Back to Journals » Journal of Pain Research » Volume 14
Combined Percutaneous Kyphoplasty/Pediculoplasty by Posterolateral Transpedicular Approach for Painful Cervical Spine Metastases: A Single-Center Prospective Study
Authors Xia Y, Zhai H, Wang X, Wang Y, Feng B
Received 17 March 2021
Accepted for publication 26 May 2021
Published 10 June 2021 Volume 2021:14 Pages 1699—1706
DOI https://doi.org/10.2147/JPR.S310446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Yonghui Xia, Huan Zhai, Xinlei Wang, Yudong Wang, Bo Feng
Department of Radiology, The First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Bo Feng
Department of Radiology, The First Affiliated Hospital of China Medical University, No. 155 Nanjing North Street, Heping District, Shenyang, Liaoning, 110001, People’s Republic of China
Tel +8613898121986
Email [email protected]
Purpose: In patients requiring percutaneous kyphoplasty (PKP) for painful cervical spine metastases (PCSMs), the surgical approach is of utmost importance. Anterolateral and transoral routes are generally used at present, whereas PKP as well as percutaneous pediculoplasty (PPP) via posterolateral transpedicular approach (PTPA) has yet to be pursued in the treatment of PCSMs. The study was designed to evaluate safety and efficacy of PKP procedures combined with PPP via PTPA as treatment of PCSMs.
Patients and Methods: The patients with PCSMs were enrolled and housed in a database. The pain intensity of enrolled patients was gauged by Visual Analog Scale (VAS), ranging from 0 (none) to 10 (extreme). After preprocedural imaging assessment, combined PKP/PPP via PTPA was performed under the guidance of CT and fluoroscopic monitoring. Postprocedural VAS scores, complications, cement dosage, and hospitalization were recorded in the database for analysis. All cases were followed up for 6 months.
Results: Adult enrollees (7 women, 4 men) with PCSMs successfully underwent PKP/PPP via PTPA between February 2019 and January 2020, injected with 3.7± 0.7 mL (range, 2.5– 4.8 mL) of cement on average. Other than a single instance of asymptomatic cement leakage into paravertebral soft tissues, no complications ensued. Significant analgesic effects observed 24 hours after procedures were sustained for up to 6 months in follow-up surveys. Postprocedural hospitalizations were as brief as 2.2± 0.8 days.
Conclusion: Combined PKP/PPP via PTPA is safe and effective as treatment of PCSMs, enabling quick pain relief and patient recovery.
Keywords: percutaneous vertebral augmentation, safety, efficacy, Visual Analog Scale
Introduction
Lesions of the cervical spine account for 8–15% of all spinal metastases1,2 causing intractable pain or numbness and markedly undermining quality of life. Percutaneous vertebral augmentation (PVA), including percutaneous kyphoplasty (PKP) and percutaneous vertebroplasty (PVP), is a minimally invasive and promising technique for treating painful cervical spine metastases (PCSMs)3–6 cited in international guidelines.7–9 Percutaneous pediculoplasty (PPP), as a non-classical PVA technique, has been applied to patients with vertebral pedicle involvement.10–14 Currently, PVA is more often used for thoracolumbar metastases than for PCSMs due to problematic puncture strategies. In patients with PCSMs, PVA is largely performed via anterolateral or transoral approach, although the risk of injury to vital organs abutting cervical spine is high by either route. The present single-center prospective study was undertaken to evaluate posterolateral transpedicular access, a seldom-used alternative in patients submitting to combined PKP/PPP procedures for PCSMs, assessing operative safety and efficacy up to 6 months.
Patients and Methods
Our study protocol was approved by the ethics committee at the First Affiliated Hospital of China Medical University. Enrollees granted consent after appropriate preprocedural consultation and disclosure. This study was conducted in accordance with the Declaration of Helsinki.
All patient information was housed in a database created in February 2019. Radiographic studies were obtained at baseline included enhanced magnetic resonance imaging (MRI) and enhanced 3D-computed tomography (3D-CT) to assess the lesions and distinguish the organs at risk including vertebral arteries and spinal canal during the procedures. Visual Analog Scale (VAS), scored from 0 (none) to 10 (extreme), served to assess pain intensity.
Inclusion criteria were as follows: (1) VAS score ≥6, with/without numbness, (2) failed drug treatment, (3) definitive single PCSM, and (4) adult age, 18–85 years. Exclusion criteria were the following: (1) infection or coagulation dysfunction, (2) osteogenic metastases, (3) severe underlying disorders, such as cardiovascular or cerebrovascular disease, (4) high-level paraplegia, (5) the height or width of the cervical pedicle is less than 3.4 mm, and (6) dyscrasia.
Combined PKP/PPP via PTPA
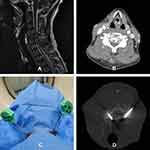
PKP was performed aseptically under local anesthesia and analgesics were added intraoperatively if necessary. To ensure intraoperative postural stability and optimize accuracy of puncture, patients were trained in the prone position 1 day before the procedures and were immobilized in vacuum pads during the procedures. Each received cefuroxime sodium (1.5 g) as prophylaxis, delivered by intravenous drip 30 min before PKP commencing. We used 7G trocar needles with triangular-edged tips (Guanlong Medical Utensils Co., Ltd, Shandong, China) to puncture uni- or bilateral pedicles at targeted zones under CT guidance, leaving one or two 10-F working cannulas in place after removal of the needles. During puncture procedures, injuries to vertebral artery and spinal canal were carefully avoided by real-time comparison between preoperative enhanced 3D-CT images and intraoperative CT guidance images, as well as slowly advancing the needles (see Figure 1). A CT rescan was performed to identify the direction once the tips of the needles were fixed at the cortex of pedicles.
After the needles reached the desired position in vertebral body, Patients were moved to an examining table for fluoroscopic monitoring (Artis zeego; Siemens, Munich, Germany), and small balloons (9-mm across, 10-mm long; Guanlong Medical Utensils Co, Ltd, Shandong, China) were inserted into targeted lesions. Balloons were inflated with contrast medium to shape cavities for low-pressure injection of polymethyl methacrylate (PMMA) cement (Heraeus Medical GmbH, Wehrheim, Germany). Prepared cement (toothpaste consistency) was subsequently infused at a slow rate into vertebral bodies under fluoroscopy, monitored by anteroposterior and lateral observation in real-time. Once satisfactorily filled, PPP immediately followed, injecting additional pasty cement into punctured pedicles at low pressure. The cementitious content of vertebral bodies and pedicles was thereby interconnected. Afterwards, CT rescan was performed to assess the diffusion and leakage of cement (see Figure 2). VAS scoring of pain intensity (patient generated) was recorded 24 hours after procedures and 6 months later by way of follow-up phone surveys. Volumes of injected cement, complications, changes in pain and numbness, and duration of hospitalization were chronicled for analysis.
Statistical Analysis
All computations were driven by standard software (SPSS v20.0; IBM Corp, Armonk, NY, USA), expressing ranked data as median (interquartile range) values and measured data as mean±standard deviation. One-way analysis of variance (ANOVA) was used to analyze changes in pain intensity (VAS), setting significance at p<0.05.
Results
Patient Characteristics at Baseline
Eleven patients (women, 7; men, 4) with PCSMs were enrolled for the study from February 2019 to January 2020. The average age was 62.5±15.1 years (range, 35–83 years). Primary tumor sites included breast (n=4), lung (n=3), liver (n=2), kidney (n=1), and thyroid gland (n=1). Spinal metastases involved C2 (n=2), C4 (n=2), C5 (n=5), C6 (n=1), and C7 (n=1). Four patients also showed pedicle invasion, producing numbness of upper limbs (unilateral, n=3; bilateral, n=1). Mean VAS score at baseline was 7 (range, 6–8). Patient characteristics are fully detailed in Table 1.
 |
Table 1 Enrolled Patients’ Characteristics |
Therapeutic Outcomes
Combined PKP/PPP was successfully performed via PTPA in all eleven patients with PCSMs. On average, 3.7±0.7 mL (range, 2.5–4.8 mL) of PMMA cement was injected. All enrolled patients completed a 6-month follow-up. Mean VAS scores 24 hours and 6 months after procedures were 1 (interquartile range, 1–2) and 2 (interquartile range, 1–2), respectively. Relative to baseline scores, significant analgesic effects were achieved at both 24-hour (95% CI: 4.94–6.52; p<0.001) and 6-month (95% CI: 4.39–5.97; p<0.001) time points; and in comparing postprocedural VAS scores (24 hours vs 6 months), there was no significant difference (95% CI: −1.34 to 0.25; p=0.169). The four patients with numbness prior to treatment also reported substantial relief after 24 hours that did not diminish during the 6-month follow-up period. Mean postprocedural hospitalization was 2.2±0.8 days (range, 1–3 days). The data of outcomes is detailed in Table 2.
 |
Table 2 The Outcomes of Combined PKP/PPP via PTPA for PCSMs |
Complications
CT examination disclosed one instance (9.09%) of cement leakage into paravertebral soft tissues (see Figure 3), devoid of clinical symptoms. No other complications (ie, neurologic defects, infections, or hemorrhage) were observed after procedures or during follow-up monitoring.
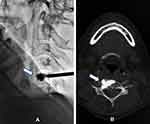 |
Figure 3 Cement leakage. (A) Asymptomatic cement leakage into paravertebral soft tissues was observed by fluoroscopy and (B) Post-procedural CT scan. |
Discussion
In treating patients with PCSMs, the approach used for PKP is the most critical determinant of technical success. Unlike the thoracolumbar spine, the cervical segment is notably smaller and has transverse foramina surrounding the vertebral artery. It is also flanked by a number of vital structures, including carotid artery, jugular vein, trachea, esophagus, and thyroid gland. Despite the palliative potential in this setting, these issues are problematic for cervical PKP, explaining why thoracolumbar applications are generally preferred. Although PKP has been conducted for PCSMs by anterolateral, translateral, or transoral approach,15–19 PTPA has not been attempted to date.
In this particular trial, however, we successfully tested PTPA in combined PKP/PPP procedures for PCSMs, regularly achieving satisfactory VAS-scored analgesia for up to 6 months. The exact mechanisms of action are not entirely clear, but it is presumed that PKP and PPP confer vertebral stability while exerting cytotoxic and thermal effects on tumor cells and nerve endings.20 To our knowledge, this is the first effort and the largest patient series involving PKP/PPP in combination via PTPA as treatment of PCSMs.
Anterolateral and transoral approaches have been used by others in treating PCSMs, although PVP and PKP are still high-risk complex procedures under these circumstances. Both methods are fairly harsh, often requiring general anesthesia. Transoral procedures carry a high risk of infection to the organs including the throat, brain, and cervical spine. In anterolateral procedures, manual traction of carotid artery is needed, and prolonged overextension is taxing for obese patients and for those in severe pain.21 Translateral CT-guided PVP of upper cervical spine, as reported by Guo, is yet another mode of puncture that unfortunately still places carotid artery and jugular vein in jeopardy.22 To properly expose puncture sites at vertebral targets, some have advocated anterolateral surgical incisions prior to kyphoplasty.23–26 However, these measures are not truly percutaneous and inflict unnecessary injury.
PTPA is seldom used in clinical practice, encountered in just four patients treated by PVP (not PKP) during a search of the literature27–30 (see Table 3), and apparently never invoked for PKP. This approach differs from alternative strategies in that there is a safe space between the puncture site and the posterior bone cortex of the pedicle allowing the needle to be fixed between the transverse foramen and the spinal canal, under CT guidance. Then, the only two structures at risk (vertebral artery and spinal canal) lie on either side of the needle passageway, rather than directly ahead of needle tip. Advancing the needle into vertebral body through the pedicle is the most perilous step, calling for extreme vigilance. CT monitoring guidance is a highly advisable adjunct to ensure the safety of puncture. From our perspective, PTPA seemed to be the most concise and most secure method of accessing cervical spine. Despite the opposing view that PTPA is particularly difficult and dangerous, given the proximity of vertebral arteries and the small width of cervical pedicles,15 morphometric analysis indicates that implants as small as 4 mm are safely treated;31–34 and in our cohort, we routinely used a 7G (3.4 mm) needle.
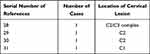 |
Table 3 The List of 4 Literatures About Case Reports of PVP (Not PKP) via PTPA for Cervical Spine Lesions |
It is true that secondary fracture of the pedicle remains a potential hazard and an inherent disadvantage of PTPA. PPP was thus added to PKP as a precaution. By injecting cement into cervical pedicles, no secondary fractures resulted. In past studies, PPP has been described as a vertebroplasty-complementary technique for treating traumatic or metastatic lesions of thoracolumbar pedicles.10–14 We recommend PPP as reinforcement, whether or not the cervical pedicle itself is involved, combined with PKP in treating PCSMs via PTPA.
As shown in our patients, complementary PPP may also relieve numbness by stabilizing pedicles that harbor metastatic disease. Injury to the vertebral artery is another serious complication of PTPA in this clinical context. Enhanced CT examination of cervical spine is essential in advance of therapeutic intervention to delineate the position of vertebral artery.
Cement leakage is a common complication of PVP and PKP that is asymptomatic in most cases. Still, such leakage should be kept to a minimum in the confined cervical space. PKP and PVP may be equally effective in analgesia, but PKP is superior in terms of cement leakage and restoring vertebral height.35 Considering the high safety threshold for PCSMs, we prefer a PKP/PPP combination procedure performed via PTPA. In our cohort, only one patient experienced asymptomatic leakage of cement into paravertebral soft tissues.
Local anesthesia was used in all of our patients and served adequately. In addition, all patients complied with and benefitted from preprocedural positional exercise36 and neck splinting by vacuum pad. An antibiotic given 30 min before and was also sufficient as prophylaxis, no postoperative dosing thereafter. Procedure-related infections were not a problem. Compared with traditional open surgery, percutaneous procedures allow faster patient recovery and shorter hospital stays, ours averaging 2.2±0.8 days.
Certain study limitations deserve mention. This single-center prospective trial of PKP via PTPA in patients with PCSMs lacked a control group. The cohort we recruited was also exceedingly small.
In conclusion, PKP and PPP performed in combination via PTPA is a viable treatment for patients with PCSMs, proven safe and effective in this preliminary study.
Acknowledgments
The authors thank the nurses from interventional operating room at the First Affiliated Hospital of China Medical University for their cooperation. This study did not receive any funding or financial support.
Disclosure
The authors declare no conflicts of interest.
References
1. Mesfin A, Buchowski JM, Gokaslan ZL, Bird JE. Management of metastatic cervical spine tumors. J Am Acad Orthop Surg. 2015;23(1):38–46. doi:10.5435/JAAOS-23-01-38
2. Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31(4):515–528. doi:10.1016/S0030-5898(05)70171-1
3. Clarençon F, Fahed R, Cormier E, et al. Safety and effectiveness of cervical vertebroplasty: report of a large cohort and systematic review. Eur Radiol. 2020;30(3):1571–1583. doi:10.1007/s00330-019-06525-w
4. Cohen M, Zeitoun D, Blanpain S, Brochard C, Lellouche J, Deramond H. Percutaneous vertebroplasty of the C2 body and dens using the anterior oblique ascending transdiscal approach. J Neuroradiol. 2013;40(3):211–215. doi:10.1016/j.neurad.2013.03.002
5. De la Garza-ramos R, Benvenutti-Regato M, Caro-Osorio E. Vertebroplasty and kyphoplasty for cervical spine metastases: a systematic review and meta-analysis. Int J Spine Surg. 2016;10:7. doi:10.14444/3007
6. Masala S, Anselmetti GC, Muto M, Mammucari M, Volpi T, Simonetti G. Percutaneous vertebroplasty relieves pain in metastatic cervical fractures. Clin Orthop Relat Res. 2011;469(3):715–722. doi:10.1007/s11999-010-1550-y
7. Tsoumakidou G, Too CW, Koch G, et al. CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Intervent Radiol. 2017;40(3):331–342. doi:10.1007/s00270-017-1574-8
8. Chandra RV, Meyers PM, Hirsch JA, et al.; Society of Neurointerventional Surgery. Vertebral augmentation: report of the standards and guidelines committee of the society of neurointerventional surgery. J Neurointerv Surg. 2014;6(1):7–15. doi:10.1136/neurintsurg-2013-011012
9. Shah LM, Jennings JW, Kirsch CFE, et al. ACR appropriateness criteria eq \o\ac(○,R)management of vertebral compression fractures. J Am Coll Radiol. 2018;15(11S):S347–S364. doi:10.1016/j.jacr.2018.09.019
10. Eyheremendy EP, De Luca SE, Sanabria E. Percutaneous pediculoplasty in osteoporotic compression fractures. J Vasc Interv Radiol. 2004;15(8):869–874. doi:10.1097/01.RVI.0000136969.96466.99
11. Singh J, Baker MD, Morris PP, Whitlow CT. Percutaneous pediculoplasty for traumatic pedicle fracture. A technical case report. Interv Neuroradiol. 2012;18(2):221–226. doi:10.1177/159101991201800216
12. Ke ZY, Wang Y, Zhong YL, Chen L, Deng ZL. Percutaneous vertebroplasty combined with percutaneous pediculoplasty for lytic vertebral body and pedicle lesions of metastatic tumors. Pain Physician. 2015;18(3):E347–353.
13. Martin JB, Wetzel SG, Seium Y, et al. Percutaneous vertebroplasty in metastatic disease: transpedicular access and treatment of lysed pedicles–initial experience. Radiology. 2003;229(2):593–597. doi:10.1148/radiol.2292020976
14. Gailloud P, Beauchamp NJ, Martin J-B, Murphy KJ. Percutaneous pediculoplasty: polymethylmethacrylate injection into lytic vertebral pedicle lesions. J Vasc Interv Radiol. 2002;13(5):517–521. doi:10.1016/S1051-0443(07)61533-4
15. Lykomitros V, Anagnostidis KS, Alzeer Z, Kapetanos GA. Percutaneous anterolateral balloon kyphoplasty for metastatic lytic lesions of the cervical spine. Eur Spine J. 2010;19(11):1948–1952. doi:10.1007/s00586-010-1465-z
16. Bao L, Jia P, Li J, et al. Percutaneous vertebroplasty relieves pain in cervical spine metastases. Pain Res Manag. 2017;2017:3926318. doi:10.1155/2017/3926318
17. Seo SS, Lee DH, Kim HJ, Yoon JW, Kwon OS, Kim KH. Percutaneous vertebroplasty at C7 for the treatment of painful metastases -a case report. Korean J Anesthesiol. 2013;64(3):276–279. doi:10.4097/kjae.2013.64.3.276
18. Anselmetti G, Manca A, Montemurro F, et al. Vertebroplasty using transoral approach in painful malignant involvement of the second cervical vertebra (C2): a single-institution series of 25 patients. Pain Physician. 2012;15:35–42. doi:10.36076/ppj.2012/15/35
19. Monterumici DA, Narne S, Nena U, Sinigaglia R. Transoral kyphoplasty for tumors in C2. Spine J. 2007;7(6):666–670. doi:10.1016/j.spinee.2006.08.007
20. Xie P, Zhao Y, Li G. Efficacy of percutaneous vertebroplasty in patients with painful vertebral metastases: a Retrospective Study in 47 cases. Clin Neurol Neurosurg. 2015;138:157–161. doi:10.1016/j.clineuro.2015.08.026
21. Wang KW, Wang HK, Lu K, Liang CL, Chen YW, Liliang PC. Fluoroscopically guided C2 percutaneous vertebroplasty: a surgical technique note on an anterior ascending approach. Pain Physician. 2016;19(4):E625–629.
22. Guo WH, Meng MB, You X, et al. CT-guided percutaneous vertebroplasty of the upper cervical spine via a translateral approach. Pain Physician. 2012;15(5):E733–741.
23. Blondel B, Adetchessi T, Demakakos J, Pech-Gourg G, Dufour H, Fuentes S. Anterolateral kyphoplasty in the management of cervical spinal metastasis. Orthop Traumatol Surg Res. 2012;98(3):341–345. doi:10.1016/j.otsr.2012.01.004
24. Quraishi NA, Elsayed S. A traumatic, high-energy and unstable fracture of the C5 vertebra managed with kyphoplasty: a previously unreported case. Eur Spine J. 2011;20(10):1589–1592. doi:10.1007/s00586-011-1858-7
25. Druschel C, Schaser KD, Melcher I, Haas NP, Disch AC. Minimally invasive combined anterior kyphoplasty for osteolytic C2 and C5 metastases. Arch Orthop Trauma Surg. 2011;131(7):977–981. doi:10.1007/s00402-011-1270-0
26. Sebaaly A, Najjar A, Wang Z, Boubez G, Masucci L, Shedid D. Anterolateral cervical kyphoplasty for metastatic cervical spine lesions. Asian Spine J. 2018;12(5):823–829. doi:10.31616/asj.2018.12.5.823
27. Dang D, Baig MN, Christoforidis G, Chiocca EA, Gabriel J. C2/C3 pathologic fractures from polyostotic fibrous dysplasia of the cervical spine treated with percutaneous vertebroplasty. Eur Spine J. 2007;16(Suppl 3):250–254. doi:10.1007/s00586-007-0434-7
28. Sun G, Jin P, Li M, et al. Percutaneous vertebroplasty for treatment of osteolytic metastases of the C2 vertebral body using anterolateral and posterolateral approach. Technol Cancer Res Treat. 2010;9(4):417–422. doi:10.1177/153303461000900411
29. Sun HY, Lee JW, Kim KJ, Yeom JS, Kang HS. Percutaneous intervention of the C2 vertebral body using a CT-guided posterolateral approach. Am J Roentgenol. 2009;193(6):1703–1705. doi:10.2214/AJR.09.2783
30. Cianfoni A, Distefano D, Chin SH, Varma AK, Rumboldt Z, Bonaldi G. Percutaneous cement augmentation of a lytic lesion of C1 via posterolateral approach under CT guidance. Spine J. 2012;12(6):500–506. doi:10.1016/j.spinee.2012.05.012
31. Sheng SR, Xu HZ, Wang YL, et al. Comparison of cervical spine anatomy in calves, pigs and humans. PLoS One. 2016;11(2):e0148610. doi:10.1371/journal.pone.0148610
32. Moser M, Farshad M, Farshad-Amacker NA, Betz M, Spirig JM. Accuracy of patient-specific template-guided versus freehand cervical pedicle screw placement from C2 to C7: a Randomized Cadaveric Study. World Neurosurg. 2019;126:e803–e813. doi:10.1016/j.wneu.2019.02.152
33. Mahiphot J, Iamsaard S, Sawatpanich T, Sae-Jung S, Khamanarong K. A Morphometric Study on subaxial cervical pedicles of Thai people. Spine. 2019;44(10):E579–E584. doi:10.1097/BRS.0000000000002920
34. Westermann L, Spemes C, Eysel P, et al. Computer tomography-based morphometric analysis of the cervical spine pedicles C3–C7. Acta Neurochir. 2018;160(4):863–871. doi:10.1007/s00701-018-3481-4
35. Wang B, Zhao CP, Song LX, Zhu L. Balloon kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral compression fracture: a meta-analysis and systematic review. J Orthop Surg Res. 2018;13(1):264. doi:10.1186/s13018-018-0952-5
36. Li G, Liu H, Wang Q, Zhong D. Preoperative prone position exercises: a simple and novel method to improve tolerance to kyphoplasty for treatment of single level osteoporotic vertebral compression fractures. BMC Musculoskelet Disord. 2017;18(1):472. doi:10.1186/s12891-017-1843-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.