Back to Journals » Journal of Inflammation Research » Volume 14
Combining Preoperative and Postoperative Inflammatory Indicators Can Better Predict the Recurrence of Hepatocellular Carcinoma After Partial Hepatectomy
Authors Wu M , Yang S, Feng X, Li C, Liu X, Zhang Z, Xiao Y, Liu C, Dong J
Received 16 April 2021
Accepted for publication 4 June 2021
Published 13 July 2021 Volume 2021:14 Pages 3231—3245
DOI https://doi.org/10.2147/JIR.S316177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Meilong Wu,1,2,* Shizhong Yang,1,2,* Xiaobin Feng,1,2 Chengquan Li,1,2 Xiangchen Liu,1,2 Zhenyu Zhang,1,2 Ying Xiao,1,3 Chuchu Liu,4 Jiahong Dong1,2
1School of Clinical Medicine, Tsinghua University, Beijing, 100084, People’s Republic of China; 2Hepato-pancreato-biliary Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, 102218, People’s Republic of China; 3Department of pathology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, 102218, People’s Republic of China; 4Hepato-pancreato-biliary Surgery of Affiliated Hospital of Qinghai University, Affiliated Cancer Hospital of Qinghai University, Xining, Qinghai, 810001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiahong Dong
Hepato-pancreato-biliary Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, No. 168 Litang Road, Changping District, Beijing, 102218, People’s Republic of China
Tel/Fax +86-10-56118888
Email [email protected]
Purpose: Previous studies have shown that various preoperative inflammatory indicators can predict the prognosis of hepatocellular carcinoma (HCC), but the role of postoperative inflammatory indicators remains unclear. This study aimed to explore the prognostic value of postoperative inflammatory indicators and whether combining preoperative and postoperative inflammatory indicators can improve the predictive performance of the prognostic model.
Patients and Methods: Eighty-eight patients with primary HCC were included in this study. A preoperative model, postoperative model, and combined model that integrated preoperative and postoperative inflammatory indicators were established. The prognostic value of the models was evaluated by the area under the curve of time-dependent receiver operating characteristic curves (td-AUC).
Results: Multivariate analysis of preoperative and postoperative inflammatory indicators and clinicopathological indicators found that tumor number, alpha-fetoprotein (AFP) level, and the preoperative platelet-lymphocyte ratio (prePLR), preoperative prognostic nutritional index (prePNI), and postoperative neutrophil-lymphocyte ratio (postNLR) were independent prognostic factors for the disease-free survival. The prognostic efficacy of the postNLR at 2 years and 3 years was better than that of tumor number, AFP level, and the prePLR, and prePNI. The combined model had higher td-AUC values than the preoperative model, postoperative model, American Joint Committee on Cancer 8th edition stage, and Barcelona Clinic Liver Cancer stage at 2 years (0.814 vs 0.754, 0.765, 0.513 and 0.527, respectively), and 3 years (0.786 vs 0.749, 0.753, 0.509 and 0.529, respectively). The predictive performance of the combined model was better than that of the preoperative model, postoperative model, and traditional clinical stage.
Conclusion: Postoperative inflammatory indicators were valuable prognostic indicators. The combination of preoperative and postoperative inflammatory indicators improved the predictive performance of the prognostic model. We should pay more attention to postoperative inflammatory indicators.
Keywords: hepatocellular carcinoma, inflammation, postoperative, neutrophil-lymphocyte ratio, disease-free survival
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide.1 Partial hepatectomy is the main and most common treatment option for HCC. Unfortunately, the 5-year disease-free survival (DFS) rate after partial hepatectomy for HCC is only approximately 30–40%.2,3 The American Joint Committee on Cancer 8th edition Tumor, Node, Metastasis (AJCC TNM (8th)) and Barcelona Clinic Liver Cancer (BCLC) staging systems are commonly used for HCC risk stratification and determining treatment options.4 Accurate risk stratification is critical for treatment decisions. However, the current staging systems incorporate limited clinicopathological indicators, and the predictive power is obviously insufficient.5 Many factors affect tumor occurrence and progression, such as inflammation, viral infection, and the tumor macro-and microenvironment. Unlike most other malignancies, HCC is a typical inflammation-related cancer, more than 90% of HCC cases occur in the context of chronic inflammation.6 Inflammation promotes the development of HCC by promoting proliferation and survival signal transduction, evading immune surveillance, inducing angiogenesis and genomic instability, and promoting invasion and metastasis.7
The prognostic value of preoperative inflammatory indicators in HCC was extensively studied, including the preoperative platelet-lymphocyte ratio (prePLR), preoperative lymphocyte-monocyte ratio (preLMR), preoperative prognostic nutritional index (prePNI), preoperative neutrophil-lymphocyte ratio (preNLR) and preoperative derived NLR (predNLR).8–13 However, the treatment process for tumors is complicated and long, and we should not only pay attention to the influence of preoperative indicators on the prognosis of HCC but also to postoperative indicators. Previous studies showed that the immune status of HCC patients changed after the primary tumor burden was reduced,14,15 and a high postNLR was associated with poor long-term prognosis.16,17 However, the value of many postoperative inflammatory indicators was not clarified. No study has reported which of the preoperative and postoperative inflammatory indicators has better prognostic performance.
This study aimed to explore the prognostic value of postoperative inflammation indicators and compared the prognostic efficacy of a prognostic model that combined preoperative and/or postoperative inflammation indicators with the current clinical staging systems.
Patients and Methods
Patient Selection
This study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the ethics committees of the hospital (ethics number: 20001–01). All patients signed an informed consent form before surgery. Patients with primary HCC who underwent partial hepatectomy from December 2014 to December 2019 at the Beijing Tsinghua Changgung Hospital were included in the study. This study was registered in the National Hepatobiliary Standard Database of China (registration number: CDR/20210099).18
We screened qualified patients based on inclusion and exclusion criteria. The inclusion criteria were as follows: (1) primary HCC diagnosed by pathology; (2) partial hepatectomy in the study hospital as the initial treatment; (3) no history of other tumors and (4) no infection during peripheral blood collection. The exclusion criteria were as follows: (1) previous chemotherapy, intervention, radiofrequency ablation and other treatments; (2) incomplete clinical pathological data. Finally, 88 patients with primary HCC who underwent partial hepatectomy were enrolled (Figure 1).
 |
Figure 1 Selection of patients included in the analysis. |
Clinicopathological Indicators
We collected peripheral blood at admission and before discharge. If the time from the last blood collection after surgery was less than 3 days, we collected peripheral blood at the first follow-up after surgery. On the basis of previous studies, the following definitions of inflammatory indicators included in this study: PLR, LMR, and NLR were the ratios of corresponding inflammatory cells; dNLR was calculated as (white blood cell- neutrophil)/lymphocyte, and PNI was defined as albumin (g/L) + 5 × lymphocyte (109/L).8–13
DFS was the main endpoint of this study and was defined as the time from the day of surgery to recurrence. Follow-up examination included an alpha-fetoprotein (AFP) test, ultrasound and/or abdominal enhanced computed tomography, and magnetic resonance, and the patients were followed up every 3 months for 2 years and every 6 months thereafter.19 Recurrence was defined as a significant increase in AFP levels or tumor lesions on the image after surgery.
Statistical Analysis
The optimal cutoff values of inflammatory indicators were obtained from maximally selected rank statistics with the “survminer“ package. Survival curves were drawn by the Kaplan-Meier method and compared using the Log rank test. A Cox regression model was used for univariate and multivariate analyses. The independent risk factors in multivariate analysis were used to construct a nomogram by using the “rms” package. The concordance index (C-index) was used to measure the discriminative power of the model. The ability of the model to predict DFS was evaluated with the bootstrapping of 1000 resamples. Calibration plots were generated to explore the performance of the model at 2 and 3 years after surgery. The “nomogramFormula” package was used to calculate the risk score of each patient in the model, and the time-dependent receiver operating curve (ROC) was used to compare the predictive performance of different time points between models. A larger area under the curve (AUC) indicated higher predictive power. R version 4.0.3 (http://www.r‑project.org/) and SPSS 26.0 software (IBM Corp.) were used for statistical analyses. All analyses were two-sided, and P<0.05 indicated a significant difference.
Results
Baseline Characteristics
Eight-eight patients with primary HCC were included in this study, and 62 (70.5%) were male. There were 28 patients (31.8%) with microvascular invasion (MVI) and 7 patients (8.0%) with satellite lesions. Sixty-four patients who were AFP positive (>20 ng/mL) before surgery (Table 1).
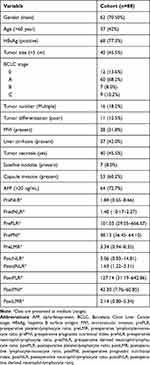 |
Table 1 Clinicopathological Features of HCC Patients |
Optimal Cutoff Value of Inflammation Indicators
The optimal cutoff values of the preNLR, predNLR, prePLR, prePNI, and preLMR were calculated using the maximum selection rank statistics of 3.22, 1.29, 61.07, 44.35, and 2.16, respectively (Table 2). The optimal cutoff values for the postoperative neutrophil-lymphocyte ratio (postNLR), postoperative derived NLR (postdNLR), postoperative platelet-lymphocyte ratio (postPLR), postoperative prognostic nutritional index (postPNI), and postoperative lymphocyte-monocyte ratio (postLMR) were 3.25, 1.44, 91.53, 41.75, and 2.48, respectively (Table 2).
 |
Table 2 Optimal Cutoff Values for Preoperative and Postoperative Inflammation Indicators |
Survival analysis indicated that the difference in DFS between the high and low preoperative inflammatory indicator groups was statistically significant (Figure 2). There was also a significant difference in DFS between the high and low postoperative inflammatory indicator groups, except for postPLR (Figure 3).
 |
Figure 2 Kaplan‑Meier survival curves of DFS based on preoperative inflammatory indicators. (A) preNLR, (B) predNLR, (C) preLMR, (D) prePLR, (E) prePNI. |
 |
Figure 3 Kaplan‑Meier survival curves of DFS based on postoperative inflammation indicators. (A) postNLR, (B) postdNLR, (C) postLMR, (D) postPLR, (E) postPNI. |
Preoperative Model
As shown in Table 3, univariate analysis showed that tumor number, MVI, satellite nodule number, AFP level, preNLR, predNLR, prePLR, prePNI, preLMR, postNLR, postdNLR, postPNI, and postLMR were risk factors for poor DFS after partial hepatectomy in primary HCC patients (P<0.05).
 |
Table 3 Univariate and Multivariate Analysis of DFS |
We incorporated the preoperative inflammatory indicators and clinicopathological indicators into the multivariate analysis to construct the preoperative prognostic model, and the multivariate analysis found that tumor number (hazard ratio (HR) =2.731, confidence interval (CI)=1.303–5.727, P=0.008), AFP level (HR=2.294, 95% CI=1.020–5.160, P=0.045), and prePLR (HR=0.236, 95% CI=0.101–0.553, P=0.001), prePNI (HR=0.338, 95% CI=0.168–0.682, P=0.002), and preLMR (HR=0.331, 95% CI=0.132–0.825, P=0.018) were independent prognostic factors of DFS (Table 4).
 |
Table 4 Multivariate Analysis of Preoperative and Postoperative Models |
The C-index of the preoperative model was 0.725. The nomogram of the preoperative prognosis model is shown in Figure 4. The calibration curve showed that the prediction of the 2-and 3-year DFS rate from the preoperative model based on preoperative inflammation indicators and clinicopathological indicators had good agreement with the actual observation (Figure 5).
 |
Figure 4 Nomogram of the preoperative model. |
 |
Figure 5 Calibration curve of the preoperative model for (A) 2 years and (B) 3 years. |
Postoperative Model
Multivariate analysis of postoperative inflammatory and clinicopathological indicators found that tumor number (HR=2.336, 95% CI=1.156–4.721, P=0.018), satellite nodule number (HR=2.565, 95% CI=1.051–6.259, P=0.038), AFP level (HR=2.229, 95% CI=1.036–4.798, P=0.040) and the postNLR (HR=3.189, 95% CI=1.736–5.860, P<0.001) were independent prognostic factors for patients with poor DFS rates (Table 4). These indicators were further included in the construction of a postoperative prognostic model (Figure 6).
 |
Figure 6 Nomogram of the postoperative model. |
The C-index of the postoperative model was 0.722. The calibration curve showed that the predictions of the 2- and 3-year DFS from the postoperative model had good agreement with the actual observation (Figure 7).
 |
Figure 7 Calibration curve of the postoperative model for (A) 2 years and (B) 3 years. |
Combined Model
We integrated the preoperative and postoperative inflammatory indicators and clinicopathological indicators to construct a combined prognostic model. Multivariate analysis suggested that tumor number (HR=3.378, 95% CI=1.603–7.117, P=0.001), AFP level (HR=3.340, 95% CI=1.561–5.576, P=0.002), the prePLR (HR=0.231, 95% CI=0.099–0.538, P=0.001), prePNI (HR=0.281, 95% CI=0.143–0.554, P<0.001), and postNLR (HR=3.580, 95% CI=1.906–6.725, P<0.001) were independent risk factors for poor DFS (Table 3), and these indicators were included to construct a combined model (Figure 8).
 |
Figure 8 Nomogram of the combined model. |
The C-index of the combined model was 0.765. The calibration curve showed that the prediction of the 2-and 3-year DFS rate from the combined model based on both preoperative and postoperative inflammation and clinicopathological indicators had good agreement with the actual observation (Figure 9).
 |
Figure 9 Calibration curve of the combined model for (A) 2 years and (B) 3 years. |
A time-dependent ROC curve was used to compare the prognostic efficacy of independent prognostic factors in the combined model at 2 years and 3 years. Our results indicated that the prognostic efficacy of the postNLR at 2 years (0.666) was better than that of tumor number (0.597), AFP level (0.595), and the prePLR (0.437) and prePNI (0.366) (Figure 10A). Similarly, the prognostic efficacy of the postNLR at 3 years showed the same trend and was better than that of other preoperative inflammatory and clinicopathological indicators (Figure 10B). In addition, the C-index of the postNLR was 0.627, which was higher than that of the AFP level (0.605) and the prePLR (0.551), prePNI (0.596), and tumor number (0.568).
 |
Figure 10 Different independent prognostic factors of the combined model and the area under the time-dependent ROC for predicting (A) 2-year and (B) 3-year DFS. |
Comparison of the Models
The C-indexes of the combined model, preoperative model, postoperative model, AJCC TNM (8th) stage, and BCLC stage for predicting HCC DFS were 0.765, 0.725, 0.722, 0.504, and 0.545, respectively. The prognostic performance of the combined model was superior to that of the preoperative model (0.765 vs 0.725, P=0.090), postoperative model (0.765 vs 0.722, P=0.115), AJCC TNM (8th) stage (0.765 vs 0.504, P=0.031), and BCLC stage (0.765 vs 0.545, P<0.001). The prognostic performance of the preoperative prognostic model was significantly better than that of the AJCC TNM (8th) stage (0.725 vs 0.504, P=0.047), and BCLC stage (0.725 vs 0.545, P=0.003). The postoperative model better predicted the prognosis of HCC than the AJCC TNM (8th) stage (0.722 vs 0.504, P=0.051), and BCLC stage (0.722 vs 0.545, P=0.002).
A time-dependent ROC was used to compare the prognostic efficacy of the different models at different time points. We found that the predictive performance of the combined model at 2 and 3 years (0.814 and 0.786, respectively) was better than that of the preoperative model (0.754 and 0.749, respectively), the postoperative model (0.765 and 0.753, respectively), AJCC TNM (8th) stage (0.513 and 0.509, respectively), and BCLC stage (0.527 and 0.529, respectively) (Figure 11). We also found that the prognostic efficacy of the models combined with inflammatory indicators was better than traditional clinical staging at 2 and 3 years. The predictive performance of the postoperative model at 2 and 3 years (0.765 and 0.753, respectively) was slightly better than that of the preoperative model (0.754 and 0.749, respectively).
 |
Figure 11 Different models and the area under the time-dependent ROC for predicting (A) 2-year and (B) 3-year DFS. |
Discussion
The influence of inflammation on the biological behavior of tumors is complex. Different inflammatory cells have pro- and antitumor properties. Neutrophils promote tumor progression by enhancing angiogenesis and promoting tumor proliferation and metastasis.20 Platelets protect tumor cells from attack and mediate the arrest of tumor cell platelet emboli at the blood vessel wall and further pass through capillaries to accelerate metastasis.21 Lymphocytes are typical antitumor cells that inhibit or kill tumor cells via cytotoxicity.22,23 Albumin levels are positively correlated with the efficacy of immunotherapy, and patients with high albumin levels have a better prognosis.24 The prognostic value of a single inflammatory indicator is limited. Combining inflammatory indicators with different effects may better reflect whether the immune balance is in a pro- or antitumor state.
Many studies combined different inflammatory indicators to explore the prognostic value of inflammatory indicators; in particular, preoperative inflammation indicators have been extensively studied.8–13 However, the prognostic value of postoperative inflammatory indicators in patients with HCC is still unclear. To the best of our knowledge, this study is the first to compare the prognostic efficacy of traditional clinical staging systems and models that integrated preoperative and postoperative inflammation indicators. The efficacy of postoperative inflammatory indicators, preoperative inflammatory indicators and clinicopathological indicators was compared for the first time. Our study found that the preNLR, predNLR, prePLR, prePNI, preLMR, postNLR, postdNLR, postPNI, and postLMR were risk factors for poor DFS, and the prePLR, prePNI, and postNLR were independent risk factors. Among the independent prognostic factors, the prognostic efficacy of the postNLR was better than that of the other preoperative inflammatory indicators and clinicopathological indicators. The introduction of postoperative inflammatory indicators improved the predictive performance of the prognostic model for DFS, and it was better than the preoperative model, postoperative model and traditional staging systems.
The treatment process for tumors is long and complicated. An increasing number of researchers have paid attention to the influence of postoperative inflammatory indicators on the long-term prognosis of patients with different solid tumors.16,17,25–28 HCC is a typical inflammation-related cancer, and inflammation has a significant impact on the prognosis of patients with HCC.6 Therefore, the influence of postoperative inflammation on the prognosis of patients HCC deserves more attention. One study found that a high postNLR after curative hepatectomy was significantly associated with poorer overall survival and DFS.17 Another study on radiofrequency ablation of HCC showed that a high postNLR had a negative effect on long-term prognosis.16 Similarly, our study found that a high postNLR was significantly associated with poor DFS. One breast cancer study found that the immune status of the patient changed after the main primary tumor was removed.14 Our previous study confirmed this finding and revealed that a high preNLR was associated with a greater tumor burden, but there was no significant correlation between the postNLR and tumor burden.15 Tumor recurrence is affected by many factors. Even after the HCC tumor is completely removed or ablated, the microenvironment of the remaining carcinogenic tissue in the liver can also cause recurrence of de novo HCC tumors.29 It is generally believed that recurrence after 2 years is caused by the carcinogenic microenvironment.30 Our research indicated that the prognostic model combined with postoperative inflammatory indicators was better than the preoperative model and traditional clinical staging systems in predicting 2- and 3-year DFS, and the calibration curve also showed good consistency. Notably, the predictive power of the postNLR for 2- and 3-year DFS was better than that of all of the other independent prognostic factors, including preoperative inflammatory indicators and commonly used clinicopathological indicators. We speculate that the possible reason for this result is that high postoperative inflammation can activate potential micrometastases and plays an important role in the carcinogenic microenvironment.
Although previous studies explored the prognostic value of postoperative inflammatory indicators, there were differences in postoperative acquisition time, which ranged from 1 day to several months.16,17,25–28 In response to tissue damage, neutrophils migrate forward and backward in the damaged tissue and vasculature.31 Therefore, the incision healing process may have a potential impact on inflammatory indicators in the peripheral blood. One study focused on the prognostic value of the postNLR and postPLR at 1, 3, 5, and 7 days and at 3, 6, and 12 months and revealed that the prognostic value of these indicators stabilized 3 days after liver transplantation.32 A colorectal cancer study revealed that the postNLR on day 7 was an independent prognostic factor for DFS, but the postNLR on day 1 was not an independent prognostic indicator.33 These two studies demonstrated that postoperative inflammatory indicators increased rapidly on the first day then declined rapidly.32,33 Neutrophils are transient effector cells that undergo apoptosis in damaged tissues.31 Therefore, due to the impact of incision and surgical stress in the early postoperative period, the inflammatory indicators in the peripheral blood cannot accurately reflect the patient’s immune status, and the prognostic value of early postoperative inflammatory indicators is limited. Taken together, these results suggest that it could be reasonable to select postoperative inflammatory indicators after 3 days.
There are some limitations to address. First, this study was a retrospective study with potential bias. Second, this study was a single-center study, and further multicenter, large-sample studies are needed. Finally, most patients in this study had hepatitis viral infection, and the incidence of this infection may vary across ethnicities.
Conclusion
Postoperative inflammatory indicators are valuable prognostic indicators, and postNLR can better predict DFS of patients with HCC after partial hepatectomy than preoperative inflammatory indicators and clinicopathological indicators. The prognostic model that combined preoperative and postoperative inflammatory indicators had improved predictive performance and was better than the AJCC TNM (8th) stage and BCLC stage. Future research should pay more attention to postoperative inflammatory indicators.
Acknowledgments
The work is supported by the National Natural Science Foundation of China (81930119), Natural Science Foundation of Beijing Municipality (Z190024), Medical-Engineering Project of Tsinghua University School of Software (MESR201912-3), and CAMS Innovation Fund for Medical Sciences (2019-l2M-5-056).
Disclosure
The authors declare that there are no conflicts of interest for this work.
References
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
2. Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104(13):1775–1784. doi:10.1002/bjs.10677
3. Di Sandro S, Centonze L, Pinotti E, et al. Surgical and oncological outcomes of hepatic resection for BCLC-B hepatocellular carcinoma: a retrospective multicenter analysis among 474 consecutive cases. Updates Surg. 2019;71(2):285–293. doi:10.1007/s13304-019-00649-w
4. Duseja A. Staging of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S74–79. doi:10.1016/j.jceh.2014.03.045
5. Chen LJ, Chang YJ, Chang YJ. Survival predictability between the american joint committee on cancer 8th edition staging system and the barcelona clinic liver cancer classification in patients with hepatocellular carcinoma. Oncologist. 2021;26(3):e445–e453. doi:10.1002/onco.13535
6. Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. doi:10.1038/s41590-018-0044-z
7. Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. doi:10.1038/s41698-018-0048-z
8. Dharmapuri S, Ozbek U, Lin JY, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020;9(14):4962–4970. doi:10.1002/cam4.3135
9. Wu SJ, Lin YX, Ye H, Li FY, Xiong XZ, Cheng NS. Lymphocyte to monocyte ratio and prognostic nutritional index predict survival outcomes of hepatitis B virus-associated hepatocellular carcinoma patients after curative hepatectomy. J Surg Oncol. 2016;114(2):202–210. doi:10.1002/jso.24297
10. Ismael MN, Forde J, Milla E, Khan W, Cabrera R. Utility of inflammatory markers in predicting hepatocellular carcinoma survival after liver transplantation. Biomed Res Int. 2019;2019:7284040.
11. Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. 2017;44(3):967–981. doi:10.1159/000485396
12. Wang Z, Wang J, Wang P. The prognostic value of prognostic nutritional index in hepatocellular carcinoma patients: a meta-analysis of observational studies. PLoS One. 2018;13(10):e0202987. doi:10.1371/journal.pone.0202987
13. Song W, Tian C, Wang K, Zhang RJ, Zou SB. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: a meta-analysis. Sci Rep. 2017;7:46601. doi:10.1038/srep46601
14. Rashid OM, Nagahashi M, Ramachandran S, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153(6):771–778. doi:10.1016/j.surg.2013.02.002
15. Wu M, Yang S, Feng X, Yu F, Liu X, Dong J. Preoperative plus postoperative neutrophil-lymphocyte ratio for predicting overall survival following partial hepatectomy for hepatocellular carcinoma. Oncol Lett. 2020;20(6):375. doi:10.3892/ol.2020.12238
16. Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8:3. doi:10.1371/journal.pone.0058184
17. Peng W, Li C, Wen T-F, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. Journal of Surgical Research. 2014;192(2):402–408. doi:10.1016/j.jss.2014.05.078
18. National Hepatobiliary Standard Database of China. Primary Hepatocellular Carcinoma Dataset of Beijing Tsinghua Changgung Hospital 2014-2019; 2021. Available from: http://www.udimed.org/CDR/20210099.
19. Ji GW, Zhu FP, Xu Q, et al. Radiomic features at contrast-enhanced CT predict recurrence in early stage hepatocellular carcinoma: a Multi-Institutional Study. Radiology. 2020;294(3):568–579. doi:10.1148/radiol.2020191470
20. Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C
21. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. doi:10.1186/s13045-018-0669-2
22. Yunger S, El A B, Zeltzer LA, et al. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology. 2019;8(12):e1672494. doi:10.1080/2162402X.2019.1672494
23. Zhang E, Yang P, Gu J, et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. J Hematol Oncol. 2018;11(1):102. doi:10.1186/s13045-018-0646-9
24. Jiang Y, Tu X, Zhang X, et al. Nutrition and metabolism status alteration in advanced hepatocellular carcinoma patients treated with anti-PD-1 immunotherapy. Support Care Cancer. 2020;28(11):5569–5579. doi:10.1007/s00520-020-05478-x
25. Jakubowska K, Koda M, Kisielewski W, Kanczuga-Koda L, Grudzinska M, Famulski W. Pre- and postoperative neutrophil and lymphocyte count and neutrophil-to-lymphocyte ratio in patients with colorectal cancer. Molecular and Clinical Oncology. 2020;13(5):56. doi:10.3892/mco.2020.2126
26. Jin F, Han A, Shi F, Kong L, Yu J. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Oncotargets and Therapy. 2016;9:6529–6537. doi:10.2147/OTT.S117290
27. Min KW, Kwon MJ, Kim DH, et al. Persistent elevation of postoperative neutrophil-to-lymphocyte ratio: a better predictor of survival in gastric cancer than elevated preoperative neutrophil-to-lymphocyte ratio. Sci Rep. 2017;7(1):13967. doi:10.1038/s41598-017-13969-x
28. He W, Wei M, Yang X, et al. Do inflammatory markers predict prognosis in patients with synchronous colorectal cancer? Medicine. 2017;96(17).
29. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–549.
30. Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi:10.1245/s10434-019-07227-9
31. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16(6):378–391. doi:10.1038/nri.2016.49
32. Pravisani R, Mocchegiani F, Isola M, et al. Postoperative trends and prognostic values of inflammatory and nutritional biomarkers after liver transplantation for hepatocellular carcinoma. Cancers (Basel. 2021;13(3):513. doi:10.3390/cancers13030513
33. Hayama T, Hashiguchi Y, Okada Y, et al. Significance of the 7th postoperative day neutrophil-to-lymphocyte ratio in colorectal cancer. Int J Colorectal Dis. 2020;35(1):119–124. doi:10.1007/s00384-019-03463-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
