Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Comorbidity of Pulmonary Fibrosis and COPD/Emphysema: Research Status, Trends, and Future Directions --------- A Bibliometric Analysis from 2004 to 2023
Authors Fang H , Dong T , Han Z , Li S , Liu M, Liu Y , Yang Q , Fu M, Zhang H
Received 12 July 2023
Accepted for publication 1 September 2023
Published 12 September 2023 Volume 2023:18 Pages 2009—2026
DOI https://doi.org/10.2147/COPD.S426763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Hanyu Fang,1,2,* Tairan Dong,1,* Zhuojun Han,1 Shanlin Li,1 Mingfei Liu,1 Ying Liu,3 Qiwen Yang,1 Min Fu,4 Hongchun Zhang1,2
1Graduate School, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China; 2Department of Traditional Chinese Medicine for Pulmonary Diseases, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China; 3The Second Health and Medical Department, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China; 4Department of Infectious Diseases, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, 100029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongchun Zhang, Department of Traditional Chinese Medicine for Pulmonary Diseases, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China, Tel +86 13701226664, Email [email protected] Min Fu, Department of Infectious Diseases, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, 100029, People’s Republic of China, Tel +86 13366093706, Email [email protected]
Objective: The comorbidity of pulmonary fibrosis and COPD/emphysema has garnered increasing attention. However, no bibliometric analysis of this comorbidity has been conducted thus far. This study aims to perform a bibliometric analysis to explore the current status and cutting-edge trends in the field, and to establish new directions for future research.
Methods: Statistical computing, graphics, and data visualization tools such as VOSviewer, CiteSpace, Biblimatrix, and WPS Office were employed.
Results: We identified a total of 1827 original articles and reviews on the comorbidity of pulmonary fibrosis and COPD/emphysema published between 2004 and 2023. There was an observed increasing trend in publications related to this comorbidity. The United States, Japan, and the United Kingdom were the countries with the highest contributions. Professor Athol Wells and the University of Groningen had the highest h-index and the most articles, respectively. Through cluster analysis of co-cited documents, we identified the top 17 major clusters. Keyword analysis predicted that NF-κB, oxidative stress, physical activity, and air pollution might be hot spots in this field in the future.
Conclusion: This bibliometric analysis demonstrates a continuous increasing trend in literature related to the comorbidity of pulmonary fibrosis and COPD/emphysema. The research hotspots and trends identified in this study provide a reference for in-depth research in this field, aiming to promote the development of the comorbidity of pulmonary fibrosis and COPD/emphysema.
Keywords: COPD, pulmonary fibrosis, bibliometric analysis, VOSviewer, CiteSpace
Introduction
Chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis are prevalent lung diseases that have a significant impact on individuals’ health and mortality rates worldwide. Additionally, these chronic lung conditions impose a substantial economic and clinical burden. Epidemiological investigations estimate the overall prevalence of interstitial lung disease to be between 6.3 and 76.0 cases per 100,000 people.1 The current prevalence of COPD is estimated to range from 5% to 15%.2 Risk factors such as smoking, aging, and various environmental factors are closely associated with the development of COPD and interstitial lung disease. Furthermore, the pathogenesis of these conditions involves factors such as inflammation, oxidative stress, heredity, and genetic molecules. Therefore, it is imperative to advance research on COPD, interstitial lung disease, and their comorbidities. This includes optimizing imaging and pulmonary function tests and enhancing the level of diagnosis and treatment. By doing so, we can significantly improve individuals’ life and health while reducing the economic burden associated with these diseases.
In recent years, there has been a growing recognition of the association between comorbidities of COPD and pulmonary fibrosis/emphysema, particularly regarding pathogenic factors, pathogenesis, as well as clinical diagnosis and treatment. To gain insights into the current state and future trends in the field of comorbidities of pulmonary fibrosis and COPD/emphysema, it is valuable to employ bibliometric analysis. Bibliometrics is a scientific methodology that employs mathematical, statistical, and quantitative techniques to analyze the distribution, structure, quantitative relationships, and patterns of literature in a specific field.3 By conducting bibliometric analysis, we can visually examine and identify research directions, hot areas, and frontier directions. While bibliometric analyses have been conducted in various medical fields, there has been no such study undertaken specifically on the comorbidities of COPD and pulmonary fibrosis/emphysema. Therefore, this study aims to fill this gap by employing bibliometric analysis to identify relevant research published between 2004 and 2023. Through visual examinations, we aim to identify current research hotspots and predict potential future directions of inquiry.
Methods
Paper Retrieval and Data Extraction
In this study, a systematic literature search was conducted in the Web of Science Core Collection (WoSCC) to retrieve published articles from 2004 to 2023. The search strategy employed was TS=(“COPD” OR “chronic obstructive pulmonary disease*” OR “emphysema” OR “pneumonectasia”) AND TS=(“pulmonary fibrosis”) to gather articles and reviews pertaining to the comorbidity of pulmonary fibrosis and COPD/emphysema. The retrieval process was completed by April 28, 2023. Only English articles and reviews were included in the analysis, while other publication types such as meeting abstracts, editorial materials, letters, proceeding papers, early access articles, book chapters, corrections, and retracted publications were excluded. Duplicate publications were identified and removed using Citespace. In total, 1290 articles and 537 reviews were collected and analyzed (Figure 1).
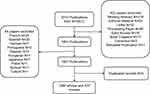 |
Figure 1 Screening flow chart. |
Bibliometric Analysis
Bibliometric indicators, including the number of publications (NP) and total citations (TC), were analyzed from the WoSCC database to assess the quality of the publications. In certain cases, the H-index was used to evaluate the scholarly achievements of regions or countries, journals, institutions, and individuals. For statistical computing, graphics, and data visualization, we employed various tools such as VOSviewer, CiteSpace, Biblimatrix, and WPS Office. VOSviewer and CiteSpace were utilized to extract and analyze potential information from the collected data, while Scimago Graphica was employed to optimize the visual graphs. Biblimatrix, which is based on R, and WPS Office were used to plot the statistical data.
Results
Annual Publication Trends
Using our search strategy, a total of 2313 papers and reviews published between 2004 and 2023 were identified, with a cumulative total of 77,394 citations. The annual publication rate exhibited fluctuations, reflecting the pace and progress of research in the field as well as the level of interest it has generated. The overall publication count can be accurately modeled by a quadratic function (R2=0.997), indicating a consistent upward trend (Figure 2). It is anticipated that this trend will continue to accelerate in the future.
 |
Figure 2 Trend of publications in comorbidity of pulmonary fibrosis and COPD/emphysema publications from 2004 to 2023. |
Country Analysis
The analysis of publications from different countries provides valuable insights into the importance placed on a research area by a country and the degree of influence it holds in that area. Table 1 displays the top ten countries receiving the most total citations (TC), which can indicate a country’s influence and publication quality in the field of comorbidity of pulmonary fibrosis and COPD/emphysema. The United States obtained the most citations (24,513) and publications (545), followed by the Japan and United Kingdom with 5662 and 4945 total citations, respectively. However, the number of citations does not always reflect the quality of publications. Mexico, United Kingdom, France produced high-quality publications with average article citations of 91.2, 48.5, and 47.8, indicating that authors from these countries focused on the hot topics of this area and produced quality papers. Conversely, China ranked third in the number of publications, but its average article citations were the lowest among the top 10 TC countries, suggesting a need to improve the quality of papers produced.
 |
Table 1 Top 10 Countries Rated by Total Citation |
We utilized Vosviewer to generate cooperation and clusters between countries of all authors, and Scimago Graphica to create a geographical map (Figure 3A). Among the different cooperative network clusters, inter-country cooperation was generally low, with only the United States exhibiting adequate inter-country cooperation in this area. This finding suggests that countries should further deepen their cooperation to promote the development of the discipline. The trends in the number of publications from each country over time are displayed in Figure 3B. The United States’ publication rate has stabilized after peaking in 2015, while China’s publication rate has been consistently rising. The publication rates of other countries have either remained stable or have shown an upward trend.
 |
Figure 3 (A) Inter-country cooperation network map. (B) Trend of publication volume by each country. |
Author and Institution Analysis
The H-index is a metric that reflects the comprehensive influence and contribution of an author in a field. Table 2 presents the top 10 authors with the highest H-index. Athol Wells from Imperial College London achieved the highest H-index with 30 publications in this area, followed by Ivan O. Rosas and David Hansell. Bartolome Celli obtained the highest number of total citations (4238). The co-occurrence network of authors and burst chart were explored using Citespace, as shown in Figure 4A and B, respectively. Authors with a greater number of citations and publications tend to collaborate more frequently. Additionally, Figure 4C visualizes the earliest publication time and publication volume of institutions, generated by Scimago Graphica. Table 3 lists the top 10 institutions with the largest number of publications. The University of Pittsburgh ranked as the most productive institution with 78 articles, followed by Brigham & Women’s Hospital with 75 articles, and Mayo Clinic with 65 articles.
 |
Table 2 Top 10 Authors Rated by h-Index |
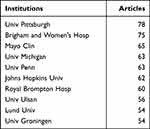 |
Table 3 Top 10 Most Publication Institutions |
Bibliometric Analysis of Journal
The VOSviewer software was utilized to identify the most prominent and productive journals in the field of comorbidity between pulmonary fibrosis and COPD/emphysema. A total of 3300 publications were found in 581 academic journals, as presented in Table 4. RESPIRATORY MEDICINE, with an impact factor (IF) of 4.582 according to Clarivate’s Journal Citation Reports, published the highest number of papers, totaling 54. CHEST, with an IF of 10.262, ranked second and published 88 papers. BMC Pulmonary Medicine was found to be the most cited journal. The journal co-occurrence map (Figure 4D) indicates that RESPIRATORY MEDICINE and CHEST are the two largest nodes.
 |
Table 4 Top 10 Most Publication Journal |
Reference Analysis
The co-citation analysis of references plays a pivotal role in CiteSpace, facilitating the extraction of cluster labels based on the cited documents.4 These cited and citing documents serve as a representation of the forefront of research and knowledge base.5 Consequently, the analysis of typical clusters enables us to gain insights into the fundamental information in this research field, as well as its evolution and development. In our investigation on the comorbidity of pulmonary fibrosis and COPD/emphysema, we employed a scaling factor of k = 25 and utilized the g-index to identify homogeneous groups of highly cited documents pertinent to this field. Furthermore, we thoroughly examined and elucidated the co-citation relationships among references.
Cluster analysis offers a means to delve into the knowledge structure and research interests’ boundaries. By conducting cluster analysis on co-cited documents, we have effectively summarized the research areas within this field and explored the prevailing trends and research directions.6 From the total of 77,401 references cited in the articles, we extracted 72 distinct clusters. Subsequently, we identified the 17 major clusters based on cluster labels derived from the titles of the cited articles using the Log-likelihood ratio (LLR) and mutual information (MI) algorithm. These clusters include #0 pulmonary fibrosis, #1 cardiopulmonary exercise testing, #2 cellular senescence, #3 cigarette smoke, #4 smoking-related interstitial lung disease, #5 lung transplantation, #6 possible biomarker, #7 lung development homeostasis, #8 distinct horn, #9 thiol proteins redox modulation, #10 current status, #11 mast cell, #12 matrix metalloproteinases, #13 lung transplantation, #14 chronic lung injury, #15 stem cell treatment, and #17 elevated plasma (Figure 5A). The modularity Q value of 0.7676 and the weighted mean silhouette of each cluster of 0.9026 indicate reasonable cluster quality. Early clusters are depicted with purple contours, such as #4 and #5, while recent cluster labels are represented with yellow contours, notably #14 (Figure 5B).
 |
Figure 5 (A) Co-citation analysis and clustering network analysis of references. (B) Burst detection of co-citations. |
The size of each node within the network corresponds to the betweenness centrality, which measures the likelihood of a node lying on the shortest path between any two nodes in the network. Nodes with high betweenness centrality play a crucial role as mediators between different clusters or within clusters, facilitating the exchange of research topics and paradigms.7,8 Table 5 presents the top ten items ranked by centrality. Topping the list in terms of centrality is Alder JK (2011) in Cluster #8, with a centrality score of 0.31. The second-ranked item is Lettieri CJ (2006) in Cluster #4, with a centrality score of 0.26. Following closely, the third-ranked item is Demedts M (2005) in Cluster #5, with a centrality score of 0.21. Gauldie Jack (2006) in Cluster #4 secures the fourth spot with a centrality score of 0.15. Hansell DM (2008) in Cluster #0 claims the fifth position with a centrality score of 0.14. Additionally, Alder JK (2015) in Cluster #2 ranks sixth with a centrality score of 0.13. Notably, Cottin V (2005) in Cluster #4 and Akagi T (2009) in Cluster #0 both secure the seventh position with a centrality score of 0.13. Furthermore, Cottin V (2009) in Cluster #0 and Cottin V (2010) in Cluster #0 occupy the ninth and tenth positions, respectively, with centrality scores of 0.12 and 0.11. Notably, four of the top ten references with the highest centrality are found in Cluster #0, while three are present in Cluster #4, indicating the influential nature of these two clusters.
 |
Table 5 Top 10 Most Centrality References |
Keywords
VOSviewer was employed to conduct keyword co-occurrence and clustering analysis, resulting in the extraction of a total of 6913 keywords (Figure 6A and B). Among these, 7 keywords appeared over 200 times, while 20 keywords appeared over 100 times. The keyword density plot enabled the identification of high-frequency co-occurring words, shedding light on research hotspots (Figure 6C). Excluding search terms, the most significant terms were “survival”, “asthma”, and “diagnosis.”
Clustering analysis was then utilized to reveal the knowledge structure within the research area.7 By assessing the strength of connections between co-occurring keywords, the network was divided into four clusters, with each cluster demonstrating clear homogeneity among its constituent terms. Cluster 1 (represented by the color red) emerged as the most important, encompassing 89 projects related to inflammation, asthma, gene expression, oxidative stress, and pathogenesis. The second cluster (green) comprised 61 projects focusing on the epidemiology of comorbidity between pulmonary fibrosis and COPD/emphysema, exploring aspects such as survival, diagnosis, mortality, and lung transplantation. Cluster 3 (blue) centered around the diagnosis of comorbidity between pulmonary fibrosis and COPD/emphysema, featuring 34 projects involving computed tomography, classification, pneumonia, alveolitis, and respiratory bronchiolitis. Lastly, Cluster 4 (yellow) primarily addressed lung cancer, encompassing 8 projects related to associations, biomarkers, genome-wide associations, mac5b promoter, and mutations.
To showcase the evolutionary process across different clusters, a timeline map of high-frequency keywords was generated using CiteSpace.19 Each keyword was positioned according to the year of its initial appearance, with the color of the link indicating the simultaneous appearance of the two keywords. Keyword bursts were employed to detect the frequency and magnitude of keyword occurrences, providing insights into the temporal relationship between clusters. Through this keyword timeline map and burst analysis, the temporal characteristics of clustering were depicted, uncovering hotspots and frontiers within the research area. In total, 25 keywords representing the research field appropriately in terms of burst intensity, duration, and timing were identified through CiteSpace’s burst analysis of keywords and timeline analysis (Figures 7A and 6D). Additionally, a trend topic analysis generated by Biblimatrix (Figure 7B) was conducted.
 |
Figure 7 (A) Timeline of keywords with different clusters. (B) Trend topics generated by bibliometrix in R. |
Discussion
In this pioneering study, we conducted a comprehensive analysis of the current status and development trends in the comorbidity of pulmonary fibrosis and COPD/emphysema using bibliometric analysis. Our search of the WoSCC database yielded a total of 2313 papers and reviews published between 2004 and April 28, 2023. To gain insights into the spatial and temporal distribution, author contributions, and journal quality of these articles, we utilized CiteSpace and VOSviewer. Additionally, we performed burst hotspot analysis, cluster analysis, and keyword analysis to identify the current areas of research focus and frontiers in the comorbidity of pulmonary fibrosis and COPD/emphysema. The analysis of annual trends in published publications revealed a dynamic change, with 18 annual publications in 2004 and a significant increase to 201 publications in 2021. Although there were decreases in the number of publications in 2006, 2019, and 2022, the overall trend indicates a rise in the number of publications. It is worth noting that the decreases in publication numbers during these years may be associated with the COVID-19 pandemic.
Through an analysis of countries and institutions, it is evident that the United States, Japan, the United Kingdom, Germany, and Italy are the top five countries in terms of citation frequency, indicating their significant influence in this field. Mexico, on the other hand, surpasses other countries in terms of average number of citations, suggesting the high quality of Mexican papers in this field. While China ranks 6th in terms of citations and 3rd in total publications, it falls within the top 10 in average citations, underscoring the need to enhance the quality of Chinese papers. Furthermore, the top 10 countries are primarily concentrated in Europe and the North America, with only the United States demonstrating sufficient national cooperation in this field. Therefore, it is recommended that countries deepen their collaborations to foster the advancement of disciplines. In terms of publication trends, the United States experienced a peak in publication rate in 2015, which then stabilized at a significantly higher level compared to other countries. Conversely, China’s publication rate has continued to rise, indicating its considerable potential for development in this field.
The analysis of the top ten authors of the H-index showed that Athol Wells from Imperial College London topped the list with an H-index of 23. He is a consultant in respiratory medicine at Royal Brompton Hospital and clinical and academic lead for the interstitial lung disease unit at Royal Brompton Hospital. His research focuses on lung fibrosis, associated autoimmune disorders, idiopathic pulmonary fibrosis, interstitial lung disease and all forms of sarcoidosis. Ivan O Rosas from Baylor College of Medicine ranked second with an H-index of 18, whose research focuses on monocytes and transforming growth factors in idiopathic pulmonary fibrosis. David Hansell from Imperial College London ranked third with an H-index of 17. He is emeritus Professor of thoracic imaging at the National Heart and Lung Institute at Imperial College London. His primary research focus is on the application of high-resolution computed tomography to characterize and quantify diffuse lung disease. They both concentrated on and researched interstitial lung disease. In terms of the most significant citation burst, Cottin Vincent’s was the earliest (2011), while Athol Wells had the longest burst (2013–2018). Maria Kokosi and Jacob Joseph experienced the strongest burst. Among them, Cho Michael H have great potential for a burst, which has persisted from four years ago until now. Co-occurrence networks among authors were analyzed, revealing that those with higher citation and publication rates exhibited greater proclivity towards active collaboration. Athol Wells emerged as a central hub within the network, likely due to its high H-index and strong citation burst. Based on the above analysis, it can be inferred that research pertaining to interstitial lung disease is likely to become a prominent area of focus in the future.
In terms of the number of publications of institution, University of Pittsburgh ranked first with 78 articles, Brigham & Women’s Hospital ranked second with 75 articles and Mayo Clinic ranked third with 65 articles. University of Pittsburgh’s researched involved basic science, including genetic and molecular studies, and therapeutic applications of pulmonary fibrosis. It paid attention to the pathology of COPD and novel therapeutic targets as well. Brigham & Women’s Hospital focused on the cellular and molecular mechanisms of fibrosis, some treatment options and their efficacy. And the global burden of disease was involved, too. The analysis of the change of publication frequency over time shown in Figure 4B found that the majority of top 10 institutions had high frequency in early 2010s, while more new institutions have emerged with increasing frequency from late 2010s. In recent years, University of Technology Sydney, Qingdao University and Charles Darwin University have shown a high publication frequency.
From the analysis of journal, RESPIRATORY MEDICINE (IF=4.582) had the most published papers and the highest total link strength which indicating its significant role in this research area. CHEST (IF=10.262) had published 53 articles ranked second while its citations was much higher than RESPIRATORY MEDICINE, suggesting a higher quality of publications. Additionally, BMC PULMONARY MEDICINE was the most cited journal with the citation of 3834, followed by MOLECULAR BIOLOGY with the citation of 2239, indicating they had a great influence in the area. From the analysis of the journal co-occurrence map, RESPIRATORY MEDICINE and EUROPEAN RESPIRATORY JOURNAL were two journals with the highest total link strength, suggesting their contribution to collaborations among institutions in this area. Besides, respiratory-related established journals had played a vital role before 2016, while some new cancer-related journals have shown an increasing publication frequency nowadays. Therefore, respiratory cancer may be a new research hotspot.
Based on the Log-likelihood ratio (LLR) and mutual information (MI) algorithm, we identified 17 major clusters from a total of 77,401 cited references in the articles. These clusters were extracted from their corresponding article titles and represent a subset of the original 72 clusters, such as #0 pulmonary fibrosis, #1 cardiopulmonary exercise testing, #2 cellular senescence, #3 cigarette smoke, #4 smoking-related interstitial lung disease, #5 lung transplantation, #6 possible biomarker, #7 lung development homeostasis, #8 distinct horn, #9 thiol proteins redox modulation, #10 current status, # 11 mast cell, # 12 matrix metalloproteinases, #13 lung transplantation, #14 chronic lung injury, #15 stem cell treatment, #17 elevated plasma. Most of the clusters were centered around research on mechanisms and clinical classification of COPD and pulmonary fibrosis, as well as treatment methods and disease prognosis. However, the focus of clustering varies.
The following clusters delve into the clinical classification, diagnosis, and treatment recommendations of respiratory diseases, specifically idiopathic fibrosis. Cluster #0 explores clinical conditions associated with pulmonary fibrosis, including idiopathic interstitial pneumonia, idiopathic fibrosis, and pulmonary fibrosis combined with emphysema. This cluster establishes more precise classification and diagnostic criteria for these conditions, distinguishing them from one another, and emphasizes the necessity of actively pursuing accurate diagnosis and treatment strategies in clinical research. The implications of these findings are significant, as they can greatly impact subsequent diagnoses, treatments, and even prevention efforts.20–23 Cluster #1 primarily focuses on the latest clinical research concerning idiopathic pulmonary fibrosis, encompassing clinical classification, diagnostic criteria, cutting-edge methodologies, the influence of comorbidities on disease progression, and the effectiveness of various drug treatment modalities. Advancements in these areas are crucial for ongoing patient care and improving prognoses.24–27
The following clusters are dedicated to studying the pathogenesis of related diseases, including idiopathic fibrosis, by investigating genes, signaling pathways, cellular mechanisms, and more. Cluster #2 focuses on elucidating the mechanisms of cellular senescence in idiopathic fibrosis. Lung senescence, characterized by an increased number of senescent cells, is believed to directly contribute to various age-related respiratory diseases. Targeting cellular senescence through therapeutic interventions could potentially delay or even reverse age-related respiratory diseases. By exploring the correlation between cellular senescence mechanisms and respiratory diseases like idiopathic fibrosis, this research significantly contributes to future clinical drug development and other therapeutic investigations.28–30 Cluster #3 primarily engages in fundamental research on the cellular and molecular mechanisms underlying pulmonary fibrosis. It investigates related genes, regulatory factors, and cellular pathways to gain a deeper understanding of the pathogenic mechanisms of this disease. These findings serve as a foundation for the development of more effective treatments.31–34 Cluster #7 delves into the intricate cellular and molecular mechanisms underlying pulmonary fibrosis. It focuses on tissue remodeling, structural alterations in fibrotic lesions, and aberrant cell populations. These insights provide novel perspectives for developing targeted therapeutics.35–38 Cluster #8 explores the cellular and molecular mechanisms of lung tissue and their implications for treatments. It demonstrates that age-related lung diseases share common mechanisms, such as telomere shortening, abnormal tissue remodeling, and functional damage. This understanding offers more preventative and therapeutic options for clinical practice, including cell therapy.9,39–41 Cluster #9 investigates the crucial role of Redox regulatory proteins, oxidative stress, and inflammatory response in the pathogenesis of pulmonary diseases, including pulmonary fibrosis. A comprehensive understanding of these mechanisms underlying lung injury may lead to effective intervention strategies to halt the progression of parenchymal lung disease and guide clinical management.42–45 Cluster #11 presents novel clinical treatment strategies based on fundamental research on genes, cellular mechanisms, and pathways associated with chronic lung diseases, including pulmonary fibrosis.46–49 Cluster #12 investigates alterations in proteases and transformation factors associated with respiratory diseases, such as COPD and pulmonary fibrosis, and identifies novel therapeutic targets for treatment.50–53 Cluster #15 focuses on exploring the clinical research and mechanisms of stem cells in chronic lung diseases, such as idiopathic fibrosis and COPD, highlighting their immense therapeutic potential in this field. This offers a novel approach to clinical treatment that complements conventional drug therapies.54–56
The following clusters focus on the clinical diagnosis and treatment of respiratory diseases, particularly idiopathic fibrosis. Cluster #5 centers around lung transplantation treatment for end-stage parenchymal or pulmonary vascular diseases. It emphasizes the importance of disease classification, diagnostic criteria, and prognostic performance to provide more precise treatment options.11,57–59 Cluster #6 showcases the efficacy of drug therapy in treating idiopathic fibrosis through clinical trials. It highlights the significance of monitoring disease progression and managing potential complications during treatment.60–63 Cluster #13 delves into the advancements and current status of lung transplantation as a treatment for respiratory diseases. It also discusses the various guidelines that dictate the requirements for this procedure. The research emphasizes the need for rigorous evaluation standards to enhance surgical outcomes and improve patient survival rates.64–67 Cluster #17 primarily focuses on mechanistic studies and explores the correlation between elevated plasma levels post-lung transplantation. This research facilitates timely assessment and early prediction in future research endeavors.68–71
The following clusters discuss the pathogenic factors and provide a current status analysis of pulmonary fibrosis and related diseases. Cluster #4 focuses on the investigation of smoking as a significant risk factor for interstitial lung disease. It proposes the assessment of COPD prognosis in combination with pulmonary fibrosis and emphysema. These findings have substantial implications for disease prevention, raising public awareness, and evaluating treatment prognosis.15,72,73 Cluster #10 reveals that chronic respiratory diseases, including COPD, remain the leading causes of death and disability globally. The etiology of these diseases varies significantly across regions and genders, necessitating ongoing attention. Studies on extracellular vesicles and PMN-derived exosomes suggest their potential as biomarkers and therapeutics.74–77 Cluster #14 establishes a strong correlation between idiopathic fibrosis and the prevalence of lung cancer based on epidemiological data.78–81 Other lung injuries also impose significant economic and clinical burdens. Clinical examinations, such as low-dose CT scans, prove effective in screening for these diseases, enabling timely clinical interventions and treatments.
Keyword co-occurrence analysis, cluster analysis, outbreak intensity analysis and time plot analysis can be utilized to explore research directions, hotspots and development lines in this field. The most frequent keywords in this field were “survival”, “asthma” and “diagnosis” based on keyword co-occurrence. The co-occurrence network was divided into four main clusters according to connection strength: #1 inflammation, asthma, gene expression, oxidative stress and pathogenesis, #2 epidemiology, #3 diagnosis of comorbidities, and #4 lung cancer. Currently, the primary research focuses in pulmonary fibrosis and emphysema are centered on disease pathogenesis and epidemiological analysis, which will enhance our comprehension of these conditions. Furthermore, improved diagnosis of comorbidities and understanding the correlation between pulmonary fibrosis and cancer will facilitate better treatment and prevention strategies for these diseases.
Based on keyword outbreak mapping analysis, physical activity, air pollution, and oxidative stress have shown notable trends over the last three years. Notably, keywords like physical activity and air pollution exhibit a pronounced upward trend that has garnered increased research focus aimed at preventing associated respiratory illnesses. Before the onset of the disease, attention should be paid to the prevention of causes and the enhancement of physical fitness. The investigation of these keywords is significantly valuable in mitigating the incidence of associated pulmonary ailments and alleviating the medical and economic burden stemming from disease treatment. The exploration of NF-κB and oxidative stress exhibits a promising trajectory for elucidating the mechanisms underlying COPD and interstitial lung disease. The proliferation of such keywords indicates the shift of related lung diseases towards the molecular stage, which is a rapidly growing area for future research in this field with promising prospects.
Through the analysis of the keyword timeline diagram, objective tracking of research hotspots and trends at different time points can be achieved. Prior to 2009, the field’s research hotspots were primarily focused on clinical symptom mechanisms. From 2009 to 2017, attention was directed towards CT and computed tomography for pulmonary fibrosis screening. At the same time, the research on the mechanism of the disease is also more in-depth and more detailed. Since 2017, the research on disease-related mechanisms has gradually deepened from the cellular level to the molecular and genetic level, and disease-related complications and prognosis have gradually attracted more and more attention. By analyzing the burst intensity of keywords at different time points, early lung transplantation emerges as the most explosive research content in this field. In recent years, increasing attention has been paid to the molecular mechanism of NF-κB and oxidative stress, which have become the focus of mechanism research. Overall, respiratory diseases and disorders remain a hot topic in recent years. Therefore, it is reasonable to speculate that in future research directions, attention should be paid to the association of mechanisms related to pulmonary fibrosis and COPD with other respiratory diseases. This will benefit the clinical diagnosis and treatment of these diseases.
We have conducted a comprehensive literature review on the correlation between chronic respiratory diseases, such as COPD and pulmonary fibrosis, and air pollution, and have drawn several conclusions. Through the examination of relevant literature on air pollution, it has been discovered that prolonged exposure to atmospheric pollutants may increase the susceptibility to respiratory illnesses such as IPF, COPD, and lung cancer. These findings serve as a reminder that we must not only be mindful of the impact of industrial air pollution but also take into account everyday sources of air contamination like kitchen fumes and vehicular exhaust. Air pollution not only increases the incidence of respiratory diseases, but also correlates with poor prognosis and heightened mortality rates. Therefore, future research should focus on elucidating the mechanisms underlying air pollution-induced illnesses, while patients must be reminded to take active interventions in clinical practice. We have primarily focused on NF-κB and oxidative stress in our literature review of the pre-correlation mechanism, from which we have drawn several conclusions. Enhanced expression of cytokines and growth factors in COPD and pulmonary fibrosis has been demonstrated to activate the NF-κB signaling pathway, thereby promoting disease onset and progression. This discovery underscores the importance of signaling pathways in health care, and it has been demonstrated that pharmacological interventions targeting these mechanisms can effectively ameliorate excessive inflammation and pulmonary fibrosis.82 This suggests that future research should prioritize investigating relevant signaling pathways in order to facilitate the development of drugs that can more effectively meet clinical needs. Relevant research has indicated that oxidative stress primarily affects patients’ related structures and functions of lung through the oxidation of proteins, activation of proteases, and reduced sensitivity to calcium. The implication of oxidative stress in the pathogenesis of this disease implies that antioxidant therapy may be a viable treatment option. Although research in this area is limited, it holds great potential for future investigation and clinical application. We conducted a comprehensive literature review on physical activity and derived several pivotal conclusions. Studies suggest that the exercise mechanism in COPD diaphragm may involve enhancing antioxidant capacity, reducing oxidase activity and improving mitochondrial function with regards to oxidative stress.83 Physical inactivity is a common occurrence among individuals with COPD and has been associated with unfavorable outcomes. Therefore, physical exercise plays a pivotal role in enhancing and preventing COPD, pulmonary fibrosis, and other related ailments. However, the significance of physical activity is often underestimated. Further endeavors are required to devise long-term strategies for sustaining improvements or averting declines in physical activity levels. According to modern medical research, COPD with pulmonary fibrosis is strongly associated with smoking, epithelial-mesenchymal transition, airway inflammation, genetic susceptibility, occupational exposure, and other factors.15,78 Airway inflammation is the main pathological process leading to COPD, and smoking and other causes cause large numbers of inflammatory cells to converge in the airways. Under the action of a variety of inflammatory factors, a large number of inflammatory mediators, such as interleukin-6, interleukin-8, C-reactive protein, TNF-α, TGF-β, etc., are released, which cause damage and repair reactions in lung parenchyma, lung interstitium and lung vessels. Traditional Chinese medicine also has a corresponding understanding of COPD, which is complicated by pulmonary fibrosis. Based on the theory of traditional Chinese medicine (TCM), a variety of pathogenesis is proposed, mainly based on root deficiency and branch excess. In addition, various diseases and syndromes have been proposed based on TCM theory. Different treatments are given according to the disease. In terms of treatment options, a variety of approaches can be chosen. Traditional Chinese medicine decoction is the principal treatment, and appropriate prescriptions are selected according to the different diseases. Additional TCM treatments are also available. For example, the combination of moxibustion and traditional Chinese medicine decoction, thread embedding and TCM application therapy can also be used.84 TCM features personalized treatment at diagnosis and has the advantage of less trauma during treatment. However, there is a lack of basic research on the treatment of COPD with traditional Chinese medicine for pulmonary fibrosis.85 Traditional Chinese medicine is an important part of complementary and alternative medicine. At present, there are some deficiencies in the clinical research or basic research of traditional Chinese medicine in the treatment of the disease, such as the course of treatment, curative effect, side effects, etc., but we believe that this will also become the next breakthrough point of traditional Chinese medicine research. Therefore, TCM treatment has great potential in the future research process of this disease.
Conclusion
Bibliometric analysis using CiteSpace and VOSviewer software was employed to gain a comprehensive understanding of the research progress, hotspots, and future trends in comorbidity of pulmonary fibrosis and COPD/emphysema over the past 19 years. The analysis revealed that there is an urgent need for enhanced cooperation and exchange among countries, institutions, and authors. This objective and quantitative approach provides crucial insights for researchers seeking to comprehend the structural and temporal dynamics of the field. By leveraging these findings, future research can focus on disease mechanisms, as well as diagnosis and treatment, in order to address the remaining challenges in the field.
Funding
This study is supported by National Natural Science Foundation of China Project: Research on the Repair Mechanism of Respiratory Injury in COPD Mice by Traditional Chinese Medicine for Strengthening the Body, Strengthening the Health, and Regulating the Skin and Hair of the Lung (82074367); National Natural Science Foundation Project: Effect of Bufei Granule on Improving the Condition of Airway Inflammation-Mucous Hyperplasia in COPD by Controlling miRNA-TLR4 Signaling Network (81703858).
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Olson AL, Patnaik P, Hartmann N, Bohn RL, Garry EM, Wallace L. Prevalence and incidence of chronic fibrosing interstitial lung diseases with a progressive phenotype in the United States estimated in a large claims database analysis. Adv Ther. 2021;38(7):4100–4114. doi:10.1007/s12325-021-01786-8
2. Global Initiative for Chronic Obstructive Lung Disease. 2020 global strategy for prevention, diagnosis and man- agement of COPD[EB/OL]; 2021. Available from: https://gold-copd.org/gold-report.
3. Yong-Mi K, Delen D. Medical informatics research trend analysis: a text mining approach. Health Informatics J. 2018;24(4):432–452. doi:10.1177/1460458216678443
4. Synnestvedt MB, Chen C, Holmes JH. Citespace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724–728. doi:10.1002/asi.20317
5. Chen CM, Ibekwe-SanJuan F, Hou JH. The structure and dynamics of cocitation clusters: a multiple-perspective cocitation analysis. J Am Soc Inf Sci Technol. 2010;61(7):1386–1409. doi:10.1002/asi.21309
6. Qin Y, Zhang Q, Liu Y. Analysis of knowledge bases and research focuses of cerebral ischemia-reperfusion from the perspective of mapping knowledge domain. Brain Res Bull. 2020;156:15–24. doi:10.1016/j.brainresbull.2019.12.004
7. Freeman LC. Centrality in social networks: conceptual clarification. SocNetw. 1979;1(3):215–239. doi:10.1016/0378-8733(78)90021-728
8. Brandes U. A faster algorithm for betweenness centrality. J Math Sociol. 2001;25(2):163–177. doi:10.1080/0022250X.2001.9990249
9. Alder JK, Guo N, Kembou F, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184(8):904–912. PMID: 21757622; PMCID: PMC3208661. doi:10.1164/rccm.201103-0520OC
10. Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129(3):746–752. PMID: 16537877. doi:10.1378/chest.129.3.746
11. Demedts M, Behr J, Buhl R, et al.; IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. PMID: 16306520. doi:10.1056/NEJMoa042976
12. Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc. 2006;3(8):696–702. PMID: 17065376; PMCID: PMC2647655. doi:10.1513/pats.200605-125SF
13. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
14. Alder JK, Hanumanthu VS, Strong MA, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci USA. 2018;115(10):E2358–E2365. doi:10.1073/pnas.1720427115
15. Cottin V, Nunes H, Brillet PY, et al. Groupe d’Etude et de Recherche sur les Maladies Orphelines Pulmonaires (GERM O P). Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–593. PMID: 16204587. doi:10.1183/09031936.05.00021005
16. Akagi T, Matsumoto T, Harada T, et al. Coexistent emphysema delays the decrease of vital capacity in idiopathic pulmonary fibrosis. Respir Med. 2009;103(8):1209–1215. doi:10.1016/j.rmed.2009.02.001
17. Cottin V, Cordier JF. The syndrome of combined pulmonary fibrosis and emphysema. Chest. 2009;136(1):1–2. doi:10.1378/chest.09-0538
18. Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–111. doi:10.1183/09031936.00038709
19. Chen CM. Science mapping: a systematic review of the literature. J Data Inf Sci. 2017;2(2):1–40. doi:10.1515/jdis-2017-0006
20. Lin H, Jiang S. Combined pulmonary fibrosis and emphysema (CPFE): an entity different from emphysema or pulmonary fibrosis alone. J Thorac Dis. 2015;7(4):767–779. PMID: 25973246; PMCID: PMC4419325. doi:10.3978/j.issn.2072-1439.2015.04.17
21. Ryerson CJ, Hartman T, Elicker BM, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest. 2013;144(1):234–240. PMID: 23370641. doi:10.1378/chest.12-2403
22. Raghu G, Collard HR, Egan JJ, et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. PMID: 21471066; PMCID: PMC5450933. doi:10.1164/rccm.2009-040GL
23. Travis WD, Costabel U, Hansell DM, et al; ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. PMID: 24032382; PMCID: PMC5803655. doi:10.1164/rccm.201308-1483ST
24. Valenzuela C, Torrisi SE, Kahn N, Quaresma M, Stowasser S, Kreuter M. Ongoing challenges in pulmonary fibrosis and insights from the nintedanib clinical programme. Respir Res. 2020;21(1):7. PMID: 31906942; PMCID: PMC6945404. doi:10.1186/s12931-019-1269-6
25. Jacob J, Bartholmai BJ, Rajagopalan S, et al. Functional and prognostic effects when emphysema complicates idiopathic pulmonary fibrosis. Eur Respir J. 2017;50(1):1700379. PMID: 28679612. doi:10.1183/13993003.00379-2017
26. Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–153. PMID: 29154106. doi:10.1016/S2213-2600(17)30433-2
27. Raghu G, Remy-Jardin M, Myers JL, et al; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. PMID: 30168753. doi:10.1164/rccm.201807-1255ST
28. Birch J, Barnes PJ, Passos JF. Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol Ther. 2018;183:34–49. PMID: 28987319. doi:10.1016/j.pharmthera.2017.10.005
29. Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. PMID: 28365056. doi:10.1016/S0140-6736(17)30866-8
30. Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. PMID: 28230051; PMCID: PMC5331226. doi:10.1038/ncomms14532
31. Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208(7):1339–1350. PMID: 21727191; PMCID: PMC3136685. doi:10.1084/jem.20110551
32. King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. PMID: 21719092. doi:10.1016/S0140-6736(11)60052-4
33. Meiners S, Eickelberg O, Königshoff M. Hallmarks of the ageing lung. Eur Respir J. 2015;45(3):807–827. PMID: 25657021. doi:10.1183/09031936.00186914
34. Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. PMID: 23583980; PMCID: PMC3677861. doi:10.1038/ng.2609
35. Liu G, Philp AM, Corte T, et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther. 2021;225:107839. PMID: 33774068. doi:10.1016/j.pharmthera.2021.107839
36. Reyfman PA, Walter JM, Joshi N, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(12):1517–1536. PMID: 30554520; PMCID: PMC6580683. doi:10.1164/rccm.201712-2410OC
37. Adams TS, Schupp JC, Poli S, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1983. PMID: 32832599; PMCID: PMC7439502. doi:10.1126/sciadv.aba1983
38. Hogan BL, Barkauskas CE, Chapman HA, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–138. PMID: 25105578; PMCID: PMC4212493. doi:10.1016/j.stem.2014.07.012
39. Huleihel L, Levine M, Rojas M. The potential of cell-based therapy in lung diseases. Expert Opin Biol Ther. 2013;13(10):1429–1440. PMID: 23984902. doi:10.1517/14712598.2013.833603
40. Chilosi M, Poletti V, Rossi A. The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res. 2012;13(1):3. PMID: 22235752; PMCID: PMC3282644. doi:10.1186/1465-9921-13-3
41. Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. PMID: 22586007. doi:10.7326/0003-4819-156-10-201205150-00004
42. Kinnula VL, Vuorinen K, Ilumets H, Rytilä P, Myllärniemi M. Thiol proteins, redox modulation and parenchymal lung disease. Curr Med Chem. 2007;14(2):213–222. PMID: 17266580. doi:10.2174/092986707779313345
43. Selman M, King TE, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136–151. PMID: 11177318. doi:10.7326/0003-4819-134-2-200101160-00015
44. Rangasamy T, Cho CY, Thimmulappa RK, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–1259. PMID: 15520857; PMCID: PMC524225. doi:10.1172/JCI21146
45. Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28(5):555–562. PMID: 12707011. doi:10.1165/rcmb.2002-0090OC
46. Baarsma HA, Königshoff M. ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax. 2017;72(8):746–759. PMID: 28416592; PMCID: PMC5537530. doi:10.1136/thoraxjnl-2016-209753
47. Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. PMID: 23921127; PMCID: PMC3696553. doi:10.1172/JCI68782
48. Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108(52):E1475–83. PMID: 22123957; PMCID: PMC3248478. doi:10.1073/pnas.1117988108
49. Parker MW, Rossi D, Peterson M, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124(4):1622–1635. PMID: 24590289; PMCID: PMC3971953. doi:10.1172/JCI71386
50. Bonniaud P, Kolb M, Galt T, et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173(3):2099–2108. PMID: 15265946. doi:10.4049/jimmunol.173.3.2099
51. Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009;13(3):287–295. PMID: 19236151. doi:10.1517/14728220902751632
52. Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L715–31. PMID: 20363851; PMCID: PMC2886606. doi:10.1152/ajplung.00361.2009
53. Demedts IK, Morel-Montero A, Lebecque S, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61(3):196–201. PMID: 16308335; PMCID: PMC2080750. doi:10.1136/thx.2005.042432
54. Tzouvelekis A, Ntolios P, Bouros D. Stem cell treatment for chronic lung diseases. Respiration. 2013;85(3):179–192. PMID: 23364286. doi:10.1159/000346525
55. Katsha AM, Ohkouchi S, Xin H, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19(1):196–203. PMID: 20842104; PMCID: PMC3017437. doi:10.1038/mt.2010.192
56. Aguilar S, Scotton CJ, McNulty K, et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One. 2009;4(11):e8013. PMID: 19956603; PMCID: PMC2779453. doi:10.1371/journal.pone.0008013
57. Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(9):1084–1090. PMID: 12917227. doi:10.1164/rccm.200302-219OC
58. Lynch JP, Saggar R, Weigt SS, Ross DJ, Belperio JA. Overview of lung transplantation and criteria for selection of candidates. Semin Respir Crit Care Med. 2006;27(5):441–469. PMID: 17072794. doi:10.1055/s-2006-954604
59. American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001, Am J Respir Crit Care Med. 2002;165(2):277–304. PMID: 11790668. doi:10.1164/ajrccm.165.2.ats01
60. King CS, Nathan SD. Idiopathic pulmonary fibrosis: effects and optimal management of comorbidities. Lancet Respir Med. 2017;5(1):72–84. PMID: 27599614. doi:10.1016/S2213-2600(16)30222-3
61. Richeldi L, du Bois RM, Raghu G, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. PMID: 24836310. doi:10.1056/NEJMoa1402584
62. Raghu G, Rochwerg B, Zhang Y, et al; American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT Clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–19. PMID: 26177183. doi:10.1164/rccm.201506-1063ST
63. King TE, Bradford WZ, Castro-Bernardini S, et al.; ASCEND Study Group. A Phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. PMID: 24836312. doi:10.1056/NEJMoa1402582
64. Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27(9):957–969. PMID: 18765187. doi:10.1016/j.healun.2008.07.018
65. Orens JB, Estenne M, Arcasoy S, et al. Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755. PMID: 16818116. doi:10.1016/j.healun.2006.03.011
66. Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. PMID: 16613597. doi:10.1111/j.1600-6143.2006.01276.x
67. Iribarne A, Russo MJ, Davies RR, et al. Despite decreased wait-list times for lung transplantation, lung allocation scores continue to increase. Chest. 2009;135(4):923–928. PMID: 19017874. doi:10.1378/chest.08-2052
68. Christie JD, Shah CV, Kawut SM, et al.; Lung Transplant Outcomes Group. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010–1015. PMID: 19661249; PMCID: PMC2778153. doi:10.1164/rccm.200901-0118OC
69. Covarrubias M, Ware LB, Kawut SM, et al.; Lung Transplant Outcomes Group. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–2578. PMID: 17908278. doi:10.1111/j.1600-6143.2007.01981.x
70. Hoffman SA, Wang L, Shah CV, et al.; Lung Transplant Outcomes Group. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389–396. PMID: 19120076; PMCID: PMC2821938. doi:10.1111/j.1600-6143.2008.02497.x
71. Diamond JM, Lederer DJ, Kawut SM, et al.; Lung Transplant Outcomes Group. Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant. 2011;11(11):2517–2522. PMID: 21883907; PMCID: PMC3206646. doi:10.1111/j.1600-6143.2011.03702.x
72. Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008;28(5):1383–1396. PMID: 18794314. doi:10.1148/rg.285075223
73. Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. PMID: 17507545. doi:10.1164/rccm.200703-456SO
74. Trappe A, Donnelly SC, McNally P, Coppinger JA. Role of extracellular vesicles in chronic lung disease. Thorax. 2021;76(10):1047–1056. PMID: 33712504; PMCID: PMC8461402. doi:10.1136/thoraxjnl-2020-216370
75. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017, Lancet Respir Med. 2020;8(6):585–596. PMID: 32526187; PMCID: PMC7284317. doi:10.1016/S2213-2600(20)30105-3
76. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. PMID: 28513453. doi:10.1016/S0140-6736(17)31222-9
77. Genschmer KR, Russell DW, Lal C, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176(1–2):113–126.e15. PMID: 30633902; PMCID: PMC6368091. doi:10.1016/j.cell.2018.12.002
78. Bezerra FS, Lanzetti M, Nesi RT, et al. Oxidative stress and inflammation in acute and chronic lung injuries. Antioxidants. 2023;12(3):548. PMID: 36978796; PMCID: PMC10045332. doi:10.3390/antiox12030548
79. JafariNezhad A, YektaKooshali MH. Lung cancer in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PLoS One. 2018;13(8):e0202360. PMID: 30114238; PMCID: PMC6095562. doi:10.1371/journal.pone.0202360
80. Ballester B, Milara J, Cortijo J. Idiopathic pulmonary fibrosis and lung cancer: mechanisms and molecular targets. Int J Mol Sci. 2019;20(3):593. PMID: 30704051; PMCID: PMC6387034. doi:10.3390/ijms20030593
81. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. PMID: 31995683. doi:10.1056/NEJMoa1911793
82. Verma S, Dutta A, Dahiya A, Kalra N. Quercetin-3-Rutinoside alleviates radiation-induced lung inflammation and fibrosis via regulation of NF-κB/TGF-β1 signaling. Phytomedicine. 2022;99:154004. doi:10.1016/j.phymed.2022.154004
83. Zhang B, Li P, Li J, Liu X, Wu W. Effect of Oxidative Stress on Diaphragm Dysfunction and Exercise Intervention in Chronic Obstructive Pulmonary Disease. Front Physiol. 2021;12:684453. doi:10.3389/fphys.2021.684453
84. Xu ZH. Clinical Study of Moxibustion-Medicine Combination in the Treatment of Chronic Obstructive Pulmonary Disease Combined with Interstitial Fibrosis [MA thesis]. Shandong University of Traditional Chinese Medicine; 2022.
85. Li Y, Wang Y, Chen Y, et al. Research status and progress of Chinese medicine in the treatment of chronic obstructive pulmonary disease combined with pulmonary fibrosis. Clin Res Tradit Chin Med. 2018;10(26):146–148.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


