Back to Journals » Cancer Management and Research » Volume 16
Comparative Analysis of Oncologic Outcomes in Patients with Squamous Cell Carcinoma of the Uterine Cervix with High-Risk Features for Para-Aortic Recurrence: Prophylactic Extended-Field versus Pelvic Chemoradiotherapy
Authors Chen CS, Wang YM, Huang EY
Received 12 December 2023
Accepted for publication 13 March 2024
Published 3 April 2024 Volume 2024:16 Pages 269—279
DOI https://doi.org/10.2147/CMAR.S451137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Chung-Shih Chen,1 Yu-Ming Wang,1– 3 Eng-Yen Huang1– 3
1Department of Radiation Oncology & Proton and Radiation Therapy Center, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung City, 833, Taiwan; 2School of Traditional Chinese Medicine, Chang Gung University, Taoyuan, Taiwan; 3Department of Radiation Oncology, Kaohsiung Chang Gung Memorial Hospital, School of Medicine, College of Medicine, National Sun Yat-Sen University, Kaohsiung City, 804, Taiwan
Correspondence: Eng-Yen Huang, School of Medicine, College of Medicine, National Sun Yat-sen University, No. 70, Lienhai Rd, Kaohsiung, 80424, Taiwan, Email [email protected]
Purpose: To compare the oncologic outcomes of prophylactic extended-field radiation therapy (EFRT) and whole pelvic radiation therapy (WPRT) in cervical patients at high risk of para-aortic lymph node (PALN) recurrence.
Patients and Methods: From July 1999 to May 2022, a total of 115 patients with cervical cancer and high-risk features of PALN recurrence based on tumor markers, positive LNs and extensive parametrial invasion were retrospectively analyzed. All patients had received EFRT or WPRT at a dose of 39.6– 45 Gy and concurrent chemotherapy. In EFRT, coverage was extended to include the para-aortic region below the level of the left renal vein or T12.
Results: Twenty-eight and 87 patients underwent EFRT and WPRT, respectively. For patients who survived, the median follow-up time was 60.8 months (range 9.2– 131.6 months) in the EFRT group and 115.9 months (range 16.9– 212.1 months) in the WPRT group. The 5-year overall survival (OS) and pelvic, extrapelvic and PALN recurrence rates were 87.7% vs 60.8% (p=0.019), 10.9% vs 25.3% (p=0.119), 18.1% vs 45.8% (p=0.011), and 0% vs 30.4% (p=0.005), respectively, between the EFRT and WPRT groups. Multivariate analysis revealed that EFRT and 2018 FIGO stage IV disease status were significant predictors of OS and extrapelvic recurrence.
Conclusion: Compared to WPRT, EFRT significantly improved OS and reduced extrapelvic and PALN recurrence in patients with cervical cancer with high-risk recurrence features.
Keywords: extended-field radiation therapy, cervical cancer, para-aortic recurrence, pelvic chemoradiotherapy
Introduction
Pelvic irradiation is one of the main treatment modalities for cervical cancer patients without extrapelvic metastasis in a definitive setting. Although there is good local control, systemic or extrapelvic failure is still a concern.1 Para-aortic lymph nodes (PALNs) are common failure sites and sanctuaries for micrometastasis beyond the pelvis.2 A previous study revealed that 11% of patients experienced PALN recurrence, and a 37% PALN recurrence rate was found in patients with positive pelvic lymph nodes, indicating that pelvic irradiation might not eradicate microscopic disease.3 In addition, despite the use of concurrent chemotherapy, the data showed that the risk of PALN failure was not eliminated.4 A previous randomized study demonstrated that the PALN recurrence and distant failure rates in patients treated with concurrent pelvic chemoradiation were 9% and 35%, respectively.4 Once patients experience PALN relapse, the prognosis is poor, with a 5-year survival rate of approximately 30%.5
Several studies have demonstrated that prophylactic para-aortic irradiation has the potential to attenuate PALN recurrence in patients without positive PALNs on imaging.6–11 The Radiation Therapy Oncology Group 79–20 trial showed that extended-field radiation therapy (EFRT) decreases distant metastasis and increases survival in patients with bulky FIGO stage IB and IIB disease.6 In addition, patients with 2018 International Federation of Gynecology and Obstetrics (FIGO) stage IIIB or IIIC1 disease were considered eligible for EFRT in other retrospective studies.9–11 Currently, the criteria for patient selection and benefit from EFRT remain controversial. In our earlier report, the risk factors for PALN relapse included a squamous cell carcinoma antigen (SCC-Ag) level >20 ng/mL, a carcinoembryonic antigen (CEA) level >10 ng/mL, advanced parametrial invasion and the presence of pelvic lymphadenopathy.3,12 Patients with any of the above risk factors have a 5-year PALN recurrence rate ranging from 20% to 46%.3,12 However, the benefit of prophylactic EFRT compared with pelvic irradiation alone has not been fully established for these patients. Hence, the aim of this study was to investigate the value of prophylactic EFRT according to tumor markers, parametrial involvement and pelvic node metastases in patients at high risk of PALN recurrence.
Materials and Methods
Patient Recruitment and Risk Classification
Our cohort study was reviewed and approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (IRB No. 202301178B0). From July 1999 to May 2022, patients with biopsy-proven FIGO stage IB-IVA cervical cancer without PALN metastases in the high- and intermediate-risk groups were retrospectively reviewed. The definition of the risk group for PALN recurrence was based on our previous studies.3,12 Briefly, intermediate risk included one of the following minor risk factors: (1) pretreatment SCC-Ag level of 20–40 ng/mL; (2) parametrial (PM) score 4–6; and (3) positive pelvic lymph nodes (LN). High risk was defined as ≥2 minor risk factors or one of the following major risk factors: (1) pretreatment SCC-Ag >40 ng/mL; and (2) pretreatment CEA ≥ 10 ng/mL. The pathology of cervical cancer in all patients was squamous cell carcinoma. The positive lymph nodes were identified based on the radiographic findings at diagnosis with a short diameter >10 mm either by computed tomography (CT) or magnetic resonance imaging (MRI). The stage previously recorded by the 1994 or 2009 FIGO staging system was converted to the 2018 FIGO stage. Patients with initial distant metastases (FIGO stage IVB) were excluded.
Primary Treatment Modality
All patients received definitive concurrent chemoradiotherapy. Weekly cisplatin was used as a first-line regimen with concurrent radiotherapy delivered at a dose of 40 mg/m2 to a maximum of 60 mg. Chemotherapy was withheld if patients had any grade 3 or higher hematological toxicity. Patients were categorized into whole-pelvic radiotherapy (WPRT) and EFRT groups. In the WPRT group, patients received 39.6–45 Gy (1.8–2 Gy/fraction) of whole-pelvis irradiation with 10–15 megavoltage (MV)-X-ray via anteroposterior parallel-opposed, four-field box radiation, intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT). The clinical target volume (CTV) included the primary cervical tumor, parametrium, pelvic lymph nodes, and superior part of the vagina. The planning target volume included the primary CTV with additional margins of 10 mm for daily setup variation. The field of the WPRT was no lower than the level of the inferior border of the L4–5 interspace but did not extend above the aortic bifurcation. In the EFRT group, the RT field additionally covered the para-aortic region below the level of the left renal vein or T12 at a dose of 39.6–45 Gy. For patients with locally advanced disease in either the EFRT or WPRT group, a parametrial or pelvic lymph node boost ranging from 50.4 to 59.4 Gy was administered.
For patients with tumor regression, intracavitary brachytherapy was performed after external beam radiotherapy (EBRT). Brachytherapy was delivered by the Henschke applicator with an Ir-192 high-dose-rate unit and an afterloading technique. Typically, the total brachytherapy dose ranged from 24 to 28 Gy in 4 to 6 fractions and was delivered to point A. In addition, the dose to the bladder and rectum points was evaluated by a 2D-based planning system with radiographic film according to the International Commission on Radiation Units and Measurements, report #38. For patients with large residual tumors after EBRT who were not suitable for brachytherapy, an EBRT boost was performed with a total dose of up to 61.2–72 Gy. Additionally, in cases where the applicator could not be inserted into the cervical canal or the cervical os was not found, an EBRT boost was employed instead of brachytherapy.
Follow-Up
Patients underwent follow-up evaluation 1–2 months after initial treatment. Thereafter, regular follow-up was performed every 3 months for the first two years and then every 4–6 months thereafter. Patients received a pelvic examination at each follow-up. In addition, tumor markers, including SCC-Ag and CEA, were also examined. Images were evaluated via pelvic MRI or CT within 3 months after the completion of radiotherapy and then annually thereafter or whenever recurrence was suspected based on clinical symptoms or elevated tumor markers.
Statistical Analysis
The baseline patient characteristics included age, comorbidities (hypertension/diabetes mellitus), FIGO stage (2009/2018), tumor marker (SCC/CEA), PM score, positive pelvic LN and risk group (intermediate/high). The categorical variables were compared between the EFRT and WPRT groups by either Pearson’s Chi-squared test or two-sided Fisher’s exact test. Independent t tests were performed to compare continuous variables. Overall survival (OS), pelvic recurrence, extrapelvic recurrence and PALN recurrence were evaluated as clinical outcomes. OS was defined as the duration from the date of initial diagnosis to the date of death or last follow-up. The time to pelvic recurrence was defined as the duration from the date of initial diagnosis to the date of tumor or regional LN recurrence below the aortic bifurcation. The time to extrapelvic recurrence, including PALN relapse and distant failure, was defined as the duration from the date of initial diagnosis to the date of tumor recurrence beyond the aortic bifurcation. The Kaplan‒Meier method was used for analysis, and the Log rank test was performed to compare the differences between groups. Multivariate analysis was performed by using the Cox regression model. Variables for multivariate analysis included age, EFRT, risk group and 2018 FIGO stage. All the statistical analyses were performed using SPSS v. 25.0 (IBM, Illinois, USA), and a two-tailed value of p<0.05 was considered indicative of statistical significance.
Results
Baseline Characteristics of the Patients
A total of 115 patients were eligible for analysis, with 28 patients receiving EFRT and 87 patients receiving WPRT. The patients’ basic characteristics are shown in Table 1. The EFRT group had significantly more patients with positive pelvic LNs (85.7% vs 34.5%, p<0.001). In addition, more patients in the EFRT group tended to be in the high-risk group (57.1% vs 37.9%, p=0.074), but the difference was not significant. On the other hand, more patients in the WPRT group than in the EFRT group had PM scores of 4–6 (21.4% vs 47.1%, p=0.016). The other characteristics in each group were not significantly different.
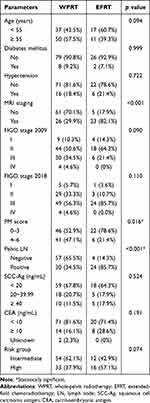 |
Table 1 Patient Characteristics (n =115) |
Oncologic Outcome and Multivariate Analysis
For surviving patients, the median follow-up time was 60.8 months (range 9.2–131.6 months) in the EFRT group and 115.9 months (range 16.9–212.1 months) in the WPRT group. The treatment outcomes are shown in Figures 1–4. The 5-year OS was greater in patients in the EFRT group than in those in the WPRT group (87.7% vs 60.8%, p=0.019; Figure 1). In addition, the 5-year pelvic recurrence rates were 10.9% and 25.3% (p=0.119, Figure 2), respectively. The incidence of 5-year extrapelvic recurrence (18.1 vs 45.8%, p=0.011; Figure 3) and PALN recurrence (0% vs 30.4%, p=0.005; Figure 4) were also significantly lower in the EFRT group than in the WPRT group.
 |
Figure 1 Overall survival in the EFRT and WPRT groups. Patients treated with EFRT had significantly better overall survival. |
 |
Figure 2 Pelvic recurrence rate in the EFRT and WPRT groups. The pelvic recurrence rate was not significantly different between the two groups. |
 |
Figure 3 Extrapelvic recurrence rate in the EFRT and WPRT groups. Patients treated with EFRT have less extrapelvic recurrence. |
 |
Figure 4 PALN recurrence rate in the EFRT and WPRT groups. EFRT significantly reduced the PALN recurrence rate. |
In addition, treatment outcomes between 2018 FIGO IIIC1 and 2018 FIGO non-IIIC1 (any stage without LN metastases) patients are shown in Table 2. For patients with FIGO IIIC1 disease, EFRT reduced 5-year extrapelvic recurrence (13.8% vs 47.9%, p=0.016) and PALN recurrence (0.0% vs 33.3%, p=0.007). For patients with FIGO non-IIIC1 disease, the 5-year extrapelvic recurrence rates and PALN recurrence rates were 33.3% vs 44.5% (p=0.474) and 0.0% vs 28.5% (p=0.318) in the EFRT and WPRT groups, respectively.
 |
Table 2 Comparison of Treatment Outcomes Between FIGO 2018 IIIC1 Patients and Non-IIIC1 Patients |
WPRT patients with FIGO non-IIIC1 tumors had more extensive parametrial invasion (PM score 4–6) than did those with IIIC1 tumors (54.4% vs 33.3%, p= 0.062). In addition, there were more patients with CEA levels >10 ng/mL in the FIGO non-IIIC1 subgroup than in the FIGO IIIC1 subgroup (21.1% vs 6.7%, p=0.070). In the WPRT group, for patients with FIGO IIIC1 and non-IIIC1 disease, the OS rates were 71.4% and 55.5% (p=0.103), respectively. The 5-year pelvic recurrence rates were 13.8% and 31.1%, respectively (p = 0.100). There was no significant difference in 5-year extrapelvic recurrence or PALN recurrence between FIGO IIIC1 and FIGO non-IIIC1 patients. The 5-year extrapelvic recurrence rates were 47.9% and 44.5% (p = 0.849), respectively. The 5-year PALN recurrence rates were 33.3% and 28.5%, respectively (p = 0.657).
Patients with MRI staging had more positive pelvic LNs than those without MRI staging (73.5% vs 27.3%, p<0.001). In addition, patients without MRI staging had more extensive parametrial invasion (PM score 4–6) (53.0% vs 24.5%, p=0.002) and locally advanced tumors (2009 FIGO stage III–IV) (42.4% vs 24.5%, p=0.046) than those with MRI staging. Additionally, there were trends toward more patients with SCC levels >40 ng/mL in the non-MRI staging group than in the MRI staging group (18.2% vs 6.1%, p=0.058). The differences in treatment outcomes between patients with and without MRI staging are presented in Table 3. In patients who underwent MRI, the 5-year OS rates were 94.1% and 80.6% (p=0.128) in the EFRT and WPRT groups, respectively. For patients with MRI staging who received EFRT or WPRT, the extrapelvic and PALN recurrence rates were 18.0% vs 34.6% (p = 0.130) and 0.0% vs 18.6% (p = 0.053), respectively. In patients without MRI staging, the 5-year OS rates were 60.0% and 53.0% (p=0.777) in the EFRT and WPRT groups, respectively. For patients without MRI data who underwent EFRT or WPRT, the recurrence rates of extrapelvic and PALN lesions were 20.0% vs 50.7% (p = 0.356) and 0.0% vs 35.7% (p = 0.230), respectively.
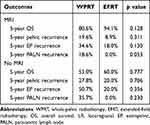 |
Table 3 Treatment Outcomes Between Patients with and without MRI Staging |
The results of multivariate analyses for OS and extrapelvic recurrence are shown in Table 4. EFRT was significantly associated with better OS (HR: 0.276, 95% CI: 0.081–0.937, p = 0.039) and less extrapelvic recurrence (HR: 0.286, 95% CI: 0.097–0.838, p = 0.023). In addition, 2018 FIGO stage IV disease independently predicted worse OS (HR: 16.781, 95% CI: 1.748–161.123, p = 0.015) and more extrapelvic recurrence (HR: 15.016, 95% CI: 2.133–105.726, p = 0.007). The other clinical factors, including age and risk group, were not associated with either OS or extrapelvic recurrence.
 |
Table 4 Multivariate Analysis of OS and EPR |
Discussion
In the present study, because patients treated with WPRT had a higher PM score of 4–6, indicating a greater extent of parametrial invasion by tumors, the pelvic recurrence rate tended to be greater in the WPRT group than in the EFRT group, but the difference was not significant. On the other hand, patients who underwent EFRT had more positive pelvic LNs, which are regarded as a significant predictor of poor prognosis. Despite baseline differences with regard to poor prognostic factors in the EFRT group, we found that EFRT led to more favorable clinical outcomes than WPRT, including better PALN control and distant control as well as longer OS. The results demonstrated that EFRT could eradicate micrometastases from pelvic lymph nodes to PALNs, thus reducing the extrapelvic and PALN recurrence rates to further improve survival.
Cervical carcinoma is reported to have a greater tendency to exhibit lymphogenous metastasis than metastasis via the hematogenous route.13 For patients with positive lymph nodes, the PALNs represent the next nodal station to which tumors metastasize in a stepwise manner. In a previous study, pelvic LN metastasis correlated with an increased incidence of PALN recurrence.3 Patients with positive pelvic LNs who underwent pelvic RT had a 5-year PALN recurrence rate of up to 37%.3 Sakaragi et al also supported the finding that a positive pelvic LN was an independent predictor of the development of PALN metastasis in the radical hysterectomy setting.14 Therefore, in view of the high risk of PALN recurrence, we chose patients with pelvic LN metastases to undergo prophylactic PALN irradiation and achieved significantly improved PALN control and overall survival. In contrast, Lee et al stratified patients with positive common iliac LNs or ≥3 pelvic LNs into a high-risk group and found that PALN irradiation improved cancer-specific survival only in those patients.9 There was no significant difference for patients in the low-risk group (ie, below the common iliac bifurcation and 1–2 pelvic LNs).9 Our findings showed that the survival benefit of prophylactic EFRT was not limited to patients with pelvic LNs and high-risk features defined in the study by Lee et al.9 Wang et al reported the treatment outcomes of 280 patients with cervical cancer with FIGO stage IIIC1 disease treated with EFRT and stratified patients into high-risk or low-risk groups based on factors including common iliac LNs or ≥3 pelvic LNs.10 The finding that EFRT significantly improved survival compared with WPRT in both the low- and high-risk groups, as determined by the location or number of pelvic LNs, was consistent with the present study results.
Most previous studies have focused on the role of EFRT combined with concurrent chemotherapy in patients with positive pelvic LNs.8–10 However, no study has explored the benefit of prophylactic EFRT in patients with high levels of tumor markers. An increase in the SCC-Ag level was found to be important for predicting distant failure and OS in patients undergoing pelvic irradiation.1,15,16 Huang et al reported that an SCC-Ag level >40 ng/mL was an independent prognostic factor for distant metastasis, DFS, and OS.16 In addition, pretreatment SCC-Ag levels strongly correlated with PALN relapse. The results of a previous study indicated that the incidence of PALN recurrence increased in patients with SCC-Ag levels >20 ng/mL.3 The five-year PALN recurrence rates in patients with SCC-Ag levels of 20–40 and >40 ng/mL were 22% and 57%, respectively.3 In our study, an SCC-Ag level >20 ng/mL was regarded as a predictive factor for PALN recurrence, and clinically PALN-negative patients were also included. Our results suggested that more aggressive therapy with prophylactic EFRT should be considered in patients with SCC-Ag levels >20 ng/mL regardless of pelvic lymph node metastasis.
In our study, the extent of parametrial invasion evaluated by the PM score was first used as an inclusion criterion for patients treated with prophylactic EFRT. In our earlier report, we showed that the PM score influenced OS, DFS, local control and the distant metastasis-free survival rate. A PM score of 4–6 represents at least bilateral parametrial involvement or extension to the pelvic sidewall with a high tumor burden.17 Huang et al showed that a PM score >4-6 increased the probability of PALN metastasis.3 A previous study also demonstrated that tumor extension to the pelvic sidewall led to an increased PALN failure rate (12.8% vs 3%) in patients treated with pelvic RT.18 In the present study, we found that patients without LN metastases but with locally advanced tumors also had a high risk of PALN recurrence and extrapelvic recurrence when treated with WPRT. The 5-year extrapelvic and PALN recurrence rates in patients with FIGO non-IIIC1 disease (mainly including extensive parametrial invasion or IIIB) were similar to those in patients with FIGO IIIC1 disease. The survival data in our study were compatible with the findings of Matsuo et al,19 who validated the revised 2018 FIGO stage. In that study,19 FIGO IIIB was associated with worse cause-specific survival than stage IIIC1, and the survival of IIIC1 patients significantly varied widely depending on T stage (5-year rates: 74.8% for T1, 58.7% for T2, and 39.3% for T3). This finding suggested that stage IIIB or extensive parametrial invasion (PM score >4-6) had an impact on both PALN recurrence and survival. Moreover, Meng et al11 demonstrated that patients with 2018 FIGO stage IIIB disease treated with EFRT had a clear improvement in OS and PALN metastasis-free survival compared with those treated with pelvic irradiation only. Our study also suggested that EFRT should be applied not only in patients with positive LNs but also in patients with negative pelvic LNs but extensive parametrial invasion.
Although EFRT provides better PALN control and OS, it is essential to be aware of the potential adverse effects. EFRT can lead to radiation-induced emesis (RIE)20 as well as renal,21 bone marrow22 and bowel toxicity20,23 due to extensive irradiation of the para-aortic region. Wang et al20 demonstrated that a sufficient volume of the small bowel receiving low-dose radiation from EFRT may trigger the vomiting reflex through the enteric nerve plexus. A higher mean bowel dose predicts a greater incidence of RIE and early onset of RIE (within 72 hours after the first fraction of EFRT). Moreover, PALN irradiation increases the radiation dose to both kidneys, potentially causing renal toxicity.21 Patients receiving EFRT also experienced acute hematologic toxicity due to increased radiation to the bone marrow.22 Additionally, in a previous RTOG 7920 trial,23 EFRT resulted in a greater rate of bowel complications than pelvic irradiation. In recent years, intensity-modulated proton therapy (IMPT) has been shown to reduce the dose to surrounding organs, especially the para-aortic region.21 The advantage of utilizing IMPT is evident in lower dose regions, and clinically relevant dose reductions are achieved for the kidneys, spinal cord and bowel.21 Therefore, EFRT using proton therapy can be considered a first option to achieve fewer side effects than photon therapy without compromising oncologic outcomes.
As a retrospective study, the present study had several limitations and inevitable biases. First, the sample size was small, and it is difficult to draw a conclusive statistical statement with a small number of patients in the subgroup analysis. There were patients who received EFRT via proton beam therapy at our institute, but the present study did not include those patients due to the short follow-up period of less than one year. Furthermore, the baseline patient characteristics were not well balanced, with more patients having a high risk of PALN recurrence and more positive lymph nodes in the EFRT group than in the WPRT group. Despite more patients with poor prognostic factors in the EFRT group, there were better treatment outcomes. Moreover, because the study covered more than 20 years, the RT technique varied with advances in technique from four-field box radiation to VMAT. This might have led to inherent biases regarding the comparison of oncologic outcomes. Finally, MRI for staging was not commonly used before 2012 because there was no reimbursement covered by national health insurance at that time. Occult PALN and pelvic LN metastases might not have been detected by CT, compromising the results of the WPRT. In our study, more positive pelvic LNs were detected in patients who underwent MRI. We observed that the EFRT group still tended to exhibit better clinical outcomes, including OS and PALN recurrence, than the WPRT group when the patients were divided into MRI and non-MRI groups, despite the statistical nonsignificance observed in the subgroup analysis. Additionally, the overall worse outcome of patients in the non-MRI group than in the MRI group might be attributed to the more locally advanced tumors in the non-MRI group. Further prospective studies might be needed to validate these findings and address the limitations of the present study.
Conclusion
The present study revealed that prophylactic EFRT improved OS and extrapelvic and PALN metastasis control in patients with intermediate to high risk of PALN recurrence based on at least one risk factor as follows: (1) SCC-Ag level >20 ng/mL, (2) CEA level>10 ng/mL, (3) PM score of 4–6, and (4) positive pelvic nodes. Further large randomized clinical trials could include those risk factors as inclusion criteria when comparing EFRT with WPRT.
Data Sharing Statement
The data are available from the corresponding author, E.Y. Huang, upon reasonable request.
Ethical Approval
All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. This retrospective study was approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (IRB No. 202301178B0).
Informed Consent for Publication
The IRB approved the waiver of the participants’ consent due to the retrospective nature of this chart review study. We confirmed that the patient data were anonymized or maintained with confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was not supported by any external funding.
Disclosure
All authors declare that there are no financial or nonfinancial competing interests.
References
1. Hong JH, Tsai CS, Chang JT, et al. The prognostic significance of pre- and posttreatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41(4):823–830. doi:10.1016/S0360-3016(98)00147-3
2. Carlson V, Delclos L, Fletcher GH. Distant metastases in squamous-cell carcinoma of the uterine cervix. Radiology. 1967;88(5):961–966. doi:10.1148/88.5.961
3. Huang EY, Wang CJ, Chen HC, et al. Multivariate analysis of para-aortic lymph node recurrence after definitive radiotherapy for stage IB-IVA squamous cell carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 2008;72(3):834–842. doi:10.1016/j.ijrobp.2008.01.035
4. Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22(5):872–880. doi:10.1200/JCO.2004.07.197
5. Chen CS, Ou YC, Lin H, et al. Analysis of prognostic factors and clinical outcomes in uterine cervical carcinoma with isolated para-aortic lymph node recurrence. Am J Transl Res. 2019;11(12):7492–7502.
6. Rotman M, Choi K, Guze C, Marcial V, Hornback N, John M. Prophylactic irradiation of the para-aortic lymph node chain in stage iib and bulky stage ib carcinoma of the cervix, initial treatment results of RTOG 7920. Internat J Radiat Oncol Biol Phys. 1990;19(3):513–521. doi:10.1016/0360-3016(90)90475-Y
7. Asiri MA, Tunio MA, Mohamed R, et al. Is extended-field concurrent chemoradiation an option for radiologic negative paraaortic lymph node, locally advanced cervical cancer? Cancer Manag Res. 2014;6:339–348. doi:10.2147/CMAR.S68262
8. Liang JA, Chen SW, Hung YC, et al. Low-dose, prophylactic, extended-field, intensity-modulated radiotherapy plus concurrent weekly cisplatin for patients with stage IB2-IIIB cervical cancer, positive pelvic lymph nodes, and negative para-aortic lymph nodes. Int J Gynecol Cancer. 2014;24(5):901–907. doi:10.1097/IGC.0b013e31829f4dc5
9. Lee J, Lin JB, Chang CL, et al. Impact of para-aortic recurrence risk-guided intensity-modulated radiotherapy in locally advanced cervical cancer with positive pelvic lymph nodes. Gynecol Oncol. 2018;148(2):291–298. doi:10.1016/j.ygyno.2017.12.003
10. Wang W, Meng Q, Zhou Y, et al. Prophylactic extended-field irradiation versus pelvic irradiation in patients with cervical cancer with 2018 FIGO stage IIIC1 disease. Pract Radiat Oncol. 2023;13:e409–e415. doi:10.1016/j.prro.2023.03.010
11. Meng Q, Liu X, Wang W, et al. Evaluation of the efficacy of prophylactic extended field irradiation in the concomitant chemoradiotherapy treatment of locally advanced cervical cancer, stage IIIB in the 2018 FIGO classification. Radiat Oncol. 2019;14(1):228. doi:10.1186/s13014-019-1431-9
12. Huang EY, Huang YJ, Chanchien CC, et al. Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix. Radiat Oncol. 2012;7(1):13. doi:10.1186/1748-717X-7-13
13. Sakurai H, Mitsuhashi N, Takahashi M, et al. Analysis of recurrence of squamous cell carcinoma of the uterine cervix after definitive radiation therapy alone: patterns of recurrence, latent periods, and prognosis. Int J Radiat Oncol Biol Phys. 2001;50(5):1136–1144. doi:10.1016/S0360-3016(01)01573-5
14. Sakuragi N, Satoh C, Takeda N, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85(7):1547–1554. doi:10.1002/(SICI)1097-0142(19990401)85:7<1547::AID-CNCR16>3.0.CO;2-2
15. Hong JH, Tsai CS, Lai CH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(1):249–257. doi:10.1016/j.ijrobp.2004.02.044
16. Huang EY, Hsu HC, Sun LM, et al. Prognostic value of pretreatment carcinoembryonic antigen after definitive radiotherapy with or without concurrent chemotherapy for squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2011;81(4):1105–1113. doi:10.1016/j.ijrobp.2010.07.011
17. Hsu HC, Leung SW, Huang EY, et al. Impact of the extent of parametrial involvement in patients with carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1998;40(2):405–410. doi:10.1016/S0360-3016(97)00766-9
18. Wang W, Wang D, Liu X, et al. Risk factors associated with para-aortic lymph node failure after pelvic irradiation in patients with cervical cancer. J Cancer. 2020;11(17):5099–5105. doi:10.7150/jca.45520
19. Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152(1):87–93. doi:10.1016/j.ygyno.2018.10.026
20. Wang YM, Chen YF, Lee PY, Ho MW, Huang EY. Radiation-induced emesis (RIE) in extended-field radiotherapy for gynecological malignancies: dosimetric and non-dosimetric factors. Curr Oncol. 2021;28(5):3602–3609. doi:10.3390/curroncol28050308
21. van de Sande MA, Creutzberg CL, van de Water S, Sharfo AW, Hoogeman MS. Which cervical and endometrial cancer patients will benefit most from intensity-modulated proton therapy? Radiother Oncol. 2016;120(3):397–403. doi:10.1016/j.radonc.2016.06.016
22. Hara JHL, Jutzy JMS, Arya R, et al. Predictors of acute hematologic toxicity in women receiving extended-field chemoradiation for cervical cancer: do known pelvic radiation bone marrow constraints apply? Adv Radiat Oncol. 2022;7(6):100998. doi:10.1016/j.adro.2022.100998
23. Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky ib and iia cervical carcinomas: ten-year treatment results of RTOG 79-20. JAMA. 1995;274(5):387–393. doi:10.1001/jama.1995.03530050035029
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
