Back to Journals » Clinical Epidemiology » Volume 15
Community Use of Repurposed Drugs Before and During COVID-19 Pandemic in the Netherlands: An Interrupted Time-Series Analysis
Authors Zhou G , de Vos S, Schuiling-Veninga CC , Bos J , Oude Rengerink K, Pasmooij AMG, Mol PG, de Bock GH , Hak E
Received 20 April 2023
Accepted for publication 27 July 2023
Published 4 September 2023 Volume 2023:15 Pages 923—937
DOI https://doi.org/10.2147/CLEP.S418069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lars Pedersen
Guiling Zhou,1 Stijn de Vos,1 Catharina CM Schuiling-Veninga,1 Jens Bos,1 Katrien Oude Rengerink,2 Anna Maria Gerdina Pasmooij,2 Peter GM Mol,2,3 Geertruida H de Bock,4 Eelko Hak1
1Unit of Pharmaco-Therapy, -Epidemiology and -Economics (PTEE), Department of Pharmacy, University of Groningen, Groningen, the Netherlands; 2Medicines Evaluation Board, Utrecht, the Netherlands; 3Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; 4Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Correspondence: Guiling Zhou, Unit of Pharmaco-Therapy, -Epidemiology and -Economics (PTEE), Department of Pharmacy, University of Groningen, Antonius Deusinglaan 1, Groningen, 9713 AV, the Netherlands, Tel +31 503638707, Fax +31 503632772, Email [email protected]
Background: Repurposing registered drugs could reduce coronavirus disease (COVID-19) burden before novel drugs are authorized. Little is known about how the pandemic and imposed restrictions changed their dispensing. We aimed to investigate the impact of COVID-19 pandemic on repurposed drugs dispensing in the Netherlands.
Methods: We performed interrupted time-series study using University of Groningen prescription database IADB.nl to evaluate dispensing trends of 24 repurposed drugs before (2017-February 2020) and after (March 2020– 2021) the pandemic’ start. Primary outcomes were monthly prevalence and incidence rates. An autoregressive integrated moving average model assessed the effect of pandemic and stringency index (measuring strictness of government’s restriction policies).
Results: Annual number of IADB.nl population ranged from 919,697 to 952,400. Generally, dispensing of common long-term-used drugs was not significantly affected by pandemic. The prevalence of antibacterials (− 4.20 users per 1000 people), antivirals (− 0.04), corticosteroids (− 1.29), prednisolone (− 1.32), calcium channel blocker (− 0.41), and diuretics (− 1.29) was lower than expected after the pandemic’s start, while the prevalence of ivermectin (0.07), sulfonylureas (0.15), sodium-glucose co-transporter-2 (SGLT2) inhibitor (0.17), and anticoagulants (1.95) was higher than expected. The pandemic was associated with statistically significant decreases in the incidence of antibacterials (− 1.21), corticosteroids (− 0.60), prednisolone (− 0.64) and anticoagulants (− 0.02), and increases in ivermectin (0.02), aggregated antidiabetic drugs (0.13), and SGLT2 inhibitors (0.06). These trends were positively associated with pandemic and negatively associated with stringency index.
Conclusion: Dispensing of most drugs was not significantly associated with pandemic and government’s response. Despite some statistically significant disruptions, these were not necessarily clinically relevant due to small absolute differences observed.
Keywords: COVID-19, drug utilization, repurposed drug, stringency index
Introduction
Since the outbreak, more than 8.59 (out of 17.53) million people in the Netherlands have been infected with coronavirus disease (COVID-19). The COVID-19 pandemic and the government measures implemented to contain the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus have posted major challenges to healthcare systems. At the beginning of the COVID-19 pandemic, many different preventive or therapeutic registered drugs were explored as off-label use for COVID-19, such as hydroxychloroquine (HCQ), ivermectin, and corticosteroids, which were identified in previous systematic reviews and meta-analyses.1–3 Recent studies have reported that the dispensing of the repurposed drugs increased or decreased significantly after the declaration of the COVID-19 pandemic4–6 in Australia and the US, but little was known about whether the dispensing of these drugs in Europe or the Netherlands was experiencing the similar alteration as in other countries. In addition, the above studies did not take the stringency of government’s measures into account, which was not constant during the COVID-19 period. The stringency index is a real-time composite measure calculated by the Oxford Government Tracker Team that reflects the stringency of government responses to the pandemic.7 Nine metrics are used to calculate the stringency index, including school and workplace closures, public event cancellations, restrictions on public gatherings, public transportation closures, stay-at-home requirements, etc. The stringency index is a more accurate parameter than simply dividing the pandemic period into multiple time periods, as it can assess and quantify the extent to which the pandemic changes people’s dispensing behavior.
We developed two hypotheses about the impact of the pandemic and related containment measures on the dispensing of repurposed drugs in community pharmacies. Our first hypothesis was that the pandemic is associated with a decrease in drug dispensing. There is evidence that the number of consultations at both general practitioners and emergency care in the Netherlands decreased.8 Patients have been discouraged to seek medical care for mild symptoms of respiratory tract infection (RTI) and, from July 2020, have been urged to get a COVID-19 test through public health services first. In addition, dispensing of drugs used to treat infection and inflammation may have decreased during the pandemic due to reduced mobility and exposure to pathogens as a result of social distancing, school closures, hygiene measures, and fear of COVID-19 exposure.
Our second hypothesis was that the pandemic is associated with an increase in drug dispensing. Many registered drugs have been evaluated for possible efficacy to prevent or treat COVID-19 in in-vitro, animal studies, clinical trials, and observational studies. Although definitive and robust evidence on the efficacy of these repurposed drugs has not yet been published, misinformation about their benefits has been widespread, which might boost the use of repurposed drugs. In addition, some of these drugs (such as angiotensin-converting enzyme inhibitors (ACEi), insulin) are important drugs for chronic diseases such as hypertension and diabetes, for which daily drug supply is crucial. Patients who rely on these drugs to manage their health conditions may stockpile these drugs due to concerns about limited access to pharmacies during COVID-19 pandemic.
It is crucial to determine the extent to which the drug dispensing in the Netherlands has been affected by the COVID-19 pandemic, and to gain some insight into how to improve the healthcare system in preparation for the next pandemic. Therefore, the primary objective of this study was to investigate the impact of the COVID-19 pandemic and the government’s response to it (stringency index) on the dispensing of repurposed drugs in the Netherlands. Drug dispensing can be determined based on the prevalence and incidence rates. In the context of the COVID-19 pandemic, the prevalence rate is indicative of the availability of drugs, the possibility of stockpiling, and the level of care, while the incidence rate is useful for understanding how treatment patterns are changing in response to the pandemic and any newly issued guidelines.
Method
Study Design and Data Source
We conducted an interrupted time-series study to evaluate the impact of the pandemic (the interruption) on drug utilization. Data were obtained from the IADB.nl pharmacy prescription database of the University of Groningen, which contains dispensing data from approximately 120 community pharmacies and covers over 1,120,000 individuals for more than 27 years in the northern region of the Netherlands. The dispensing data include basic patient characteristics and complete information on the prescribed drug (Anatomical Therapeutic Chemical (ATC) classification), the prescription date, the prescribed daily dose, and the number of drug units dispensed. Over-The-counter (OTC) drugs and in-hospital prescriptions were not included. The data were anonymized, and no individual patients were identifiable from the data. The IADB.nl database is based on community pharmacies in the Netherlands and has been proven to be representative of the whole Dutch population in terms of age distribution and the prevalence of drug use.9
Study Population
The study period covered a pre-pandemic period from January 2017 to February 2020 and the pandemic period from March 2020 to December 2021 (the first COVID-19 case in the Netherlands was diagnosed on February 27th 2020). The prevalent users were defined as individuals aged 18 years and older who were registered in IADB.nl and had at least one dispensing for the drugs of our interest. For prevalent users, age was defined on 31 December each year. The incident users were defined as adults who initiated the dispensing of corresponding drugs within the study period and had no corresponding dispensing 365 days before the index date. The index date was the date of the first dispensing during the study period. For incident users, age was defined at index date. Patients were required to be included in the database at least one year before their first dispensing of drugs of our interest to select new users, estimate incidence, and select chronic disease drug users. Chronic disease drug users were defined as people with at least two corresponding dispensings for diabetes, hypertension, cancer, cardiac arrhythmia, chronic airway diseases, dementia, or depression in each calendar year (Table S1).10–12
Drugs Researched for Repurposing
Based on the findings from previous systematic reviews,1–3 we focused on 24 individual drugs or drug classes that have been repurposed for the prevention or treatment of COVID-19. These 24 individual drugs or drug classes included antiparasitic drugs (hydroxychloroquine (HCQ), chloroquine (CQ), ivermectin), statin, antidiabetic drugs (metformin, insulin, sulfonylurea, thiazolidinedione (TZD), dipeptidyl peptidase-4 (DPP-4) inhibitor, glucagon-like peptide-1 (GLP-1) receptor agonist, sodium-glucose transport protein 2 (SGLT2) inhibitor), anti-infectious and anti-inflammatory drugs (antibacterials for systemic use, antivirals, corticosteroids for systemic use, prednisolone for systemic use, non-steroidal anti-inflammatory drug (NSAID)), antihypertensive drugs (angiotensin-converting enzyme inhibitor (ACEi), angiotensin II receptor blocker (ARB), calcium channel blocker (CCB), diuretics, beta-blocker), anticoagulant, immunomodulating agents (immunosuppressant, immunostimulant) (Table S2).
Outcome Measures
The primary outcomes were the monthly prevalence rate (dispensing rate) and the incidence rate, which were calculated as the number of prevalent users or incident users in each month per 1000 persons for each corresponding year, respectively. The population estimate is annual, and we assume that the population is a dynamic stationary cohort throughout the year, but not necessarily across study years.
Statistical Analysis
Annual baseline characteristics of the source population from 2017 to 2021 were summarized. Descriptive analyses of age, sex, and chronic drug use were performed. Continuous variables were summarized using mean and standard deviation (SD), while categorical variables were summarized using proportions.
We used an autoregressive integrated moving average (ARIMA) model to analyze our interrupted time-series data and account for autocorrelation, seasonality, underlying trends, and to assess the impact of the pandemic and the stringency index. The stringency index is a real-time composite measure that reflects the stringency of government responses to the pandemic.7 The stringency index can range from 0 to 100, with the higher the value, the more stringent the government’s response. In the ARIMA model, the pandemic dummy variable (pre-pandemic period was coded as 0 and during-pandemic period was coded as 1) and the monthly mean stringency index were included as exogenous variables.
To visualize the impact of the pandemic on prevalence and incidence, a separate ARIMA model was first fitted using only pre-pandemic monthly data for the period of 1 January 2017 through 1 March 2020. In this pre-pandemic model, the pandemic dummy variable and monthly mean stringency index were not included as exogenous variables. This model was then used to forecast the expected values after 1 March 2020, assuming no pandemic (counterfactual scenario). For each drug, the absolute difference between the mean of observed value and the mean of expected value was calculated. Because the absolute difference tends to be affected by the level of baseline prevalence and incidence, the relative difference in percentage was also calculated as the absolute difference divided by the mean expected value multiplied by 100%.
We used the auto.arima() function in R to obtain the best-fit ARIMA model, combining unit root tests, minimization of Akaike’s information criteria (AIC), and maximum likelihood estimation (MLE). We examined the model fit by using the Ljung-Box test to check for autocorrelation of the residuals. A well-fitting model should have no autocorrelation of residuals (p > 0.05).
Analyses were performed using R, version 4.2.1. The significance threshold for all statistical tests was two-tailed 0.05.
Results
From 2017 to 2021, the annual number of underlying populations ranged from 919,697 to 952,400. In 2019 (intermediate year), the mean age was 49.73 years (SD: 19.05 years) and the male proportion was 49.0%. The distribution of age, sex, and chronic drug users (diabetes, chronic airway disease, cancer, arrhythmia, dementia, depression, and hypertension) did not vary substantially over the five years (Table S3).
Table 1 presents the results of the ARIMA model assessing the impact of COVID-19 pandemic and the stringency of government’s measures on the prevalence, while Table 2 presents the incidence. Table S4 presents the number of prevalent users along with the prevalence rate (per 1000 persons) for drugs of interest from 2017 to 2021, while Table S5 presents the number of incident users and corresponding incidence rate (per 1000 persons).
 |
Table 1 ARIMA Model Results Summarizing the Association of the COVID-19 Pandemic and Stringency Index with Drug Dispensing in the Netherlands |
 |
Table 2 ARIMA Model Results Summarizing the Association of the COVID-19 Pandemic and Stringency Index with New Drug Dispensing in the Netherlands |
Antiparasitic Drugs
Compared with the counterfactual scenario of no pandemic, the prevalence of aggregated chloroquine drug classes (including HCQ and CQ) increased by 0.02 users per 1000 persons (relative difference: 3.3%), while the incidence decreased by 0.01 users per 1000 persons (relative difference: −24.0%). These changes were not significantly associated with the pandemic or stringency index. Upon the outbreak of the COVID-19 pandemic, ivermectin experienced a pronounced increase in both prevalence (0.07 users per 1000 persons, relative difference: 89.8%) and incidence rate (0.02 users per 1000 persons, relative difference: 80.3%). These steep increases mainly happened in late 2021 (Figure 1). The trend in ivermectin dispensing was significantly associated with the pandemic (prevalence: 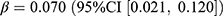 , p = 0.005; incidence:
, p = 0.005; incidence:  , p < 0.001).
, p < 0.001).
Anti-Infectious and Anti-Inflammatory Drugs
As for anti-infectious and anti-inflammatory drugs, the prevalence and incidence of all drugs, including antibacterials, antivirals, NSAID, corticosteroids, and prednisolone, decreased noticeably compared with no pandemic. For antibacterials, the difference between observed and expected prevalence was −4.20 users per 1000 persons (relative difference: −14.9%). Significant associations were found between prevalence of antibacterials and pandemic (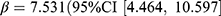 ), p < 0.001) and the stringency index (
), p < 0.001) and the stringency index (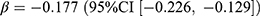 , p < 0.001). The above changes and associations were also observed for the incidence of antibacterials. The prevalence of antivirals decreased by 0.04 users per 1000 persons (relative difference: −3.1%), negatively associated with the stringency index (
, p < 0.001). The above changes and associations were also observed for the incidence of antibacterials. The prevalence of antivirals decreased by 0.04 users per 1000 persons (relative difference: −3.1%), negatively associated with the stringency index (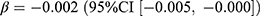 , p = 0.030). The decrease in prednisolone dispensing (prevalence: −1.32 users per 1000 persons; incidence: −0.64 users per 1000 persons) accounted for most of the decrease in corticosteroids (prevalence: −1.29 users per 1000 persons; incidence: −0.60 users per 1000 persons). Both the prevalence and incidence of corticosteroids and prednisolone were significantly associated with the pandemic and the stringency index (Table 1 and 2).
, p = 0.030). The decrease in prednisolone dispensing (prevalence: −1.32 users per 1000 persons; incidence: −0.64 users per 1000 persons) accounted for most of the decrease in corticosteroids (prevalence: −1.29 users per 1000 persons; incidence: −0.60 users per 1000 persons). Both the prevalence and incidence of corticosteroids and prednisolone were significantly associated with the pandemic and the stringency index (Table 1 and 2).
Antihypertensive Drugs
Regarding the antihypertensive drugs, the change in aggregated prevalence was negligible (0.01 users per 1000 persons, relative difference: 0.01%). The prevalence of CCB and diuretics showed some decrease compared to no pandemic (relative difference: −1.5% and −4.0%), which was negatively associated with the mean stringency index (CCB: 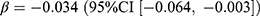 , p = 0.031; diuretics:
, p = 0.031; diuretics: 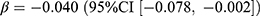 , p = 0.038). The overall new dispensing of antihypertensive drugs declined by 0.31 users per 1000 persons (relative difference: −6.7%) after the pandemic had started, mainly in ACEi, ARB, and diuretics. However, no statistically significant associations between these incidences and the pandemic were observed.
, p = 0.038). The overall new dispensing of antihypertensive drugs declined by 0.31 users per 1000 persons (relative difference: −6.7%) after the pandemic had started, mainly in ACEi, ARB, and diuretics. However, no statistically significant associations between these incidences and the pandemic were observed.
Antidiabetic Drugs
In terms of the antidiabetic drugs, we observed an increased overall prevalence (0.46 users per 1000 persons, relative difference: 1.7%) and incidence (0.13 users per 1000 persons, relative difference: 12.9%). A positive association was found between overall incidence and pandemic (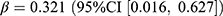 , p = 0.039). Both the prevalence (0.15 users per 1000 persons, relative difference: 1.8%) and incidence (0.03 users per 1000 persons, relative difference: 11.2%) of sulfonylureas increased compared to no pandemic, while only the increase in prevalence was associated with the pandemic (
, p = 0.039). Both the prevalence (0.15 users per 1000 persons, relative difference: 1.8%) and incidence (0.03 users per 1000 persons, relative difference: 11.2%) of sulfonylureas increased compared to no pandemic, while only the increase in prevalence was associated with the pandemic (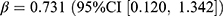 , p = 0.019) and the stringency index (
, p = 0.019) and the stringency index (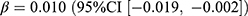 , p = 0.019). SGLT2 inhibitors showed similar changing patterns with sulfonylureas. The prevalence of SGLT2 inhibitors increased by 0.17 users per 1000 persons (relative difference: 40.0%), with significant associations with the pandemic (
, p = 0.019). SGLT2 inhibitors showed similar changing patterns with sulfonylureas. The prevalence of SGLT2 inhibitors increased by 0.17 users per 1000 persons (relative difference: 40.0%), with significant associations with the pandemic (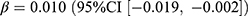 , p = 0.040) and the stringency index (
, p = 0.040) and the stringency index (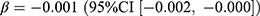 , p = 0.004). The incidence of SGLT2 inhibitors increased by 0.06 users per 1000 persons (relative difference: 189.0%), with a significant association with the pandemic (
, p = 0.004). The incidence of SGLT2 inhibitors increased by 0.06 users per 1000 persons (relative difference: 189.0%), with a significant association with the pandemic (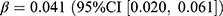 , p < 0.001).
, p < 0.001).
Anticoagulants
An increase of 1.95 users per 1000 persons (relative difference: 4.2%) and a decrease of 0.02 users per 1000 persons (relative difference: −1.2%) were observed for the prevalence and incidence of anticoagulants, respectively. The prevalence was associated with the pandemic ( , p = 0.022), whereas the incidence was associated with both the pandemic (
, p = 0.022), whereas the incidence was associated with both the pandemic (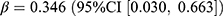 , p = 0.032) and the stringency index (
, p = 0.032) and the stringency index (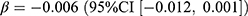 , p = 0.017).
, p = 0.017).
Figures 1–3 show how the monthly prevalence and incidence of drugs changed from 2017 to 2021. For most of the drugs, the declaration of the COVID-19 pandemic (gray vertical dashed line) coincided with an abrupt increase in prevalence, but a sharp decrease in incidence (Figures 1 and 2). However, regarding aggregated chloroquine drug class, there was an immediate rise in both the prevalence and incidence following the onset of COVID-19. As for ivermectin, an obvious increase of dispensing occurred in late 2020. Dispensing of antivirals has shown a steady upward trend since 2017, but this trend was interrupted by the COVID-19 pandemic (Figure 3). Dispensing of both antibacterials and corticosteroids fell below the expected trend. The decrease was greater than expected for seasonal declines, and the seasonal peak in drug use during winter time was not seen since the outbreak of the COVID-19 pandemic. Seasonal fluctuations of the use of antibacterials and corticosteroids seemed to slightly resume in 2021 winter (Figure 3).
Discussion
This study demonstrated that the trend of common registered long-term used drugs such as ACEi, beta-blocker, statin, metformin, insulin, etc. deviated slightly from the expected trend, probably due to the temporary restructuring of the healthcare system during the pandemic. Their dispensings were not affected significantly by the pandemic on average. However, we also observed that the prevalence of antibacterials, antivirals, corticosteroids, prednisolone, CCB, diuretics, and DPP-4 inhibitor was slightly lower than expected after the onset of the COVID-19 pandemic, while the prevalence of ivermectin, sulfonylureas, SGLT2 inhibitor, and anticoagulants was slightly higher than expected. In addition, we found that the pandemic was associated with small decreases in the incidence of CQ, antibacterials, corticosteroids, prednisolone and anticoagulants, and increases in the incidence of ivermectin, aggregated antidiabetic drugs, and SGLT2 inhibitors. These trends in changes, albeit small, were positively associated with the pandemic and negatively associated with the government responses to the pandemic.
Different healthcare systems around the world were affected differently by the pandemic, depending on the severity of the endemic and the level of government restrictions. Mathieu reported that the COVID-19 pandemic has a significant impact on the dispensing of cardiovascular and antidiabetic drugs in France, mostly in the form of a decrease in treatment initiations during the lockdown period in 2020.13 The healthcare systems of the Netherlands and France are both well-regarded and comprehensive, with similar endemic and stringency profiles between these two countries. The remarkable reduction in the incidence of cardiovascular and antidiabetic drugs was not replicated in our study, presumably because their study period only focused on the first lockdown period and a short post-lockdown period (until 20 September, 2020), while our study included a longer pandemic period, taking into account the long-term effect of the pandemic. In addition, the Dutch government did not restrict the access to pharmacies after the declaration of the COVID-19 pandemic. Outside of Europe, Aboulatta also observed that the initiations of cardiovascular, antidiabetics, and respiratory agents were disrupted greatly by the pandemic in Manitoba, Canada.14 This could be explained by the consistently higher stringency index, therefore more disruptions to drug dispensing in Canada.15
The most commonly studied repurposed drugs for COVID-19 are HCQ, CQ, and ivermectin. The marked increase in the prevalence of aggregated chloroquine drug class (HCQ and CQ) coincided with the declaration of the COVID-19 pandemic and the publication of the first in vitro study supporting the use of HCQ for COVID-19 in March 2020,16 suggesting the possibility of off-label use for COVID-19. Nevertheless, the average prevalence of aggregated chloroquine drug class was not significantly associated with the pandemic and related containment measures. In contrast, ivermectin dispensing needs more attention. Since the first supportive in vitro study of ivermectin in COVID-19 published in June 2020,17 the prevalence of ivermectin has increased dramatically, mainly driven by the increase in new users. Similar increasing trends of ivermectin have also been observed in Australia5 and the USA.18,19 Although the use of ivermectin for the prevention or treatment of COVID-19 outside of randomized clinical trials (RCTs) is not recommended by the European Medicines Agency (EMA) on 22 March 2021,20 the increasing trend did not discontinue. According to the findings of a recently published RCT, the use of ivermectin in patients with mild to moderate COVID-19 is not supported.21 Therefore, the EMA should keep an eye on the prescription of ivermectin for COVID-19.
Early COVID-19 studies emphasize that most people are highly vulnerable and express various flu-like symptoms,22 which could potentially increase the dispensing of specific drugs such as NSAID, antivirals, or antibacterials. Contrary to this, we observed that the prevalent and incident use of anti-infective and anti-inflammatory drugs decreased substantially during the COVID-19 period, with seasonal fluctuations flattening out. However, the effect of the pandemic does not appear to be long-lasting, with the use of antibacterials and corticosteroids returning gradually to pre-pandemic levels from the winter of 2021. In general, the decrease can be attributed to reduced mobility and exposure to pathogens, and reduced transmission of non-COVID-19 diseases as a result of social distancing, school closures, and hygiene measures. During the first year of the COVID-19 pandemic in the Netherlands, a decrease in the occurrence of common infections and antibiotic prescribing was observed.23,24 The prevalence and incidence rate of both oral corticosteroids and prednisolone were lower than expected level, but the magnitude of the decrease was greater for prednisolone. The reduced dispensing of prednisolone may indicate that there were fewer exacerbations of respiratory diseases such as asthma in the community after the onset of the COVID-19 pandemic. Similar findings of decreased antibiotics and corticosteroids dispensing after the COVID-19 pandemic have been reported in the UK,25 the Netherlands,23 and Australia.26
Our study found that the prevalence of anticoagulants increased, while the incidence of anticoagulants decreased slightly. Two other studies also showed an increase in oral anticoagulant (OAC) possession27 and below pre-pandemic level of incidence of OAC28 during the pandemic. The increase in prevalence is perhaps due to the tendency of patients to stockpile drugs. As for new dispensing, based on our ARIMA model, the change in incidence of anticoagulants is negatively associated with the stringency index, implying that policy restrictions could probably reduce the incidence to some extent. Anticoagulants are used to prevent thrombosis in patients with venous thromboembolism (VTE) or stroke. Although the evidence on the effect of anticoagulants on the prognosis of COVID-19 is inconsistent, there are several RCTs demonstrating the beneficial effect of anticoagulants on improving the COVID-19-related prognosis, VTE and death in hospitalized patients.29,30 Off-label use for COVID-19 does not appear to be obvious in our study, presumably because we do not have the dispensing data in the hospital, and the off-label use of anticoagulants occurred mainly in moderate to high risk of COVID-19 patients requiring hospitalization.
We also found that both the prevalence and incidence of SGLT2 inhibitors increased dramatically after 2021 (Figure S1). This is probably because clinical trial data on the benefits of SGLT2 inhibitors in heart failure became available,31 and physicians began to prescribe SGLT2 inhibitors to patients with cardiovascular disease, not just those with diabetes.
Strengths
To our best knowledge, this is the first study that quantified the strictness of government’s policy and incorporated this stringency index as continuous variable in the interrupted time-series analysis. The stringency index reflects real-time changes in the strictness of government policy, and provides a more accurate association between the pandemic and drug dispensing. Furthermore, the ARIMA model we used in this study is appropriate for analyzing the effects of the pandemic on a population-wide scale, as it takes seasonality and autocorrelation into account. The sample used in this study is representative for the Dutch population as a whole and covers a long period before and after the start of pandemic to adjust for underlying trend.
Limitations
We acknowledge that our study has some limitations. First, the indication of the drug dispensed is not available in the IADB.nl data source, therefore we are uncertain about for which diseases the drugs are dispensed and whether the drugs were prescribed as an off-label use for COVID-19. Second, more accurate coefficient estimates from the ARIMA model, which involves differencing of time series data and adjusting for autocorrelation and seasonality, come at the cost of decreased model interpretability, especially the interpretability of coefficients. Even so, the ARIMA model is a good choice to identify the association between pandemic and drug dispensing, and capture the fluctuations and complex patterns of drug dispensing. Third, we only consider the drug dispensing in the community, therefore over-The-counter, hospital, and emergency care dispensing are not part of our study. As a result, we are unable to identify the dispensing of the repurposed drugs that are used in hospital settings, probably resulting in an underestimation of the increased dispensing after the pandemic. Fourth, because of the nature of interrupted time-series analysis, this useful quasi-experimental design could only provide valuable insights into the association of pandemic and the drug dispensing, but it cannot infer any definitive causality on its own. Finally, anti-SARS-CoV-2 vaccination could influence the drug dispensing as well. On the one hand, the post-vaccination adverse events such as muscle pain and fever32 can increase the usage of drug. On the other hand, the vaccination could induce cross immunity against other pathogens, which could probably decrease the drug usage. However, we did not incorporate the vaccination information into our analysis.
Conclusion
Overall, the dispensing of most repurposed drugs for COVID-19, including some common long-term therapies, was not clinically relevantly affected by the pandemic and related measures in the Netherlands, as the access to pharmacies appeared not to be severely restricted during this period. We believe that our study makes a substantial contribution to the understanding of the community use of repurposed drugs during the COVID-19 pandemic in the Netherlands. The findings have important implications for healthcare providers and policy makers, as they suggest that access to essential drugs was largely maintained during the pandemic. As a recent perspective predicted, the post-pandemic society will be extremely vulnerable to emerging infectious diseases because of the pandemic related restrictions applied.33 Given these potential vulnerabilities, strengthening healthcare systems, maintaining robust surveillance for emerging diseases, and ensuring that populations have access to routine healthcare and vaccinations will all be key strategies for reducing vulnerability to future pandemics.
Data Sharing Statement
Additional tables and figures supporting our findings are available in the Supplementary Material. The study protocol is available upon request by emailing the corresponding author. Informed consent was not required as no personal information was used in our article. The SQL and R code for data analysis can be shared by emailing the corresponding author.
Ethics Approval and Informed Consent
The University of Groningen IADB.nl community pharmacy dispensing database contains data that is collected in accordance with the Dutch and European guidelines on privacy requirements (GDPR) for handling human data. Approval of the medical ethics committee was not needed nor required for this study.
Acknowledgment
We are grateful to Dr. Susan Hahne from the National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu, RIVM) for providing the initial concept of the stringency index and information related to the COVID-19 pandemic in the Netherlands.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is funded by internal funding. GZ received a scholarship (file number: 202107720033) from the China Scholarship Council (CSC) for her PhD at the University of Groningen, Groningen, The Netherlands. The funder of this review had no role in study design, data extraction, data synthesis, data interpretation, or writing of the report.
Disclosure
All authors declare no competing interests in this work.
References
1. Smit M, Marinosci A, Agoritsas T, Calmy A. Prophylaxis for COVID-19: a systematic review. Clin Microbiol Infect. 2021;27(4):532–537. doi:10.1016/j.cmi.2021.01.013
2. Andrade BS, Rangel FDS, Santos NO, et al. Repurposing approved drugs for guiding COVID-19 prophylaxis: a systematic review. Front Pharmacol. 2020;11:590598. doi:10.3389/fphar.2020.590598
3. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:1–12. doi:10.1136/bmj.n949
4. Gouin KA, Creasy S, Beckerson M, et al. Trends in prescribing of antibiotics and drugs investigated for Coronavirus Disease 2019 (COVID-19) treatment in US nursing home residents during the COVID-19 pandemic. Clin Infect Dis. 2022;74(1):74–82. doi:10.1093/cid/ciab225
5. Schaffer AL, Henry D, Zoega H, Elliott JH, Pearson SA, Yon DK. Changes in dispensing of medicines proposed for re-purposing in the first year of the COVID- 19 pandemic in Australia. PLoS One. 2022;17(6):1–13. doi:10.1371/journal.pone.0269482
6. Mian M, Sreedharan S, Giles S. Increased dispensing of prescription medications in Australia early in the COVID-19 pandemic. Med J Aust. 2021;214(9):428–429. doi:10.5694/mja2.51029
7. Hale T, Angrist N, Goldszmidt R, et al. A global panel database of pandemic policies (Oxford COVID-19 government response tracker). Nat Hum Behav. 2021;5(4):529–538. doi:10.1038/s41562-021-01079-8
8. Heins M, Hek K, Hooiveld M, Hendriksen J, Korevaar J. Impact Coronapandemie op Zorgvraag Bij Huisartsen (Factsheet A) (Impact of the Corona Pandemic on Care Demand at General Practitioners) . Nivel Accessed; 2021.
9. Visser ST, Schuiling-Veninga CC, Bos JH, De Jong-Van Den Berg LT, Postma MJ. The population-based prescription database IADB.nl: its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):285–292. doi:10.1586/ERP.13.20
10. Pratt NL, Kerr M, Barratt JD, et al. The validity of the Rx-risk comorbidity index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) classification system. BMJ Open. 2018;8(4):e021122. doi:10.1136/BMJOPEN-2017-021122
11. Holvik K, Hjellvik V, Karlstad Ø, et al. Contribution of an extensive medication-based comorbidity index (Rx-Risk) in explaining the excess mortality after hip fracture in older Norwegians: a NOREPOS cohort study. BMJ Open. 2022;12(5):e057823. doi:10.1136/BMJOPEN-2021-057823
12. Daniels B, Tervonen HE, Pearson SA. Identifying incident cancer cases in dispensing claims: a validation study using Australia’s Repatriation Pharmaceutical Benefits Scheme (PBS) data. Int J Popul Data Sci. 2020;5(1):1152. doi:10.23889/ijpds.v5i1.1152
13. Mathieu C, Pambrun E, Bénard-Laribière A, et al. Impact of the COVID-19 pandemic and its control measures on cardiovascular and antidiabetic drugs use in France in 2020: a nationwide repeated cohort study. Eur J Epidemiol. 2022;37(10):1049–1059. doi:10.1007/s10654-022-00912-2
14. Aboulatta L, Peymani P, Vaccaro C, et al. Drug utilization patterns before and during COVID-19 pandemic in Manitoba, Canada: a population-based study. PLoS One. 2022;17(11):1–15. doi:10.1371/journal.pone.0278072
15. Our World in Data. COVID-19: stringency index. Available from: https://ourworldindata.org/covid-stringency-index#citation.
16. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–739. doi:10.1093/CID/CIAA237
17. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi:10.1016/j.antiviral.2020.104787
18. Geller AI, Lovegrove MC, Lind JN, Datta SD, Budnitz DS. Assessment of outpatient dispensing of products proposed for treatment or prevention of COVID-19 by US retail pharmacies during the pandemic. JAMA Intern Med. 2021;181(6):869–872. doi:10.1001/JAMAINTERNMED.2021.0299
19. Lind JN, Lovegrove MC, Geller AI, Uyeki TM, Datta SD, Budnitz DS. Increase in outpatient ivermectin dispensing in the US during the COVID-19 pandemic: a cross-sectional analysis. J Gen Intern Med. 2021;36(9):2909–2911. doi:10.1007/s11606-021-06948-6
20. European Medicines Agency. EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials. Available from: https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-covid-19-outside-randomised-clinical-trials.
21. Naggie S, Boulware DR, Lindsell CJ, et al. Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial. JAMA. 2023;329(11):888–897. doi:10.1001/JAMA.2023.1650
22. Mouliou DS, Kotsiou OS, Gourgoulianis KI. Estimates of COVID-19 risk factors among social strata and predictors for a vulnerability to the infection. Int J Environ Res Public Health. 2021;18(16):8701. doi:10.3390/IJERPH18168701
23. van de Pol AC, Boeijen JA, Venekamp RP, et al. Impact of the covid-19 pandemic on antibiotic prescribing for common infections in the Netherlands: a primary care-based observational cohort study. Antibiotics. 2021;10(2):1–10. doi:10.3390/antibiotics10020196
24. Hek K, Ramerman L, Weesie YM, et al. Antibiotic prescribing in Dutch daytime and out-of-hours general practice during the COVID-19 pandemic: a Retrospective Database Study. Antibiotics. 2022;11(3):309. doi:10.3390/antibiotics11030309
25. Chalitsios CV, McKeever TM, Langley TE, Shaw DE. Impact of COVID-19 on corticosteroids and antibiotics prescribing in England: an interrupted time series analysis. J Public Health. 2021;43(3):517–520. doi:10.1093/pubmed/fdab017
26. Gillies MB, Burgner DP, Ivancic L, et al. Changes in antibiotic prescribing following COVID-19 restrictions: lessons for post-pandemic antibiotic stewardship. Br J Clin Pharmacol. 2022;88(3):1143–1151. doi:10.1111/bcp.15000
27. Hernandez I, Gabriel N, He M, et al. COVID-19 and anticoagulation for atrial fibrillation: an analysis of US nationwide pharmacy claims data. J Am Heart Assoc. 2021;10(24). doi:10.1161/JAHA.121.023235
28. Antonazzo IC, Fornari C, Paoletti O, et al. COVID-19 outbreak impact on anticoagulants utilization: an interrupted time-series analysis using health care administrative databases. Thromb Haemost. 2021;121(8):1115–1118. doi:10.1055/a-1523-7658
29. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612–1620. doi:10.1001/JAMAINTERNMED.2021.6203
30. ATTACC, ACTIV-4a, and REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi:10.1056/NEJMOA2105911
31. Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400(10354):757–767. doi:10.1016/S0140-6736(22)01429-5
32. Mouliou DS, Dardiotis E. Current evidence in SARS-CoV-2 mRNA vaccines and post-vaccination adverse reports: knowns and unknowns. Diagnostics. 2022;12(7):1555. doi:10.3390/DIAGNOSTICS12071555
33. Mouliou DS. The deceptive COVID-19: lessons from common molecular diagnostics and a novel plan for the prevention of the next pandemic. Diseases. 2023;11(1):20. doi:10.3390/DISEASES11010020
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



