Back to Journals » Infection and Drug Resistance » Volume 16
Comparative Analysis on Clinical Characteristics Among Patients with Acute Hepatitis A Virus (HAV) and Patients with Acute Hepatitis E Virus (HEV): A Single-Center Retrospective Study from Bulgaria
Authors Baymakova M, Kunchev M , Mihaylova-Garnizova R, Zasheva A, Plochev K, Kundurzhiev T, Tsachev I
Received 8 March 2023
Accepted for publication 9 May 2023
Published 29 May 2023 Volume 2023:16 Pages 3349—3366
DOI https://doi.org/10.2147/IDR.S411606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Magdalena Baymakova,1 Metodi Kunchev,2 Raynichka Mihaylova-Garnizova,1 Anelia Zasheva,1 Kamen Plochev,1 Todor Kundurzhiev,3 Ilia Tsachev4
1Department of Infectious Diseases, Military Medical Academy, Sofia, Bulgaria; 2Department of Virology, Military Medical Academy, Sofia, Bulgaria; 3Department of Occupational Medicine, Faculty of Public Health, Medical University, Sofia, Bulgaria; 4Department of Microbiology, Infectious and Parasitic Diseases, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria
Correspondence: Magdalena Baymakova, Department of Infectious Diseases, Military Medical Academy, Sofia, Bulgaria, Tel +359-882-28-50-87, Email [email protected]
Introduction: The acute viral hepatitis was one of the most common conditions in daily clinical practice varying in different parts of the world. The aim of the present study was to perform a comparative analysis on clinical characteristics among patients with acute hepatitis A virus (HAV) infection and patients with acute hepatitis E virus (HEV) infection admitted to the Military Medical Academy (MMA), Sofia, Bulgaria.
Methods: A retrospective study was performed at MMA, between 1 January 2016 and 31 December 2021. The etiological diagnosis was confirmed by enzyme-linked immunosorbent assay (ELISA) HAV/HEV IgM serology assays.
Results: The current survey included 231 patients with mean age 45.11 ± 16.08 years (95% confidence interval: 43.04– 47.19). According to the case definition, inclusion and exclusion criteria, persons were divided into two groups: patients with acute HAV infection (68.4%; 158/231) and patients with acute HEV infection (31.6%; 73/231). Males with HEV had 3.091 times the odds of comorbidity “hypertension” than males with HAV (p = 0.032). There were almost equal odds of increased ALT (odds ratio = 0.999; p = 0.003) in men with HEV and men with HAV. Females with HEV had 5.161 times the odds of comorbidity “hypertension” compared with females with HAV (p = 0.049). We found almost equal odds for elevated ALT in women with HEV and women with HAV (OR = 0.999; p = 0.025). In the non-elderly group (< 60-year-old), HEV individuals had 4.544 and 10.560 times the odds of comorbidities “hypertension” and “cardiovascular diseases” compared with HAV patients (p < 0.05). We found almost equal odds for elevated ALT in HEV patients and HAV participants (OR = 0.998; p = 0.002).
Conclusion: The results from the current study may support the physicians daily care for patients with acute HAV and acute HEV.
Keywords: Bulgaria, clinical characteristics, hepatitis A virus, HAV, hepatitis E virus, HEV, single-center study
Introduction
The hepatitis A virus (HAV) was characterized in 1973 by scientific group with team leader Stephen M. Feinstone.1 HAV has a single-stranded positive-sense RNA genome, and it is part of Hepatovirus genus, within the Picornaviridae family.2 According to the World Health Organization (WHO) database in 2016, 7134 individuals died from HAV (this is approximately 0.5% of the mortality due to viral hepatitis).3 The virus spreads through contaminated water and food, poor sanitary conditions and living environment, worsened personal hygiene and practice of anal/oral sex.4,5 Most patients with HAV infection have no or few symptoms with mild clinical form mainly in children compared to severe clinical forms much more common in adults/elderly.6–11 Unvaccinated adults are at risk of moderate, severe, or fulminant clinical form of HAV.12,13 From an-other point of view, in developed countries the incidence of HAV is low.14 Therefore, in these countries, due to the low circulation of HAV, part of the elderly population may have never encountered HAV or have not been vaccinated and therefore be at increased risk. In this regard, vaccination against HAV is very important because it protects against illness, complications and fatal outcome. HAV does not cause chronic infection in comparison with the other viral hepatitis.15
To date in international peer-reviewed journals, there are few articles on HAV infection in Bulgaria. Dimitrova et al reported that the inclusion of the HAV vaccine in the Bulgarian National Vaccination Calendar is recommended for certain risk groups and may help to reduce the circulation of the virus in the Bulgarian population.16 In 2017, a joint Italian-Bulgarian scientific team presented the first molecular analysis of HAV among 105 patients from Bulgaria.17 These authors reported that phylogenetic analysis revealed two main sequence groups corresponding to the HAV-IA sub-genotype (74%; 78/105) and HAV-IB sub-genotype (26%; 27/105).17 In another article, Toseva et al presented data for the overall 52.7% HAV seropositivity among 110 workers in three wastewater treatment plants (WWTPs) in Bulgaria.18 In 2018, Cella et al analyzed the genetic diversity of HAV-IA and HAV-IB sub-genotypes in Bulgaria.19 The last published Bulgarian study on HAV recommended the performance of an abdominal ultrasound with high attention to the gallbladder wall in case of acute HAV infection among children.20 According to the annual epidemiological reports of the European Centre for Disease Prevention and Control (ECDC) in the last years (2011–2021), the frequency of HAV infection in Bulgaria varies widely.21 For this period Bulgaria's mean notification rate was 28.95 cases per 100,000 population, compared to European Union/European Economic Area (EU/EEA) countries’ mean notification rate of 2.52 cases per 100,000 population (Figure 1).21
 |
Figure 1 Dynamics of HAV notification rate per 100,000 population in Bulgaria (in red) and EU/EEA (in blue), 2011–2021. |
The hepatitis Е virus (HЕV) was characterized in 1983 by scientific group with team leader Mikhail S. Balayan.22 These scientists established the fecal-oral route of spread of this new liver disease. HEV is a single-stranded, positive-sense RNA virus in the Hepeviridae family.23 Human HEV belongs to Paslahepevirus balayani species, Paslahepevirus genus.23,24 According to the WHO database, every year, 20 million human HEV cases are estimated worldwide, including approximately 3.3 million people with mild, moderate or severe clinical forms of HEV, ie, people with clinical signs and symptoms.25 Furthermore, WHO estimates that this virus caused 44,000 fatal cases in 2015 (accounting for 3.3% of the mortality due to viral hepatitis).25 In developing countries HEV genotype (gt) 1 and HEV gt 2 spread via the fecal-oral route (principally via contaminated water and water sources).26 In developed countries, HEV gt 3 and HEV gt 4 have zoonotic transmission and spread mainly through the consumption of under-cooked animal meat (including pork meat, game meat, pig liver, pork sausages, pates, salami, dry sausages, and others).27,28 HEV gt 7 (camels and humans) and rat HEV-C1 (rats and humans) are zoonoses.29–32 To date, HEV gt 5, HEV gt 6 and HEV gt 8 have been only identified in animals.33,34 Usually HEV infection affects the adults (over 50 years-old).35,36 In these individuals, the infection can proceed with mild, moderate or severe clinical form. In developing countries, pregnant women are at increased risk (the HEV disease incidence rate was 8-times higher and acute liver failure occurred 13-times more often among pregnant women in comparison to non-pregnant women).37 In developed countries, immunosuppressed and immunocompromised persons are mainly at increased risk (patients with solid organ transplantation; hematologic malignancy patients; HIV-positive individuals).38–41 Chronic HEV infection has been established in immunosuppressed and immunocompromised individuals.38–41 A recombinant hepatitis E vaccine has been developed and applied in China.42
In the last years, some important studies on HEV in humans and animals were conducted in Bulgaria and were published in international peer-reviewed journals. In 2014, Teoharov et al reported an overall 9.04% (67/741; 95% CI: 6.9–11.1%) HEV IgG seroprevalence in general population from Plovdiv district, Bulgaria.43 In 2016, Baymakova et al presented a case series of 20 patients with acute HEV infection from Sofia, Bulgaria.44 A scientific team of Italian and Bulgarian researchers conducted the first molecular studies of human HEV in Bulgaria.45,46 In 2021, Baymakova et al reported high HEV seropositivity in Bulgarian blood donors – 25.9% (144/555); general hunters – 48.7% (19/39); and hunters of wild boars – 51.6% (16/31).47 During the last year, Golkocheva-Markova et al reported 10.9% (34/312) positive HEV antibodies in 312 serum samples on HIV-infected individuals.48 It is difficult to determine the frequency of acute HEV infection in Bulgaria. On the one hand, ECDC does not prepare HEV annual epidemiological reports. On the other hand, Bulgarian health authorities did not prepare annual epidemiological reports on HEV. National HEV surveillance has been started since 2019. The National Center of Infectious and Parasitic Diseases (NCIPD), Sofia, Bulgaria reported 217 cases of acute HEV in 2019; in 2020–88 cases; in 2021–46 cases; the mean incidence rate was 1.68 per 100,000 population.49
The present study aimed to do a comparative analysis on clinical characteristics among patients with acute HAV infection and patients with acute HEV infection from one hospital in Sofia, Bulgaria. To the best of our knowledge, this is the first research that makes a detailed and in-depth comparative analysis (univariate analysis and multivariable analysis) of the clinical characteristics of HAV and HEV infections in both sexes (males and females) and in two important age groups (adults under 60-years-old and adults over 60-years-old).
Materials and Methods
Study Design, Participants and Data Collection
A retrospective survey was conducted between 1 January 2016 and 31 December 2021 at Military Medical Academy (MMA), Sofia, Bulgaria. To date, the MMA includes five multidisciplinary hospitals for active treatment and two hospitals for long-term treatment and rehabilitation. The present study was conducted at the Department of Infectious Diseases in multidisciplinary hospital for active treatment in Sofia, part of MMA. This hospital has over 700 beds, and it is the Bulgarian reference center for liver transplants (more than 70 liver transplants have been performed to date).
Upon admission to the Department of Infectious Diseases, all patients with acute HAV and acute HEV infections were examined by a clinical team including a physician (infectious disease specialist) and nurse. The physician performed the following activities: medical history (personal and family); physical examination; laboratory tests (according to evidence-based medicine for viral hepatitis); and imaging and invasive investigations (according to evidence-based medicine for viral hepatitis). The nurse assisted the physician with the administrative activities of filling out the patient’s electronic medical record and settling in the hospital room.
Participants in this study were selected based on medical history, laboratory tests, serological assays, inclusion and exclusion criteria (Figure 2). The necessary information for the survey was extracted from the electronic medical records of patients. For demographics and baseline characteristics, data were collected for sex (male, female); age; length of hospital stay; type of patients (civilians, soldiers); exposure history (travel abroad, contact with ill persons, contact with animals); comorbidities (hypertension, diabetes mellitus, cardiovascular disease, etc.). Clinical signs and symptoms were recorded on the day of admission and included data on dark urine; fatigue; abdominal pain; jaundice; nausea/vomiting; pale stools; and others. The laboratory parameters were extracted from patient records at admission and discharge, and included the following laboratory tests: aspartate aminotransferase (AST); alanine aminotransferase (ALT); gamma-glutamyl transferase (GGT); alkaline phosphatase (AP); bilirubin (total, direct); protein (total); albumin; fibrinogen; international normalized ratio (INR); white blood cells (WBC); thrombocytes; and C-reactive protein (CRP).
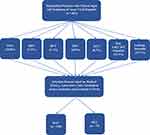 |
Figure 2 Flow chart showing the selection process of research participants. |
Case Definition
Acute HAV infection was defined by the presence of clinical signs and symptoms for viral hepatitis, AST/ALT elevation, and positive anti-HAV IgM serology.
Acute HEV infection was defined by the presence of ALT/AST elevation with clinical signs and symptoms for viral hepatitis, and positive results from HEV IgM serology assay.
Inclusion and Exclusion Criteria
The inclusion criteria for the current research: (a) individuals older than 18 years; (b) adults sought primary or secondary medical care in hospital; (c) persons with clinical signs and symptoms for viral hepatitis established by a physician (infectious disease specialist); (d) individuals with elevated liver enzymes; (e) department of virology which has confirmed HAV and/or HEV infections (HAV IgM and/or HEV IgM antibody testing); (f) obtained written informed consent from each patient to participate in a scientific survey.
The exclusion criteria for the present study include: (a) individuals younger than 18 years; (b) persons without clinical signs and symptoms for acute viral hepatitis; (c) individuals with liver enzymes in reference range; (d) negative result for HAV or HEV infection; (e) patients with solid organ transplantation; (f) pregnant women; (g) individuals with persistence of HEV IgM antibodies ≥6 months.
HAV and HEV Serological Tests
Serological diagnosis and analysis were performed in the Department of Virology, MMA, Sofia, Bulgaria, by a highly qualified team led by a clinical virology specialist (physician). From each patient, 5 mL of serum was taken, after that HAV IgM ELISA test and HEV IgM ELISA test according to the instructions of the manufacturer were conducted at the virology department. The collection, transportation and storage of all serum samples were done according to the regulatory requirements of the hospital and the country.
For diagnosis of acute HAV infection, we used the HAVIgM enzyme-linked immunosorbent assay (ELISA) on Dia.Pro (Milan, Italy). The test was used for the determination of IgM class antibodies to HAV in human plasma or sera with the “capture” system. The assay was based on the principle of “IgM capture” where IgM antibodies were first captured by the solid phase coated with anti-IgM antibody. The HАV IgM ELISA test (Dia.Pro, Milan, Italy) has a 100% sensitivity and >98% specificity.
The diagnosis of HEV infection was performed with HEV IgM ELISA test (Dia.Pro, Milan, Italy).50,51 The test was used for the determination of IgM antibodies to HEV in human plasma or sera. The system “capture” and the principle of “IgM capture” was performed as in the diagnostic process of HAV. The HEV IgM ELISA test (Dia.Pro, Milan, Italy) has a 100% sensitivity and ≥95% specificity.
Ethical Considerations
All patients signed a written informed consent to participate in scientific research upon admission to the hospital. All individuals gave written informed consent for the imaging and invasive procedures conducted on them. Every patient had access to their medical records during the hospital stay and after discharge. The physicians and scientists participating in this research conducted their activities according to the ethical principles of Declaration of Helsinki (adopted in June 1964, last revision in October 2013). The current survey was approved by the Local Ethics Committee of MMA, 1606 Sofia, Bulgaria, who confirmed that the research was in full accordance with all ethical principles and practices.
Statistical Analysis
Statistical analysis was conducted and verified by medical statistics specialist (full professor). Data analysis was performed with the help of SPSS Statistics 21.0 (IBM Corp., Armonk, NY, USA) and Excel 2007 (Microsoft, Redmond, WA, USA). Data were entered and arranged in MS Excel. The distribution of continuous variables was tested using the Kolmogorov–Smirnov test. Normally distributed data were presented as mean ± standard deviation (SD), whereas non-normally distributed data – as median and interquartile range (IQR). Categorical variables were presented in percentage terms. All clinical characteristics obtained from the electronic medical records were compared by Chi-square test, Fisher’s exact test, Mann–Whitney test, or t-test. Univariate logistic regression was used to assess the risk regarding the type of hepatitis (HAV or HEV) and different indicators. Multivariate logistic regression (enter method) was applied to assess the independent influence of some parameters on the type of hepatitis (HAV or HEV). A p-value <0.05 was considered statistically significant. The final results obtained from the statistical analysis were checked by the other scientists involved in this study.
Results
The current survey included 231 patients with mean age 45.11 ± 16.08 years (95% CI: 43.04–47.19); male – 46.15 ± 16.22 years (95% CI: 43.42–48.88); and female – 43.63 ± 15.83 years (95% CI: 40.44–46.81). The male sex dominated among analyzed individuals (sex ratio: male/female = 1/0.69). According to the case definition, inclusion and exclusion criteria, persons were divided into two groups: patients with acute HAV infection (68.4%; 158/231) and patients with acute HEV infection (31.6%; 73/231). The male sex dominated in persons with HAV (male vs female = 54.4% vs 45.6%) and individuals with HEV (male vs female = 68.5% vs 31.5%) (Table 1). HAV infection had affected younger people compared to HEV infection (HAV vs HEV = 40.7 years vs 54.4 years; p < 0.001). Participants over 50-years-old with HAV were 27.2% (43/158) and those with HEV – 67.2% (49/73). HAV and HEV infections varied during different months of the year (Figure 3). The seasonal distribution of HAV infection compared to HEV infection was as follows: spring – 15.2% vs 31.5% (24/158 vs 23/73); summer – 22.1% vs 17.8% (35/158 vs 13/73); autumn – 39.9% vs 20.6% (63/158 vs 15/73); and winter – 22.8% vs 30.1% (36/158 vs 22/73). In the group of HEV patients, there were more comorbidities than in the group of HAV patients: hypertension (43.8%/11.4%; p < 0.001), diabetes mellitus (19.2%/5.7%; p = 0.003), cardiovascular diseases (19.2%/5.1%; p = 0.002), and others. The first three most common clinical signs and symptoms in individuals with HAV were dark urine (89.2%), fatigue (77.8%), and abdominal pain (67.7%). The first three most common clinical signs and symptoms in participants with HEV were fatigue (93.2%), dark urine (83.6%), and abdominal pain (72.6%). At admission, AST/ALT was 2.3/3.3 times higher in individuals with HAV compared to patients with HEV (p < 0.001); total bilirubin/direct bilirubin was 1.4/1.7 times higher in HAV vs HEV (p = 0.012). We found the following clinical outcome: discharged with recommendation for outpatient control – HAV/HEV = 157/68 patients; transferred to other hospital unit – HAV/HEV = 1/4; lethal outcome – HAV/HEV = 0/1 (acute liver failure).
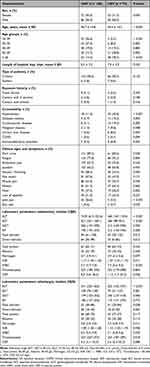 |
Table 1 Clinical Characteristics of Bulgarian Patients with Acute Hepatitis A Virus (HAV) Infection and Acute Hepatitis E Virus (HEV) Infection |
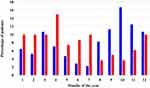 |
Figure 3 The monthly incidence rates of acute HAV (in blue) and acute HEV (in red). |
In our analysis, participants (n = 231) were divided into two groups by sex: male (n = 136) and female (n = 95) (Table 2). By the conducted univariate logistic regression, we found for the male sex the following: HEV patients had 5.523 times the odds of comorbidity “hypertension” compared with HAV individuals (p < 0.001); HEV participants had 3.761 times the odds of comorbidity “diabetes mellitus” than HAV patients (p = 0.015); and HEV individuals had 4.211 times the odds of comorbidity “cardiovascular diseases” compared with HAV participants (p = 0.007). Furthermore, the clinical sign “fever” was more common in male with HAV than in male with HEV (p = 0.002; OR = 0.274). In addition, upon admission HEV patients had 1.002/1.010 times the odds of elevated AST/ALT than HAV individuals (p ≤ 0.002).
 |
Table 2 Univariate Logistic Regression Showing the Association Between the Type of Viral Hepatitis (HAV and HEV) and Various Parameters Among Male and Female |
Regarding sex affiliation, a multivariable logistic regression was conducted to assess the relationship between the type of viral hepatitis and some parameters among male and female (Table 3). Males with HEV had 3.091 times the odds of comorbidity “hypertension” than males with HAV (p = 0.032). Furthermore, there was almost equal odds of increased AST (OR = 1.000) and ALT (OR = 0.999) in men with HEV and men with HAV. Females with HEV had 5.161 times the odds of comorbidity “hypertension” compared with females with HAV (p = 0.049).
 |
Table 3 Multivariable Logistic Regression Showing the Relationship Between the Type of Viral Hepatitis and Some Parameters Among Male and Female |
The second main distribution of the study participants was into two groups, according to their age: non-elderly (<60-year-old; n = 180) and elderly (≥60-year-old; n = 51) (Table 4). By the univariate logistic regression, we found for the non-elderly group the following: HEV participants had 5.687 times the odds of comorbidity “hypertension” than HAV individuals (p < 0.001); HEV patients had 4.094 times the odds of comorbidities “diabetes mellitus” and “cardiovascular diseases” compared with HAV participants (p = 0.042). The clinical sign “malaise” was more common in non-elderly with HEV compared with non-elderly with HAV (p = 0.040; OR = 2.118), while “fever” was more common in HAV individuals than in HEV patients (p = 0.049; OR = 0.493). Upon admission, HEV participants had 1.002 times the odds of elevated AST/ALT compared with HAV individuals (p ≤ 0.001); while elevated total bilirubin and direct bilirubin were more common in HAV patients than in HEV participants (p ≤ 0.005). By the univariate logistic regression, for the elderly group the following was established: HEV individuals had 3.284 times the odds of comorbidity “hypertension” than HAV patients (p = 0.043). The clinical sign “fatigue” was more common in HEV participants compared with HAV individuals (p = 0.016; OR = 14.400), while “pale stools” was more common in HAV patients than in HEV participants (p = 0.042; OR = 0.278).
 |
Table 4 Univariate Logistic Regression Showing the Association Between the Type of Viral Hepatitis (HAV and HEV) and Various Parameters Among Age Groups (Non-Elderly and Elderly) |
Regarding age affiliation, a multivariable logistic regression was conducted to assess the relationship between the type of viral hepatitis and some parameters in age groups (Table 5). In the non-elderly group (<60-year-old), HEV individuals had 4.544 times the odds of comorbidity “hypertension” compared with HAV patients (p = 0.011). Also, HEV participants had 10.560 times the odds of comorbidity “cardiovascular diseases” than HAV individuals (p = 0.010). In the elderly group (≥60-year-old), HEV participants had 12.592 times the odds of fatigue compared with HAV individuals (p = 0.029).
 |
Table 5 Multivariable Logistic Regression Showing the Relationship Between the Type of Viral Hepatitis and Some Parameters Among Age Groups (Non-Elderly and Elderly) |
Discussion
Acute viral hepatitis is one of the most common infectious illness. Infectious disease units in hospitals provide care for patients with this condition. In this regard, various authors share their experiences with these two infections. HAV infection affects younger individuals compared to HEV infection. Chau et al reported a median HAV age of 27 (12–45) years and median HEV age of 53 (29–73) (p < 0.01).52 Korean authors presented in their paper a mean HAV age of 28 (22–34) years and mean HEV age of 49 (41–60) years (p < 0.001).53 Suzuki et al reported a mean HEV age of 57.3 (18–83) years and mean HAV age of 45.2 (22–69) years (p = 0.032).54 Researchers from Romania presented found that the mean age of HAV participants was 13.3 ± 8.5 years compared with the mean age of HEV persons of 54.3 ± 3.0 years.55 In several other studies, the reported mean age of HAV patients was lower – 38.0 ± 14.3/29.0 /33.3 ± 7.7 years,56–58 than the mean age of HEV individuals – 42.5 (28.0–66.0)/46.0 (20.0–84.0)/57.0 (14.0–86.0) years.59–61 Regarding our study, the estimated mean ages (HAV patients = 40.7 ± 14.8 years and HEV patients = 54.4 ± 14.5 years; p < 0.001) were similar to the results from studies conducted in Akita, Aomori, Iwate, Miyagi prefectures, Japan,54 Ogaki city, Japan,56 and Brno city, Czech Republic.61
The review of the scientific literature showed that men may get both infections (HAV and HEV) more often than women. Romanian scientists presented a survey in which male were 100% affected by HEV and 53.9% by HAV.55 Japanese authors reported in their manuscript that sex distribution male:female was 80:20% for HAV and 78.2:21.8% for HEV.54 A scientific team from Hong Kong, China, found 25% females with HEV infection vs 75% males with HEV and 34.3% females with HAV infection vs 65.7% males with HAV in their study.52 Sridhar et al reported that 75% of participants with positive HEV-IgM ELISA were men and only 25% were women.59 Oh et al found that 69.6% of patients with HEV were males and 67.3% of persons with HAV were men.53 Regarding the sex distribution among patients with HAV infection in present study, our results (males = 54.4%, 86/158; females = 45.6%, 72/158) were similar to data obtained from a study conducted in Iasi, Romania.55 As far as HEV gender distribution in the current survey, our data (males = 68.5%, 50/73; females = 31.5%, 23/73) were similar to results established in a study conducted in Zhejiang province, China.60
The seasonal and monthly distribution of both infections (HAV and HEV) varies in different regions of the world and in different studies. Our study was conducted among patients living in the temperate climate zone. To date, two studies of clinical characteristics among individuals with HAV and/or HEV living in climatic conditions similar to those of our research have been published by other authors.54,58 Suzuki et al and Kwon et al carried out their researches in Japan and South Korea, respectively.54,58 Geographically, these two countries are located on the border between the temperate climate zone and the subtropical climate zone. Therefore, we could potentially and provisionally assume that the studies of Suzuki et al and Kwon et al were conducted in climatic conditions similar to ours. Suzuki et al from Iwate and three neighboring prefectures in Japan reported for an equal distribution of HAV and HEV cases during the warm months (April–September) and cold months (October–March) of a year.54 Kwon et al noted the peak of HAV cases in the period of April to August in their nationwide multicenter survey of 4218 participants.58 The results of our survey showed that the majority of HAV and HEV cases are observed in the cold months (October–March) of the year. The reasons for the varieties between our results and those reported by other authors may be different. First, the respective study designs varied. Second, different hygiene and eating habits exist in different parts of the world. Third, the serological assay may influence the data obtained. Fourth, the vaccination policy against HAV differs in different countries around the world. Fifth, different tourist preferences and period of visit to high-risk or low-risk areas for contracting HAV or HEV infection. Due to the diversity of all these reasons, it is very hard to determine the risk factors for seasonal and monthly distribution of HAV and HEV infections.
The different scientists reported different leading clinical signs and symptoms among patients with acute HAV or acute HEV. A Romanian study on clinical characteristics of HAV and HEV presented different clinical symptoms.55 Mihai et al noted that HAV participants had clinical symptoms only (n = 269).55 HEV participants were only three and therefore no clinical signs were announced.55 Consequently, we could hardly make an adequate comparison of our results and those from Romanian study. Mihalcin et al from The Czech Republic reported the main clinical symptoms in 173 patients with acute HEV infection: jaundice, myalgia, arthralgia, low-grade fever, and abdominal pain.61 Our results were similar to those found in the scientific literature. We found that statistically significant clinical signs and symptoms in our patients with acute HAV and acute HEV were fatigue (p = 0.004), fever (p = 0.002), and joint pain (p < 0.001).
Researchers from different parts of the world reported elevation of some laboratory characteristics in acute HAV and acute HEV infections. Chau et al found that few laboratory parameters were statistically significant in HAV patients compared with HEV patients: bilirubin – 79 (6–469) µmol/L vs 153 (33–680) µmol/L, p < 0.01; prothrombin time – 10 (10–18) sec vs 13 (10–26) sec, p < 0.01; prolonged prothrombin time – 8 (8)% vs 7 (30)%, p = 0.01; and hypoalbuminemia – 3 (3)% vs 4 (17)%, p = 0.02.52 Scientists from Gyeongsang province, South Korea presented that important laboratory factors for acute HAV and acute HEV infection were AST – 1337 (674–2578) U/L vs 292 (103–852) U/L, p < 0.001, and ALT – 2357 (1156–2603) U/L vs 525 (109–1062) U/L, p < 0.001.53 Suzuki et al reported high levels of liver indicators among individuals with acute HAV (AST – 2459 U/L; ALT – 3081 U/L; total bilirubin – 44 µmol/L) and those with acute HEV (AST – 1267 U/L; ALT – 1483 U/L; total bilirubin – 38 µmol/L).54 Authors from Zhejiang province, China conducted a retrospective survey among 78 patients with acute HEV infection.60 These scientists divided the participants into two groups: ≥60-year-old (elderly) and <60-year-old (non-elderly).60 The authors found the following statistically significant results when comparing the laboratory parameters between the two groups (elderly vs non-elderly): ALT – 1084 ± 1100 U/L vs 2493 ± 1664 U/L (p = 0.008); total bilirubin – 256 ± 162 µmol/L vs 73 ± 70 µmol/L (p < 0.001); and direct bilirubin – 170 ± 117 µmol/L vs 41 ± 38 µmol/L (p = 0.001).60
Our results were similar to the results of some authors, but also differed from other studies on this topic. The analysis of the literature on the subject (HAV and HEV infections) found both common characteristics and differences. For example, all surveys established that males were more commonly affected than females. In addition, the mean age of individuals with acute HAV was lower than the mean age of persons with acute HEV (most often the difference ranged from 10–15–20 years).52–55 Consequently, people in childhood or young age more frequently encountered HAV. Then clinical symptoms were noted, and mild, moderate or severe clinical forms of the illness were developed. Also, the improvement of hygiene and living conditions were a potential way, which leading to an increase in age getting HAV. But the older age was a risk factor for a severe clinical form of acute HAV infection. Furthermore, a serious risk factor for the spread of HAV infection is contaminated food. For example, Tominaga et al reported on HAV outbreak associated with a revolving sushi bar occurred in Chiba, Japan.62 In this regard, contaminated food, poor sanitary conditions and worsened personal hygiene can be important risk factors for the spread of HAV infection. An advantage of HAV infection is that the severe complications and liver failure are rare compared to other viral liver infections.
HEV infections are more frequently presented with clinical signs and symptoms in individuals over 50 years of age.35,63,64 The consumption of under-cooked animal meat (incl. pork meat, pig liver, etc.) was the route of HEV transmission, therefore we could reasonably conclude that all age groups were at risk of HEV infection. However, individuals younger than 50 years of age were unlikely to present a clinical form of HEV compared with individuals over 50 years of age. One of the potential reasons for this could be that the elderly had more common comorbidities (hypertension, diabetes mellitus, etc.) and this was a prerequisite for a more severe course of the infection. Another potential reason was the better immune system response in younger individuals compared with older individuals. So, it could be a possible reason for asymptomatic or mild clinical form of HEV in young individuals. These are scientific hypotheses that could be confirmed or rejected in future research on this topic.
The current survey has some limitations that need to be addressed. First, this is a retrospective study; therefore, data were obtained from electronic medical records, ie, additional clarifying information cannot be implemented. Second, we present a single-center experience. In this regard, it would be better to realize a multicenter study, which would reduce the possibility of omissions and errors. Third, the research involved a small number of participants. This limitation cannot be avoided because the topic of the study is acute HAV and acute HEV, ie, the participants were individuals with a clinical form (disease) of these two infections. Despite these limitations, this research has its merits. This is the first survey that makes a detailed and in-depth comparative analysis (univariate analysis and multivariable analysis) of the clinical characteristics of HAV and HEV infections in both sexes (males and females) and in two important age groups (non-elderly and elderly). To date, all studies on the topic have only made a general comparison between the two infections (HAV and HEV).52–55 Therefore, our results add to the knowledge of the clinical characteristics in patients with acute HAV and acute HEV.
Conclusion
Acute viral hepatitis may cause a severe clinical form of illness, serious complications and a fatal outcome. In this regard, the analysis of clinical characteristics among patients with acute HAV and acute HEV has important implications for daily clinical practice. It could optimize medical care for these patients. By our results, the comorbidity “hypertension” and ALT were important when comparing males and females with HAV and HEV infections. We found statistically significant results for comorbidities (hypertension and cardiovascular disease) and ALT in non-elderly patients (<60-year-old) comparing HAV and HEV. In the elderly group (≥60-year-old), fatigue was a significant parameter for patients with HAV infection compared with HEV infection. Furthermore, another important conclusion – HEV was associated more frequently with comorbidities. So, the results from the current study may support the physicians daily care for patients with acute HAV and acute HEV. Although these infections rarely lead to a severe clinical form, they continue to have an important place in infectious disease specialists’ work. Therefore, improving knowledge about acute viral hepatitis is a good prerequisite for improving medical care for these patients.
Ethical Approval
This study was conducted in accordance with the Military Medical Academy, Sofia, Bulgaria research ethical guidelines and according to ethical principles included in the Declaration of Helsinki (adopted in June 1964, last revision in October 2013). Ethical approval for this survey was obtained from the Local Ethics Committee of Military Medical Academy, 1606 Sofia, Bulgaria (MMA, 02/2022).
Acknowledgments
We are grateful to all physicians, nurses, laboratory staff, and patients who participated in the study. We thank the staff of the Military Medical Academy, Sofia, Bulgaria, for the medical care and treatment of the patients. In addition, we thank our families for providing us with the time and support needed to write this survey in a timely manner.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Article Publishing Charge (APC) was funded by a scientific project “Development of Research and Innovation at Trakia University in Service of Health and Sustainable Well-Being”, Funding organization – Bulgarian Ministry of Education and Science (Grant number: BG-RRP-2.004-0006). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Disclosure
The authors declare no conflicts of interest.
References
1. Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a virus like antigen associated with acute illness. Science. 1973;182:1026–1028. doi:10.1126/science.182.4116.1026
2. International Committee on Taxonomy of Viruses (ICTV). Family: picornaviridae. Available from: https://ictv.global/.
3. World Health Organization (WHO). Hepatitis A. Available from: https://www.who.int/.
4. Migueres M, Lhomme S, Izopet J. Hepatitis A: epidemiology, high-risk groups, prevention and research on antiviral treatment. Viruses. 2021;13:1900. doi:10.3390/v13101900
5. Dudareva S, Faber M, Zimmermann R, et al. Epidemiologie der virushepatitiden A bis E in Deutschland [Epidemiology of viral hepatitis A to E in Germany]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022;65:149–158. German. doi:10.1007/s00103-021-03478-8
6. Braccio S, Irwin A, Riordan A, et al. Acute infectious hepatitis in hospitalised children: a British Paediatric Surveillance Unit study. Arch Dis Child. 2017;102:624–628. doi:10.1136/archdischild-2016-311916
7. Gargouri S, Fki Berrajah L, Ayadi I, et al. Epidemiological and clinical analysis of hepatitis virus A infections during three successive outbreaks in Sfax (Tunisia) between 2007 and 2010. Med Sante Trop. 2016;26:159–164. doi:10.1684/mst.2015.0491
8. Gotlieb N, Moradpour D, Shouval D. Hepatitis A and E – differences and commonalities. J Hepatol. 2020;72:578–580. doi:10.1016/j.jhep.2019.05.011
9. Severi E, Georgalis L, Pijnacker R, et al. Severity of the clinical presentation of hepatitis A in five European countries from 1995 to 2014. Int J Infect Dis. 2022;118:34–43. doi:10.1016/j.ijid.2022.01.053
10. Lemon SM, Walker CM. Hepatitis A virus and hepatitis E virus: emerging and re-emerging enterically transmitted hepatitis viruses. Cold Spring Harb Perspect Med. 2019;9:a031823. doi:10.1101/cshperspect.a031823
11. Pinto RM, Perez-Rodriguez FJ, Costafreda MI, et al. Pathogenicity and virulence of hepatitis A virus. Virulence. 2021;12:1174–1185. doi:10.1080/21505594.2021.1910442
12. Lemon SM. Hepatitis A: current view of an ancient disease. J Hepatol. 2022;77:243–244. doi:10.1016/j.jhep.2021.09.028
13. Castaneda D, Gonzalez AJ, Alomari M, et al. From hepatitis A to E: a critical review of viral hepatitis. World J Gastroenterol. 2021;27:1691–1715. doi:10.3748/wjg.v27.i16.1691
14. Hu X, Collier MG, Xu F. Hepatitis A outbreaks in developed countries: detection, control, and prevention. Foodborne Pathog Dis. 2020;17:166–171. doi:10.1089/fpd.2019.2648
15. Shin EC, Jeong SH. Natural history, clinical manifestations, and pathogenesis of hepatitis A. Cold Spring Harb Perspect Med. 2018;8:a031708. doi:10.1101/cshperspect.a031708
16. Dimitrova M, Petrova G, Tachkov K, et al. Economic consequences of the vaccination against hepatitis A in the Bulgarian healthcare setting. Biotechnol Biotechnol Equip. 2014;28:366–371. doi:10.1080/13102818.2014.909654
17. Bruni R, Taffon S, Equestre M, et al. Hepatitis A virus genotypes and strains from an endemic area of Europe, Bulgaria 2012–2014. BMC Infect Dis. 2017;17:497. doi:10.1186/s12879-017-2596-1
18. Toseva EI, Atanasova MV, Turnovska TH. Seroprevalence of anti-HAV total antibodies among workers in wastewater treatment plants. Int J Occup Med Environ Health. 2018;31:307–315. doi:10.13075/ijomeh.1896.01161
19. Cella E, Golkocheva-Markova EN, Trandeva-Bankova D, et al. The genetic diversity of hepatitis A genotype I in Bulgaria. Medicine. 2018;97:e9632. doi:10.1097/MD.0000000000009632
20. Velev V, Popov M, Tomov L, et al. Involvement of the gallbladder in the course of viral hepatitis A in childhood. Trop Doct. 2019;49:271–273. doi:10.1177/0049475519858893
21. European Centre for Disease Prevention and Control (ECDC). Hepatitis A: an Annual Epidemiological Reports (AERs), 2011–2021. Available from: https://www.ecdc.europa.eu/en.
22. Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi:10.1159/000149370
23. International Committee on Taxonomy of Viruses (ICTV). Family: hepeviridae. Available from: https://ictv.global/.
24. Purdy MA, Drexler JF, Meng XJ, et al. ICTV virus taxonomy profile: hepeviridae 2022. J Gen Virol. 2022;103:001778. doi:10.1099/jgv.0.001778
25. World Health Organization (WHO). Hepatitis E. Available from: https://www.who.int/.
26. Nelson KE, Labrique AB, Kmush BL. Epidemiology of genotype 1 and 2 hepatitis E virus infections. Cold Spring Harb Perspect Med. 2019;9:a031732. doi:10.1101/cshperspect.a031732
27. Mooij SH, Hogema BM, Tulen AD, et al. Risk factors for hepatitis E virus seropositivity in Dutch blood donors. BMC Infect Dis. 2018;18:173. doi:10.1186/s12879-018-3078-9
28. Pepovich R, Baymakova M, Pishmisheva M, et al. Current knowledge on hepatitis E virus infection. Vojnosanit Pregl. 2019;76:733–739. doi:10.2298/VSP170815159P
29. Lee GH, Tan BH, Teo EC, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016;150(2):355–7.e3. doi:10.1053/j.gastro.2015.10.048
30. Sridhar S, Yip CCY, Wu S, et al. Rat hepatitis E virus as cause of persistent hepatitis after liver transplant. Emerg Infect Dis. 2018;24:2241–2250. doi:10.3201/eid2412.180937
31. Sridhar S, Yip CC, Wu S, et al. Transmission of rat hepatitis E virus infection to humans in Hong Kong: a clinical and epidemiological analysis. Hepatology. 2021;73:10–22. doi:10.1002/hep.31138
32. Sridhar S, Yip CCY, KHY L, et al. Hepatitis E virus species C infection in humans, Hong Kong. Clin Infect Dis. 2022;75:288–296. doi:10.1093/cid/ciab919
33. Kenney SP. The current host range of hepatitis E viruses. Viruses. 2019;11:452. doi:10.3390/v11050452
34. Denner J. Hepatitis E virus (HEV) – the future. Viruses. 2019;11:251. doi:10.3390/v11030251
35. European Centre for Disease Prevention and Control (ECDC). Hepatitis E in the EU/EEA, 2005–2015. Stockholm: ECDC; 2017.
36. Baymakova M, Popov GT, Pepovich R, et al. Hepatitis E virus infection in Bulgaria: a brief analysis of the situation in the country. Open Access Maced J Med Sci. 2019;7:458–460. doi:10.3889/oamjms.2019.073
37. Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61–69. doi:10.1046/j.1365-2893.2003.00398.x
38. Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi:10.1056/NEJMoa0706992
39. Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi:10.1053/j.gastro.2011.02.050
40. Mallet V, van Bommel F, Doerig C, et al. Management of viral hepatitis in patients with haematological malignancy and in patients undergoing haemopoietic stem cell transplantation: recommendations of the 5th European Conference on Infections in Leukaemia (ECIL-5). Lancet Infect Dis. 2016;16:606–617. doi:10.1016/S1473-3099(16)00118-3
41. Jagjit Singh GK, Ijaz S, Rockwood N, et al. Chronic hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect. 2013;66:103–106. doi:10.1016/j.jinf.2011.11.027
42. Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, Phase 3 trial. Lancet. 2010;376:895–902. doi:10.1016/S0140-6736(10)61030-6
43. Teoharov P, Kevorkyan A, Raycheva R, et al. Data on the prevalence of hepatitis E virus in Bulgaria. Comptes Rendus Acad Bulg Sci. 2014;67:1427–1432.
44. Baymakova M, Sakem B, Plochev K, et al. Epidemiological characteristics and clinical manifestations of hepatitis E virus infection in Bulgaria: a report on 20 patients. Srp Arh Celok Lek. 2016;144:63–68. doi:10.2298/sarh1602063b
45. Bruni R, Villano U, Equestre M, et al. Hepatitis E virus genotypes and subgenotypes causing acute hepatitis, Bulgaria, 2013–2015. PLoS One. 2018;13:e0198045. doi:10.1371/journal.pone.0198045
46. Cella E, Golkocheva-Markova E, Sagnelli C, et al. Human hepatitis E virus circulation in Bulgaria: deep Bayesian phylogenetic analysis for viral spread control in the country. J Med Virol. 2019;91:132–138. doi:10.1002/jmv.25296
47. Baymakova M, Terzieva K, Popov R, et al. Seroprevalence of hepatitis E virus infection among blood donors in Bulgaria. Viruses. 2021;13:492. doi:10.3390/v13030492
48. Golkocheva-Markova E, Kevorkyan A, Raycheva R, et al. Assessment of hepatitis E seropositivity among HIV-infected patients in Bulgaria. Braz J Infect Dis. 2022;26:102329. doi:10.1016/j.bjid.2022.102329
49. National Center of Infectious and Parasitic Diseases (NCIPD). Sofia, Bulgaria. Analysis of acute infectious diseases in Bulgaria, 2011–2021. Available from: https://ncipd.org/index.php?lang=bg.
50. Pas SD, Streefkerk RH, Pronk M, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58:629–634. doi:10.1016/j.jcv.2013.10.010
51. Avellon A, Morago L, Garcia-Galera Del Carmen M, et al. Comparative sensitivity of commercial tests for hepatitis E genotype 3 virus antibody detection. J Med Virol. 2015;87:1934–1939. doi:10.1002/jmv.24251
52. Chau TN, Lai ST, Tse C, et al. Epidemiology and clinical features of sporadic hepatitis E as compared with hepatitis A. Am J Gastroenterol. 2006;101:292–296. doi:10.1111/j.1572-0241.2006.00416.x
53. Oh HW, Cha RR, Lee SS, et al. Comparing the clinical features and outcomes of acute hepatitis E viral infections with those of acute hepatitis A, B, and C infections in Korea. Intervirology. 2017;60:109–117. doi:10.1159/000480506
54. Suzuki K, Kataoka K, Miyamoto Y, et al. Clinical and molecular analyses of sporadic acute hepatitis A and E and the specific viral genotypes isolated in Iwate and three neighboring prefectures in the northern part of Honshu, Japan, between 2004 and 2013. Hepatol Res. 2015;45:714–727. doi:10.1111/hepr.12406
55. Mihai IF, Manciuc C, Hunea IM, et al. Enterically transmitted hepatitis in the third millennium in Northeastern Romania. Exp Ther Med. 2021;21:274. doi:10.3892/etm.2021.9705
56. Toyoda H, Kumada T, Kiriyama S, et al. Clinical and molecular characteristics of hepatitis A virus infections during the years 1992–2003 in Ogaki, a centrally located city of Japan. J Clin Virol. 2009;44:145–148. doi:10.1016/j.jcv.2008.12.002
57. Moon HW, Cho JH, Hur M, et al. Laboratory characteristics of recent hepatitis A in Korea: ongoing epidemiological shift. World J Gastroenterol. 2010;16:1115–1118. doi:10.3748/wjg.v16.i9.1115
58. Kwon SY, Park SH, Yeon JE, et al. Clinical characteristics and outcomes of acute hepatitis A in Korea: a nationwide multicenter study. J Korean Med Sci. 2014;29:248–253. doi:10.3346/jkms.2014.29.2.248
59. Sridhar S, Lo SK, Xing F, et al. Clinical characteristics and molecular epidemiology of hepatitis E in Shenzhen, China: a shift toward foodborne transmission of hepatitis E virus infection. Emerg Microbes Infect. 2017;6:e115. doi:10.1038/emi.2017.107
60. Fang L, Zhang J, Chen H, et al. Epidemiological characteristics and clinical manifestations of hepatitis E in a tertiary hospital in China: a retrospective study. Front Microbiol. 2022;12:831968. doi:10.3389/fmicb.2021.831968
61. Mihalcin M, Husova L, Vasickova P, et al. Hepatitis E – epidemiology and clinical course in the largest cohort in the Czech Republic. Arch Med Sci. 2022;18:1395–1398. doi:10.5114/aoms/152338
62. Tominaga A, Kanda T, Akiike T, et al. Hepatitis A outbreak associated with a revolving sushi bar in Chiba, Japan: application of molecular epidemiology. Hepatol Res. 2012;42:828–834. doi:10.1111/j.1872-034X.2012.00988.x
63. Pishmisheva М, Golkocheva-Markova Е, Naseva Е, et al. Анализ на някои епидемиологични и клинични характеристики на ентерално предаваните хепатити А и Е (собствени наблюдения) [Analysis of some epidemiological and clinical characteristics of enterally transmitted hepatitis A and E (our own observations)]. Med Rev. 2018;54:30–35. Bulgarian.
64. Pishmisheva-Peleva М Хепатит Е: екзотика или тихо присъствие (монография) [Hepatitis E: exoticism or silent presence (monograph)]. Pazardzhik, Bulgaria: Barich & Co Ltd; 2022. Bulgarian.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
