotic (SHR [95% CI] 0.88 [0.85, 0.91]), and 12 % reduced non-hip (SHR [95% CI] 0.88 [0.84, 0.91]) fracture risk, when compared to the matched alendronate users.
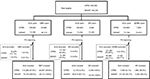
When findings from both cohorts were meta-analyzed, hip (SHR [95% CI] 1.04 [0.77, 1.40]), major osteoporotic (SHR [95% CI] 0.99 [0.76, 1.28]), and non-hip (SHR [95% CI] 0.96 [0.81, 1.14]) fracture risk appeared to be similar among OBP compared to alendronate users (Figure S1).
Alendronate versus SR users
The difference in fracture rate among alendronate and SR users within the CPRD was 1.44 and 1.92 hip fractures per 100 PYs, 2.16 and 2.77 major osteoporotic fractures, and 1.41 and 1.72 non-hip fractures, respectively. Hip, major osteoporotic, and non-hip fracture rates within the SIDIAP dataset turned out to be 0.49 and 0.59, 2.93 and 2.97, and 2.51 and 2.44 among alendronate and SR users, respectively. The probability of not having a fracture was compared between alendronate users and matched SR users as shown using a CIF (Figure 2).
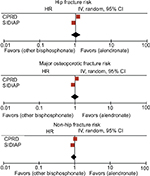
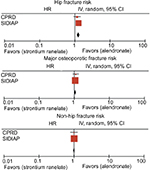
There were no statistically significant differences in fracture risk in CPRD (SHR [95% CI] 1.18 [0.94, 1.48] for hip fracture, SHR [95% CI] 1.14 [0.94, 1.39] for major osteoporotic fracture, and SHR [95% CI] 1.13 [0.89, 1.43] for non-hip fracture). However, within the SIDIAP dataset, hip and major osteoporotic fracture risk appeared to be 28% (SHR [95% CI] 1.28 [1.15, 1.42]) and 6% (SHR [95% CI] 1.06 [1.01, 1.11]) higher, respectively, among SR compared to alendronate users. Non-hip fracture risk did not show any difference between both AODs (SHR [95% CI] 1.01 [0.97, 1.07]). When findings from both cohorts were meta-analyzed, we identified a 26% (pooled SHR [95% CI] 1.26 [1.14–1.39]) and 6% (pooled SHR [95% CI] 1.06 [1.02–1.12]) higher hip and major osteoporotic fracture risk, respectively, among SR compared to alendronate users (Figure S2). We did not find any non-hip fracture risk difference between alendronate and SR users (SHR [95% CI] 1.01 [0.97, 1.05]).
Alendronate versus SERM users
All observed fracture rates turned out to be lower among SERM compared to alendronate users, within both the CPRD and SIDIAP datasets. We identified 0.31 and 0.26 hip fractures per 100 PYs at risk, 0.84 and 0.67 major osteoporotic fractures, and 0.98 and 0.74 non-hip fractures among alendronate and SERMs users registered in CPRD, respectively. In the same way, hip, major osteoporotic, and non-hip fracture rates proved to be 0.23 and 0.16, 2.32 and 1.74, and 2.11 and 1.59 within the SIDIAP database. As shown in the CIF plot in Figure 3, we compared the probability of not having a fracture between alendronate and SERM users.
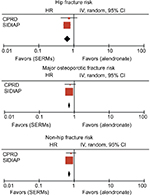
Statistically nonsignificant fracture risk differences were seen in CPRD among SERM users (SHR [95% CI] 0.86 [0.51, 1.45] for hip fracture, SHR [95% CI] 0.81 [0.57, 1.15] for major osteoporotic fracture, and SHR [95% CI] 0.76 [0.56, 1.03] for non-hip fracture). However, when assessing the fracture risk difference within the SIDIAP, we identified a 27% lower hip (SHR [95% CI] 0.73 [0.57, 0.93]), a 23% reduced major osteoporotic (SHR [95% CI] 0.77 [0.72, 0.80]), and a 23% reduction in non-hip fracture risk (SHR [95% CI] 0.77 [0.72, 0.84]) was observed among SERM compared to alendronate users.
Lastly, as shown in Figure S3, all observed fracture risks appeared to be lower among SERM compared to alendronate users after meta-analyzing data from both UK and Catalan populations. We found a 25% hip (SHR [95% CI] 0.75 [0.60, 0.94]), 23% major osteoporotic (SHR [95% CI] 0.77 [0.72, 0.83]), and 23% non-hip (SHR [95% CI] 0.77 [0.72, 0.83]) fracture risk reduction among SERM compared to alendronate users.
Although the observed ranges of RRs for SR were not significant in the UK population, the NNT to avoid an additional hip fracture over 3 to 5 years of treatment with alendronate compared to SR was calculated to be 69 to 42, respectively. For those patients included in the SIDIAP, the estimated NNT would be 333 and 200. Moreover, the NNT to avoid an additional major osteoporotic fracture at 5 years of treatment with alendronate when compared to SR within the CPRD and SIDIAP datasets was calculated to be 33 and 500, respectively.
On the other hand, the NNTs to avoid one hip, major osteoporotic, and non-hip fracture at 5 years of follow-up associated with SERM use within the CPRD were calculated to be 400, 117, and 83, respectively. For those patients included in the SIDIAP, the corresponding NNTs would be 286, 34, and 38.
Analysis of interactions
We performed tests for interaction in order to assess the association between different anti-osteoporosis medications and fracture risk in relation to patients’ age, BMI, gender, oral steroid use, and previous fracture history. Detailed findings are reported in Table S1, and most clinically relevant and consistent (in both CPRD and SIDIAP) results are summarized here.
SERMs appeared to have a significantly different anti-fracture effectiveness depending on whether a previous fracture had been reported or not. Within CPRD, we demonstrated a 37% (SHR 0.63 [95% CI 0.40–0.99]) lower major osteoporotic fracture risk among SERM users in primary prevention, that was not replicated in secondary prevention (SHR 1.33 [95% CI 0.74–2.40]). Within SIDIAP, hip and major osteoporotic fracture risk turned out to be 39% (SHR 0.61 [95% CI 0.48–0.78]) and 31% (SHR 0.69 [95% CI 0.64–0.74]) lower, respectively, among users of SERMs in primary prevention, which was again not replicated among patients in secondary fracture prevention (SHR 0.94 [95% CI 0.54–1.63] and SHR 0.85[95% CI 0.70–1.03], respectively).
Sensitivity analysis
Within the CPRD, Rosenbaum bounds analyses found a “critical” γ of 1.1 for hip and 1.0 for major osteoporotic fractures among SR versus alendronate users and 1.1 for both types of fracture among SERM versus alendronate users. Within the SIDIAP, the sensitivity analysis found a “critical” γ of 1.1 for hip and 1.2 for major osteoporotic fractures among SR versus alendronate users and 1.2 for both type of fractures among users of SERMs versus alendronate.
Discussion
We report on the anti-fracture effectiveness of different AODs while used in “real life” conditions in the UK and Catalan primary care settings. First, risk of fracture (of any location studied) while on treatment with alendronate was similar to that while on treatment with OBP. Second, anti-fracture effectiveness was better for alendronate compared to SR, with a 26% hip and 6% major fracture risk increase among users of the latter. Finally, SERMs appeared to be more effective at reducing fractures among lower risk patients, with an 25% hip and 23% major osteoporotic fracture risk reduction compared to matched alendronate users of similar characteristics.
According to most of the literature reviewed, no significant anti-fracture effectiveness difference has been reported between alendronate and OBP users, which is consistent with our results. Two meta-analysis assessing the efficacy of alendronate and risedronate (most commonly used “other bisphosphonate”) relative to placebo, resulted in an RR of vertebral fracture of 0.55 (95% CI 0.45–0.67) and 0.61 (95% CI 0.50–0.76), an RR of hip fracture of 0.60 (95% CI 0.40–0.92) and 0.74 (95% CI 0.59–0.94), and an RR for other non-vertebral fractures of 0.84 (95% CI 0.74–0.94) and 0.80 (95% CI 0.72–0.90), respectively.27,28 However, the meta-analysis carried out by Freemantle et al, comparing the anti-fracture efficacy of osteoporosis therapies, demonstrated a significant hip fracture reduction with risedronate compared to placebo (RR 0.74 [95% CI 0.59–0.94]) that was not replicated with alendronate (RR 0.65 [95% CI 0.41–1.03]).29 Our findings provide further evidence regarding the comparative anti-fracture effectiveness of OBP and demonstrate that risedronate can remain as an effective alternative when alendronate is not tolerated, as recommended in current National Institute for Health and Care Excellence guidelines.2,3
Regarding SR, hip and major osteoporotic fracture risk appeared to be increased (by 26% and 6%, respectively) among its users compared to matched participants who received alendronate. These findings are supported by the results from previous RCTs: while placebo-controlled trials failed to demonstrate hip fracture risk reduction efficacy with SR (with the exception of a post hoc subgroup analysis in high risk groups),30,31 data from the Fracture Intervention Trial (FIT) RCT found that alendronate was efficacious at reducing hip fracture risk by 53%.32 According to our data on “real life” users of these drugs in the UK NHS, one hip fracture would occur for each 42 subjects treated with SR instead of alendronate for 5 years (typical treatment duration). However, it should also be noted that, since August 2017, SR has been discontinued worldwide by manufacturers alluding to commercial reasons and it is no longer available for patients.33
On the other hand, we demonstrate that – in a lower risk population, with annual fracture rates of approximately 3/1,000 PYs at the hip and 8/1,000 PYs at “major osteoporotic” sites – a 25% RR reduction in hip and an almost 23% reduction in major osteoporotic fracture risk is seen among SERMS compared to alendronate users. Such improved anti-fracture effectiveness was only seen in primary prevention, with no significant differences in secondary prevention settings. According to some clinical specialists, the SERM raloxifene might be a beneficial option particularly for younger postmenopausal woman, due to its potential and simultaneous benefit on vertebral fracture and breast cancer prevention.2,34 Our findings suggest that in younger patients (see baseline characteristics of SERM users compared to, for example, users of other bisphosphonates) and when a previous fracture has been ruled out (primary prevention), SERMs might be preferable to alendronic acid. However, the largest trial (the “Multiple Outcomes of Raloxifene Evaluation” [MORE] study) comparing raloxifene to placebo did not demonstrate any significant hip fracture risk reduction, being just effective in vertebral fracture prevention (30% RR reduction for the 60 mg/day and 50% for the 120 mg/day group).34 This might be explained by the different risk profile (hip fracture cumulative incidence of 1.5% after 36 months in the RCT compared to 1.1% and 3.5% hip fractures in 3 years within the CPRD and SIDIAP SERM users, respectively), differences in compliance resulting in lack of anti-fracture effectiveness among bisphosphonate users, and/or limited power of the MORE trial compared to our study participants.
Strengths and limitations
Our study has some limitations. First, the observational nature of our data can be a source of potential confounding, due to the lack of randomization. Despite our attempt to minimize such confounding by indication using PS matching methods, unobserved confounders might be present, including (but not only) differences in baseline bone mineral density. To assess the potential impact of such confounding, a sensitivity analysis (Rosenbaum boundaries) was conducted, which suggested that both our findings of differential anti-fracture effectiveness of SERM and SR (compared to alendronate) were sensitive to the presence of imbalanced unobserved confounders, with an estimated γ of 1.20: this means that for any unmeasured confounder with a strong association with fracture risk (i.e., that almost perfectly predicted fracture/s), that confounder would need to be associated with a 20% increase in the probability of receiving SERM/SR (rather than alendronate) to explain the study findings. Such imbalances in the prevalence of, for instance, severe osteoporosis in users of SR or SERMs compared to alendronate are not unlikely, and thus our findings must be interpreted with caution and confirmed by further head-to-head RCTs. On the other hand, information on secondary care outpatient therapies was available in SIDIAP, but not in CPRD. This could partially explain some of the observed discrepancies in the results obtained from both databases. Differences in prescribing patterns between both countries might also be a potential source of unobserved confounding and should, accordingly, be considered. Although routinely collected data enable better knowledge of adherence and persistence to therapies, drug use within registry may not equate drug compliance. In addition, registration errors by GPs could underestimate the real fracture incidence, although large samples of data from CPRD have previously been successfully used to assess fracture risk,35,36 the diagnoses of fracture (and specially hip fracture) have been validated previously in both CPRD37 and SIDIAP,38 and the rates of fractures seen are similar to those expected from the known epidemiology.39 Finally, a proportion of the fractures seen in this study might be trauma rather than fragility related. A recent study performed in a sample of such fractures in SIDIAP has demonstrated that a high proportion (92% of hip, 88% of vertebral, and 81% of all major fractures) of fractures recorded in this database are indeed of osteoporotic nature.40
In terms of strengths, the large and representative population included in the SIDIAP and CPRD datasets are the backbone of this binational cohort and meta-analysis, which allowed us to assess the anti-fracture effectiveness of the AODs as used by potentially all NHS patients in actual practice conditions. The breadth of data available allowed us to study various potential interactions with baseline characteristics in an attempt to identify the best therapy for each patient subgroup. Moreover, we used PS adjustment to accurately estimate RRs, which is currently recognized as the best analytical approach to reduce the effects of confounding by indication.41
Conclusion
In this multi-database study, we found a 26% increased hip fracture risk among users of SR compared to matched users of alendronate, which is consistent with previous placebo-controlled RCT findings. Conversely, in a lower risk population without any previously registered fragility fracture, SERM users had a 25% reduced hip fracture risk compared to alendronate users. Head-to-head RCTs are needed to confirm these novel findings.
Acknowledgments
This work was supported by the National Institute of Health Research (NIHR) Biomedical Research Centre, Oxford.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
All authors have completed the ICMJE uniform disclosure form and declare: financial support for the submitted work from the National Osteoporosis Society (UK) DPA’s research group has received a Project Grant from the National Osteoporosis Society (United Kingdom), research grants and speaker fees from Amgen, consultancy fees and research grant from UCB; and research grants from Servier Laboratoires; DPA receives funding from the National Institute of Health Research (NIHR) in the form of a Clinician Scientist award (CS-2013-13-012); AJ has received consultancy, lecture fees and honoraria from Servier, UK Renal Registry, Oxford Craniofacial Unit, Idiap Jordi Gol, Freshfields Bruckhaus Deringer, has held advisory board positions (which involved receipt of fees) from Anthera Pharmaceuticals, Inc., and received research sponsorship from ROCHE; CC has received personal fees from Alliance for Better Bone Health, Amgen, Elli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB; all other authors have no conflicts of interest to declare. Part of the work on this paper was presented as an oral communication in Rheumatology 2017 (Birmingham, 25–27 April 2017).
References
Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–650. | ||
NICE. Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal women. London: National Institute for Health and Care Excellence (NICE). 2011. Available from: https://www.nice.org.uk/guidance/ta160. Accessed July 2017. | ||
NICE. Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. London: National Institute for Health and Care Excellence (NICE). 2011. Available from: https://www.nice.org.uk/guidance/TA161. Accessed July 2017. | ||
Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8(6):611–617. | ||
Burge RT, Worley D, Johansen A, Bhattacharya S, Bose U. The cost of osteoporotic fractures in the UK: projections for 2000–2020. J Med Econ. 2001;4(1–4):51–53. | ||
Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161(10):711–723. | ||
Nakamura T, Nakano T, Ito M, et al; MOVER Study Group. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int. 2013;93(2):137–146. | ||
Curtis JR, Westfall AO, Cheng H, Saag KG, Delzell E. RisedronatE and ALendronate Intervention over Three Years (REALITY): minimal differences in fracture risk reduction. Osteoporos Int. 2009;20(6):973–978. | ||
Cadarette SM, Katz JN, Brookhart MA, Stürmer T, Stedman MR, Solomon DH. Relative effectiveness of osteoporosis drugs for preventing nonvertebral fracture. Ann Intern Med. 2008;148(9):637–646. | ||
Reyes C, Pottegård A, Schwarz P, et al. Real-life and RCT participants: alendronate users versus FITs’ trial eligibility criterion. Calcif Tissue Int. 2016;99(3):243–249. | ||
Feldstein AC, Weycker D, Nichols GA, et al. Effectiveness of bisphosphonate therapy in a community setting. Bone. 2009;44(1):153–159. | ||
Brankin E, Walker M, Lynch N, et al. The impact of dosing frequency on compliance and persistence with bisphosphonates among postmenopausal women in the UK: evidence from three databases. Curr Med Res Opin. 2006;22(7):1249–1256. | ||
Carbonell Abella C, Guañabens Gay N, Regadera Anechina L, Marín Rives JA, Taverna Llauradó E, Ayechu Redín MP; ADHEPOR. [Analysis of therapeutic compliance in women with osteoporosis]. Reumatol Clin. 2011;7(5):299–304. Spanish [with English abstract]. | ||
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82(12):1493–1501. | ||
Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. Design of the fracture intervention trial. Osteoporos Int. 1993;3 Suppl 3: S29–S39. | ||
Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005;365(9453):82–93. | ||
Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. | ||
Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10(10):1478–1487. | ||
Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104(10):1363–1374. | ||
Marie PJ, Amman P, Boivin G, Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001;69(3):121–129. | ||
Turner CH, Sato M, Bryant HU. Raloxifene preserves bone strength and bone mass in ovariectomized rats. Endocrinology. 1994;135(5):2001–2005. | ||
Austin, PC. The performance of different propensity-score methods for estimating relative risks. J Clin Epidemiol. 2008;61(6):537–545. | ||
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. | ||
Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–387. | ||
Rosenbaum PR. Observational study. In: Everitt BS, David C, editors. Encyclopedia of Statistics in Behavioral Science. Ltd, Chichester, UK. John Wiley and Sons; 2005:1809–1814. | ||
Keele L. An overview of rbounds: an R package for Rosenbaum bounds sensitivity analysis with matched data. Columbus, OH: White Paper; 2010. | ||
Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):CD001155. | ||
Wells G, Cranney A, Peterson J, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):CD004523. | ||
Freemantle N, Cooper C, Diez-Perez A, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int. 2013;24(1):209–217. | ||
Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350(5):459–468. | ||
Reginster JY, Seeman E, De Vernejoul MC. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90(5):2816–2822. | ||
Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118–4124. | ||
Strontium ranelate discontinued. Drug Ther Bull. 2017;55(8):93–94 . | ||
Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–645. | ||
Prieto-Alhambra D, Javaid MK, Judge A, et al. Fracture risk before and after total hip replacement in patients with osteoarthritis: potential benefits of bisphosphonate use. Arthritis Rheum. 2011;63(4):992–1001. | ||
Prieto-Alhambra D, Javaid MK, Judge A, et al. Bisphosphonate use and risk of post-operative fracture among patients undergoing a total knee replacement for knee osteoarthritis: a propensity score analysis. Osteoporos Int. 2011;22(5):1555–1571. | ||
Van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf. 2000;9(5):359–366. | ||
Pagès-Castellà A, Carbonell-Abella C, Avilés FF, et al. Burden of osteoporotic fractures in primary health care in Catalonia (Spain): a population-based study. BMC Musculoskelet Disord. 2012;13(13):79–85. | ||
Curtis EM, Van Der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988-2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. | ||
Martinez-Laguna D, Soria-Castro A, Carbonell-Abella C, et al. Validation of fragility fractures in primary care electronic medical records: a population-based study. Reumatol Clin. Epub 2017 Nov 28. | ||
Normand SL, Sykora K, Li P, Mamdani M, Rochon PA, Anderson GM. Readers guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. BMJ. 2005;330(7498):1021–1023. | ||
Information System for Research in Primary Care Database [www.sidiap.org]. Barcelona: Catalan Health Institute; 2014. Available from: http://www.sidiap.org. | ||
Clinical Practice Research Datalink [www.cprd.com]. London: The Medicines and Healthcare products Regulatory Agency; 2018. Available from: http://www.cprd.com. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.