Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Combined Exercise Training Promotes More Benefits on Cardiovascular Autonomic Modulation in Ovariectomized Rats Than Isolated Aerobic or Resistance Training
Authors Da Costa-Santos N , Minguta Santos Costa G , Dos-Santos A, Nascimento-Carvalho B , Ribeiro TF, Freitas SCF , Caperuto E, Irigoyen MC , De Angelis K , Scapini KB, Sanches IC
Received 20 August 2022
Accepted for publication 10 April 2023
Published 26 June 2023 Volume 2023:16 Pages 1903—1913
DOI https://doi.org/10.2147/DMSO.S386944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Nicolas Da Costa-Santos,1 Gabrielly Minguta Santos Costa,2 Adriano Dos-Santos,1 Bruno Nascimento-Carvalho,3 Thayna Fabiana Ribeiro,1 Sarah Cristina Ferreira Freitas,2 Erico Caperuto,1 Maria-Claudia Irigoyen,3 Katia De Angelis,4 Kátia Bilhar Scapini,1 Iris Callado Sanches1
1Human Movement Laboratory, São Judas University, São Paulo, Brazil; 2Laboratory of Translational Physiology, Universidade Nove de Julho (UNINOVE), São Paulo, Brazil; 3Hypertension Unit, Heart Institute, Medicine School, University of São Paulo, São Paulo, Brazil; 4Physiology Exercise Laboratory, Department of Physiology, Federal University of Sao Paulo (UNIFESP), São Paulo, Brazil
Correspondence: Iris Callado Sanches, Human Movement Laboratory, São Judas University, Rua taquari, 546, São Paulo, SP, 03166-000, Brazil, Email [email protected]
Introduction: Cardiovascular risk increase after ovarian deprivation has been extensively demonstrated by our research group through cardiovascular autonomic analysis. Interventions involving different types of exercises, such as resistance exercises or combined exercises (aerobic and resistance) have been widely recommended to prevent or minimize neuromuscular decline in postmenopausal women, which is aggravated by a sedentary lifestyle. Experimentally, the cardiovascular effects of resistance or combined training, as well as comparison between aerobic, resistance, and combined training, in ovariectomized animals are scarce.
Purpose: In this study, we hypothesized that the combination of aerobic and resistance training may be more effective in preventing muscle mass loss, as well as improving cardiovascular autonomic modulation and baroreflex sensitivity, than aerobic or resistance training individually in ovariectomized rats.
Animals and Methods: Female rats were divided into 5 groups: sedentary (C); ovariectomized (Ovx); trained ovariectomized submitted to aerobic training (OvxAT); resistance training (OvxRT); combined training (OvxCT). Exercise training lasted 8 weeks, with the combined group alternating between aerobic training and resistance training every other day. At the end of the study, glycemia and insulin tolerance were evaluated. Arterial pressure (AP) was directly recorded. Baroreflex sensitivity was assessed by heart rate response to changes in arterial pressure. Cardiovascular autonomic modulation was evaluated by spectral analysis.
Results: Combined training was the only training regime that increased baroreflex sensitivity for tachycardic response and reduced all systolic blood pressure variability parameters. Furthermore, all animals submitted to exercise training on a treadmill (OvxAT and OvxCT) presented lower systolic, diastolic, and mean pressure, as well as improvements in the autonomic modulation for the heart.
Conclusion: Combined training showed to be more effective than isolated aerobic and resistance training, mixing the isolated benefits of each modality. It was the only modality able to increase baroreflex sensitivity to tachycardic responses, reduce arterial pressure and all parameters of vascular sympathetic modulation.
Keywords: combined exercise training, resistance exercise training, aerobic exercise training, ovarian hormone deprivation, cardiovascular autonomic modulation, baroreflex sensitivity
Introduction
According to the World Health Organization (WHO), cardiovascular diseases are responsible for approximately 17 million deaths per year worldwide.1 Hypertension has been considered the major factor that causes elevated myocardial infarction risk in women compare to men,2 as well as Diabetes.3 In addition, the American heart association (AHA) demonstrated that the incidence of cardiovascular disease in women continues to increase with age, especially after menopause and in the presence of diabetes.4
Experimentally, the ovariectomized model (bilateral ovarian removal surgery) has been used in young animals to study the effects of ovarian deprivation and exclude bias for aging process. The effects of this model on blood pressure are controversial; some reports demonstrate that 8 weeks after ovariectomy surgery a statistically significant increase in arterial pressure (AP) can be observed compared to control females,5 or females with associated risk factors.6 However, other reports did not observe a statistically significant increase in blood pressure.7,8
The analysis of heart rate variability (HRV) and systolic arterial pressure variability (SAP-V) are considered measures of autonomic function.9 Beside the values of mean AP, the study of oscillations in AP over time is associated with cardiovascular and mortality outcomes;9 the SAP-V observed beat-to-beat in a rest state is the result of autonomic modulation.10 In the same way, HRV reflects the interaction of several factors that involve the influence of the autonomic nervous system on cardiac beat-to-beat modulation.11
Baroreceptors are essential controllers of AP maintenance in the short term, and they allow neural adjustments to modulate the cardiovascular autonomic response.12 Baroreflex impairment precedes cardiovascular autonomic dysfunction, and both are strongly related to cardiac mortality.13 Aerobic exercise training is considered an important non-pharmacological strategy that provides cardiovascular benefits in ovariectomized rats, such as reduced blood pressure and heart rate and increased baroreflex sensitivity.14 At the same time, clinical investigations with resistance training primarily focus on neuromuscular adaptations, such as increasing strength, muscular endurance, and functional capacity in women.15 We standardized a resistance training model for experimental studies to investigate cardiovascular effects on different conditions and diseases.16 Previously, resistance training did not reverse blood pressure, cardiovascular autonomic and baroreflex impairment in ovarian deprivation model with hypertension or metabolic dysfunction.17,18
European Society of Cardiology (ESC) and American heart association (AHA) guidelines recommend the combination of aerobic plus resistance training as level of evidence A for cardiovascular prevention in adults.19,20 Experimentally, cardiovascular effects of resistance5,17,18 or combined training in ovariectomized animals are scarce,21,22 as well as aerobic, resistance or combined training comparison in ovariectomized animals.23 Thus, the aim of this study was to compare the cardiovascular and metabolic effects of aerobic, resistance, or combined training (aerobic plus resistance) in an experimental menopause model.
Materials and Methods
Experiments were performed on 40 female Wistar rats (8-week-old) obtained from Nove de Julho University, Brazil. The rats received standard laboratory chow (Nuvital, Colombo, Brazil) and water ad libitum. The animals were housed in cages (4 animals/cage) in a temperature-controlled room (22°C) with a 12:12-h dark-light cycle (dark cycle from 6:00 am to 6:00 pm). Thus, five experimental groups were used (n=8 each group): sedentary (C) and ovariectomized (Ovx); and trained ovariectomized submitted to aerobic training (OvxAT), or resistance training (OvxRT), or combined (aerobic + resistance) exercise training (OvxCT). All surgical procedures and protocols were approved by Nove de Julho University Ethical Committee (protocol AN0023/2015). They were conducted following the recommendations of the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. Experimental design can be seen in Figure 1.
Ovariectomy
Animals were anesthetized (80mg/kg ketamine and 12mg/kg xylazine), and the oviduct was sectioned. The ovaries were removed, and the ovariectomy surgical procedure was confirmed from a previous study.24 Previous studies demonstrated that exercise training for eight weeks attenuates the deleterious effects in ovariectomized or diabetics animals.23
Aerobic Training Protocol
All animals were adapted to walk and run on a motorized treadmill (10min/day, 5 m/min) for 5 days before the initial maximal running test,25 which was used to calculate the exercise intensity. The test consists of placing the animal on the treadmill to run at a 5 m/min speed for 3 minutes. This speed was increased by 5 m/min every 3 minutes until the animal’s exhaustion. This test was performed three times: to begin the protocol (1st week), to recalculate training intensity (4th week), and to observe the animal’s performance after the training protocol (8th week). Aerobic training was a voluntary exercise protocol performed without electrical stimulus, water restriction, or food restriction performed on a treadmill (Imbramed TK-01, Brazil) at moderate intensity (50–70% maximum capacity of running) for 8 weeks (5 days per week ~1 hour/day) without electrical stimulation, water, or food restriction.6
Resistance Training Protocol
Resistance training was a voluntary exercise protocol performed on a ladder adapted for rats without electrical stimulus, water restriction, or food restriction. The animals performed the maximal load test 3 times (1st, 4th, and 8th weeks). The test consisted of an initial climb with a load of 75% of body weight. After completing the first climb, a 2-min rest period they have preceded the next climb. For the next climbs, the loads were increased by another 15% (1st-week test), 25% (4th-week test), or 40% (8thweek test) of body weight. Resistance training intensity was calculated based on the maximal load test. The intensity was like the aerobic training protocol (5070% of maximum load) for 8 weeks, 5 days a week, with 15 climbs per session and a 1min time interval between climbs.16
Combined Training Protocol
Combined exercise training was performed for 8 weeks, 5 days a week, at moderate intensity (50–70% of maximum). It was performed on alternate days: one day on the treadmill and one day on the ladder, using half of the weekly frequency of isolated aerobic and resistance training.21
Insulin Tolerance Test
At the end of the 8th week, animals’ blood glucose level was measured after a 4-hour fast with a glucometer (ACCU-CHEK Advantage, Roche, Brazil). Rats fasted for two more hours, totaling 6 hours of fasting. They were anesthetized with pentobarbital sodium (40 mg/kg) and given a caudal intravenous injection of insulin (0.75U/kg body weight) as described in another study.26,27 The glycemia values from minutes 4 to 16 were used to calculate the plasma glucose drop constant. The constant rate for plasma glucose disappearance (Kitt) was calculated from the formula 0.693/t½.28
Hemodynamic Assessment
In the 9th week, 24 hours after the last training session, two catheters filled with 0.06mL of saline were implanted into anesthetized rats’ carotid artery and jugular vein (80mg/kg ketamine and 12mg/kg xylazine). The arterial cannula was connected to a transducer (Blood Pressure XDCR; Kent Scientific), and AP signals were recorded for a 30-min period using a microcomputer equipped with an analog-to-digital converter (CODAS, 2KHz; DATAQ Instruments). The recorded data were analyzed beat-to-beat to quantify changes in mean arterial pressure (MAP), and heart rate (HR) was calculated from systolic peaks over 60 seconds.26 To avoid detraining, the hemodynamic measurements were made in conscious, freely moving rats in their cage at least 48 hours after the last training session.
Cardiovascular Autonomic Modulation
Baroreflex sensitivity was evaluated by increasing doses of phenylephrine (0.5 to 2.0 μg/mL) and sodium nitroprusside (5 to 20 μg/mL) that were given as sequential bolus injections (0.1mL) to produce pressure responses ranging from 5 to 40 mmHg for both higher and lower AP responses. A 3- to 5-min interval between doses was needed for AP to return to baseline. Baroreflex sensitivity was evaluated by a mean index, calculated as the ratio between changes in HR to the changes in MAP, allowing separate analysis of reflex bradycardia and reflex tachycardia as described elsewhere.21,29
For time and frequency domain analysis of cardiovascular autonomic modulation, more stable time series were chosen (3-time series of 5 min for each animal) of pulse interval (PI) and systolic arterial pressure (SAP). Spectral power is composed of a low-frequency band (LF, 0.20–0.75Hz) and high-frequency band (HF, 0.75–3.0Hz) calculated using a customized routine (CardioSeries V2.4) as described elsewhere.21,24 The sympathovagal ratio (LF/HF) was calculated based on normalized LF and HF.30 The normalized units (nu) were obtained by dividing the power of a given component by the total power (from which the very low frequency-VLF was subtracted) and multiplied by 100.
Tissue Samples
After cardiovascular measures, the animals were preanesthetized with ketamine and killed by decapitation. Muscle tissues (gastrocnemius, soleus, and extensor digitorum longus EDL) were extracted and weighed by the same blinded technician; their values were normalized (divided by the animal’s body weight and multiplied by 100). Likewise, adipose tissues (visceral and ovarian) were weighed, added, and their values were normalized. No animals were excluded or died during the experimental protocol.
Statistical Analysis
Data from each group were presented as mean ± standard error of the mean and included in the results section for systematic reviews with meta-analyses. GraphPad Prism 8.0.1 software was used for statistical analysis. The Shapiro–Wilk test was used to verify the normality of the variables. The control group (C) was used only to demonstrate the baseline values of fertile female rats of the same age and body weight. It was not included in the statistical comparison. One-way ANOVA, followed by Tukey’s post hoc test, was applied to compare the sedentary and trained Ovx groups. Values of p<0.05 were considered significant.
Results
The animals submitted to exercise training in the treadmill, aerobic OvxAT, and combined OvxCT groups, presented higher running capacity than the OvxRT group (Table 1). On the other hand, only trained animals on ladder showed increased performance in the maximal load test than the sedentary ovariectomized group (Ovx). In addition, OvxRT showed an additional increase compared with OvxAT (Table 1).
Notably, there was no increase in body weight in trained ovariectomized animals with a reduction in the OvxAT vs Ovx group. There was a reduction in % WAT, regardless of training modality vs Ovx group (Table 1). Regarding muscle tissue, the three types of exercise training increased soleus mass compared to the OVX group (Table 1). Gastrocnemius and EDL muscle mass were increased only in OvxRT vs Ovx group (Table 1). Although there was no difference in glycemia among the studied animals (Table 1), the insulin tolerance test showed that aerobic training alone promoted insulin sensitivity increase in the OvxAT group compared to all groups (OvxAT vs Ovx p= 0.0008 vs OvxRT p= 0.0007 and vs OvxCT p= 0.0275) (Table 1).
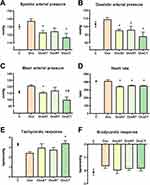
Animals submitted to exercise training on a treadmill (OvxAT and OvxCT groups) presented lower systolic pressure values compared to the Ovx group (C 130.2±2.8, Ovx 137.3±2.9, OvxAT 122.8±2.4, OvxRT 124.3±4.3, OvxCT 116.7±5.1 mmHg; OvxAT vs Ovx p= 0.0295; OvxCT vs Ovx p= 0.0010) (Figure 2A) and diastolic pressure regardless training modality (C 102.8±3.6, Ovx 108.9±2.2, OvxAT 95.3±1.4, OvxRT 95.9±4.4, OvxCT 88.0±5.1 mmHg; OvxAT vs Ovx p= 0.0040); OvxRT vs Ovx p=0.0225 OvxCT vs Ovx p=0.0001) (Figure 2B) compared to sedentary ovariectomized animals, while mean AP was reduced in both animals submitted to exercise training on a treadmill and in the OvxCT group compared to OvxRT (C 112.0±1.8, Ovx 121.5±1.7, OvxAT 110.9±1.7, OvxRT 114.1±5.1, OvxCT 99.6±4.6 mmHg; OvxAT vs Ovx p= 0.0218; OvxCT vs Ovx p<0.0001; OvxCT vs OvxRT p= 0.0235) (Figure 2C) showing an additional benefit of combined training versus OvxRT on the mean AP. The HR was lower in trained animals than in sedentary ovariectomized regardless of training modality (C 408.3±5.4, Ovx 411.0±18.2, OvxAT 342.2±8.4, OvxRT 355.3±4.7, OvxCT 351.7±4.9 bpm; OvxAT vs Ovx p=0.0212; OvxRT vs Ovx p=0.0100; OvxCT vs Ovx p= 0.0027) (Figure 2D). Baroreflex sensitivity for tachycardic responses was increased only in the OvxCT group, compared to the Ovx group C 3.30±0.12, Ovx 2.43±0.14, OvxAT 2.95±0.42, OvxRT 2.75±0.23, OvxCT 3.40±0.27 bpm/mmHg; OvxCT vs Ovx p= 0.0316) (Figure 2E). There was no difference between groups in baroreflex sensitivity for bradycardic responses (C: −2.13±0.23; Ovx: −1.66± 0.16; OvxAT: 1.98 ± 0.23; OvxRT: −1.86± 0.12; OvxCT: −1.84± 0.18 p= 0.6326) (Figure 2F). For cardiac autonomic modulation in the time domain, both OvxAT and OvxCT groups showed an increase in SD-PI (C: 5.37±0.23; Ovx: 5.39±0.23; OvxAT: 8.55±0.38; OvxRT: 7.05±0.85; OvxCT: 8.02±0.67 - ms) and VAR-PI (C: 29.34±2.39; Ovx: 29.15±2.46; OvxAT: 61.70±5.90; OvxRT: 51.07±8.12; OvxCT: 56.71±8.40 - ms2), compared to Ovx group (Figures 3A and B, respectively). In addition, the trained groups (OvxAT and OvxCT) showed an increase in RMSSD compared to the Ovx group (C: 6.39±0.24; Ovx: 5.66±0.27; OvxAT: 7.45±0.13; OvxRT: 6.18±0.29; OvxCT: 7.24±0.38 - ms) (Figure 3C). For the frequency domain, exercise training in the groups OvxRT and OvxCT reduced the LF-PI band compared to the Ovx group (C: 19.94±2.18; Ovx: 31.48±2.66; OvxAT: 21.48±0.50; OvxRT 17.43±2.13; OvxCT: 18.92±1.92 - nu), and HF-PI band was increased regardless the training modality (C 81.91±2.18, Ovx 69.80±1.74; OvxAT 78.52±0.50; OvxRT 79.76±1.45; OvxCT: 79.48±2.10 - nu) (Figures 3D and E, respectively). All trained groups (OvxAT, OvxRT, and OvxCT) reduced the LF/HF compared to the Ovx group (C 0.23±0.021; Ovx 0.34±0.025; OvxAT 0.24±0.013; OvxRT 0.24±0.013; OvxCT 0.23±0.032 (Figure 3F). Vascular sympathetic modulation parameters such as SD-SAP and VARSAP were only reduced in the combined exercise group (Figure 3G and H, respectively). The vascular sympathetic autonomic modulation, represented by the LF-SAP band, was reduced for both trained groups OvxRT and OvxCT (C 5.40±0.46; Ovx 8.45±0.98; OvxAT 6.44±1.10; OvxRT 4.83±0.54, OvxCT 3.81±0.31 mmHg2; Ovx vs C p=0.0389; OvxRT vs Ovx p=0.0082; OvxCT vs Ovx p=0.0002) (Figure 3I).
Discussion
Combined training reduced arterial pressure compared to control and OvxRT and it was the only modality to increase baroreflex sensitivity to tachycardic responses and reduce all parameters of vascular sympathetic modulation. It also combined the effects of aerobic and resistance training in all parameters. Combined training (OvxCT) was effective in associating the improvement in running capacity observed in all animals submitted to isolated aerobic training (OvxAT) with the increase in climbing strength observed in animals submitted to isolated resistance training (OvxRT). Previously, our group demonstrated that greater running capacity was correlated with VO2 max improvement.31 It has been established in the literature that VO2 max. It is a “gold standard” for assessing human functional health through caption, transport, and oxygen utilization.32
In addition, increased performance in the full load test demonstrates a specific functional improvement in the skeletal muscle system in all climbing trained animals (OvxRT and OvxCT). Previously our group demonstrated that ovaries hormone deprivation promotes reduction in gastrocnemius muscle and increases adipose tissue compared to fertile females33,34 In this sense, the three types of exercise training (OvxAT, OvxRT, and OvxCT) increased soleus muscle in relation to Ovx group indicating that can be considered a high standard strategy to avoid sarcopenia-induced by estrogen deprivation. Gastrocnemius and Extensor digitorum longus muscle increase was observed only in the resistance training group (OvxRT). The bidirectional relationship between insulin resistance and autonomic dysfunction is unquestionable. It has been widely demonstrated that the sympathetic vasoconstriction affects glucose uptake,35 while we previously demonstrated that insulin resistance through fructose overload promotes reduced parasympathetic tone.27 Weight loss has been considered a primary treatment goal to reverse metabolic dysfunctions observed in diabetes type II patients.
About 15% or more of body weight can effectively promote glucose control.36 Animals submitted only to aerobic exercise training (OvxAT) showed improved insulin tolerance with 10% less body weight than the Ovx group. However, no differences were observed in glycemia between the studied groups. The animals submitted to aerobic exercise training isolated or combined (OvxAT and OvxCT) presented lower systolic, diastolic, mean arterial pressure, and HR values compared to the ovariectomized group (Ovx), evidencing resting bradycardia, an essential marker of training efficacy. Regarding the mechanisms involved in the reduction of AP after aerobic exercise, it was shown that a reduction in cardiac output accompanies a reduction in heart rate without a reduction in total peripheral resistance in hypertensive rats.37 Thus, the bradycardia at rest observed in the present study could induce a reduction in cardiac output and contribute to a reduction in blood pressure. Furthermore, Da Palma et al18 demonstrated that resting bradycardia positively correlated with the increase in cardiac parasympathetic modulation. In the OvxRT group, resting bradycardia was not accompanied by increased cardiac parasympathetic modulation (RMSSD). This finding indicates that another pathway might explain the changes in this group, such as intrinsic heart rate change. In trained young males, resting bradycardia is associated with improved function of the sinoatrial node, the primary regulator of intrinsic heart rate.38
Baroreflex adjustments are redefined and modulated by the reciprocal interconnectivity between different nuclei of central regulation and efferent neurons.39,40 However, evidence demonstrates that coordinated activation of the vasopressinergic (VPergic) and oxytocinergic (OTergic) supra bulbar neurons is essential to adjust heart rate and cardiac output to circulatory demand at rest during exercise.41 In the present study, only animals submitted to combined exercise training (OvxCT) showed improved baroreflex sensitivity for tachycardic responses. No differences in baroreflex sensitivity were observed for bradycardic responses in any of the groups studied. The improvement in tachycardic reflex response may be related to a possible chronic effect of exercise training, increasing the efficiency of the VPergic flow, since Morris,42 observed in rats pretreated with vasopressin or V1 antagonist, significant potentiation or marked blunting of the tachycardia induced by the exercise. In contrast, the absence of improvement in reflex bradycardia can be attributed to the increased Otergic flow after 8 weeks of exercise training in elderly rats43 and rest bradycardia. Thus, preventing higher tachycardic values and not requiring further bradycardic reduction. It has also been established that a more significant sympathetic nervous system activity is related to increased cardiovascular risk. In contrast, the parasympathetic nervous system works oppositely and has been related to cardiovascular protection.21 However, whether the LF band of pulse interval (LFPI band) represents, cardiac sympathetic modulation is controversial. Although reports demonstrate that pharmacological blockade of cardiac sympathetic tone does not reduce the LF-PI band, the same experimental condition reduced the LF/HF index.44 Whereas pharmacological vagal blockade by atropine was shown to altogether, abolish the HF-PI band and affect the LF-PI band.45 Thus, the LFPI band has been considered by some researchers as a parameter not representative but predominantly of sympathetic modulation. It was recently demonstrated that the LF band in the QT interval seems to represent isolated cardiac sympathetic modulation, being suggested to be combined with the HF-PI band as a strategy to reduce the risk of bias in the analysis of autonomic cardiovascular modulation. Despite this, the results with this type of combination in different experimental models remain unknown.46 However, the results of the present study corroborate previous studies, which demonstrate that the increase in the LF-PI band and the LF / HF index is present along with conditions or diseases that affect the cardiovascular system.18,33
Regular physical activity promotes essential benefits for preventing vascular diseases in the female sex.47 Combined exercise training was able to associate LF-band reduction observed in resistance training isolated (OvxRT) with all cardiovascular autonomic modulation improvement observed with aerobic exercise isolated (OvxCT and OvxAT), such as, increase of the standard deviation and the variance of the pulse interval (SD-PI, VAR-PI) and the parasympathetic modulation through increase RMSSD, both contributing to reduce LF/HF index compared to Ovx group. Quinteiro et al48 demonstrated similar results in a study with menopausal diabetic rats. After 8 weeks of aerobic exercise training, they improved cardiovascular dysfunction, thus confirming the beneficial effects of aerobic physical training in an experimental menopause model. Previously, evidence has shown that the projections of PVN otergic neurons into the dorsal vagus motor nucleus (DMV) and ambiguous nucleus are responsible for increased cardiac parasympathetic stimulation and reduced sympathetic pre-ganglionic neuronal flow during exercise-induced tachycardia45 or pharmacological tachycardia,49 possibly suggesting, that these adaptations may become chronic as observed in the present study.
Clinical reports have been suggesting resistance exercise training in association with aerobic exercise training as an important non-pharmacological strategy for the prevention and/or attenuation of some risk factors associated with metabolic and cardiovascular diseases.18,48,50 In the present study, only animals trained in climbing reduced the LF-SAP band compared to the Ovx group. However, combined training extended these benefits to all vascular sympathetic modulation parameters, reducing SD-SAP and VAR-SAP compared to the Ovx group. Resistance training, even when performed at low intensity, promote an increase in the bioavailability of eNOS as proved by Stanisic,51 and other metabolites (adenosine, prostaglandins) that attenuate sympathetically mediated vasoconstriction, a phenomenon known as “functional sympatholytic”. This finding supports that aerobic and resistance training combination can enhance the cardiovascular health obtained in each modality.
Conclusions
In ovariectomized animals, combined training showed to be more effective than isolated aerobic and resistance training, mixing the isolated benefits of each modality. It was the only modality able to increase baroreflex sensitivity to tachycardic responses, reduce arterial pressure and all parameters of vascular sympathetic modulation.
Acknowledgments
The authors thank Ricardo Roberto Ferreira Lima for the help in the maintenance of the Animal Care Facility and the handling of animals. And to lab technician Leandro Ezequiel de Souza for his help with surgical procedures.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financed by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Ânima Institute (AI). N.C.S., A.S., B.N.C., and MAS were recipients of the CAPES-PROSUP scholarship. GMSC received a FAPESP scholarship (2016/21302-3). I.C.S. was recipient of CNPq/Universal (435123/2018-1).
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. Cardiovascular diseases (CVDs). Available from: https://www.who.int/newsroom/fact-sheets/detail/cardiovascular-diseases-(cvds).
2. Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–2438. doi:10.1016/S0140-6736(21)00684-X
3. de Jong M, Woodward M, Peters SAE. Diabetes, glycated hemoglobin, and the risk of myocardial infarction in women and men: a prospective cohort study of the UK biobank. Diabetes Care. 2020;43(9):2050–2059. doi:10.2337/DC19-2363
4. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145(8):E153–E639. doi:10.1161/CIR.0000000000001052
5. Shimojo GL, Palma RK, Brito JO, Sanches IC, Irigoyen MC, de Angelis K. Dynamic resistance training decreases sympathetic tone in hypertensive ovariectomized rats. Braz J Med Biol Res. 2015;48(6):523–527. doi:10.1590/1414431X20154387
6. Flues K, Paulini J, Brito SS, et al. Exercise training associated with estrogen therapy induced cardiovascular benefits after ovarian hormones deprivation. Maturitas. 2010;65(3):267–271. doi:10.1016/j.maturitas.2009.11.007
7. Takezawa H, Hayashi H, Sano H, Ebihara S. Estrous-related variations of blood pressure and heart rate in female rats - PubMed. Front Med Bioeng Eng. 1994;6(2):131–137.
8. Takezawa H, Hayashi H, Sano H, Saito H, Ebihara S. Circadian and estrous cycle dependent variations in blood pressure and heart rate in female rats. Am J Physiol. 1994;267(5):Pt 2. doi:10.1152/AJPREGU.1994.267.5.R1250
9. Angelis De K, Do M, Brasileiro S, et al. Sistema nervoso autônomo e doença cardiovascular. Revista da Sociedade de Cardiologia do Rio Grande do Sul. 2004;13(03):1–7. doi:10.1126/science.150.3699.971
10. Porta A, Żebrowski J. Inferring cardiovascular control from spontaneous variability. Auton Neurosci. 2013;178(1–2):1–3. doi:10.1016/j.autneu.2013.05.006
11. Joaquim LF, Salgado HC, Fazan Júnior R. Variabilidade da pressão arterial e freqüência cardíaca, e sensibilidade do barorreflexo, em animais geneticamente manipulados. Rev Bras Hipert. 2005;12(1):36–40.
12. Suarez-Roca H, Mamoun N, Sigurdson MI, Maixner W. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373. doi:10.1002/CPHY.C190038
13. Saladini F, Di Marco A, Palatini P. Autonomic dysfunction: how to identify and when to treat? High Blood Press Cardiovasc Prev. 2016;23(3):237–243. doi:10.1007/s40292-016-0162-3
14. Flores LJ, Figueroa D, Sanches IC, et al. Effects of exercise training on autonomic dysfunction management in an experimental model of menopause and myocardial infarction. Menopause. 2010;17(4):1. doi:10.1097/gme.0b013e3181cdebc9
15. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi:10.1249/MSS.0b013e3182A0c95c
16. Sanches IC, Conti FF, Sartori M, Irigoyen MC, De Angelis K. Standardization of resistance exercise training: effects in diabetic ovariectomized rats. Int J Sports Med. 2014;35(4):323–329. doi:10.1055/s-0033-1351254
17. De Oliveira brito-monzani J, Stoyell-Conti FF, Shecaira TP, et al. Aerobic or resistance training improves autonomic control of circulation in oophorectomized rats with cardiometabolic dysfunctions: impact on renal oxidative stress. Exp Gerontol. 2021:145. doi:10.1016/J.EXGER.2020.111181
18. da Palma RK, Moraes-Silva IC, da Dias DS, et al. Resistance or aerobic training decreases blood pressure and improves cardiovascular autonomic control and oxidative stress in hypertensive menopausal rats. J Appl Physiol. 2016;121(4):130. doi:10.1152/japplphysiol.00130.2016
19. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNAGuidelineforthe prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task F. Hypertension. 2018;71(6):1269–1324. doi:10.1161/HYP.0000000000000066
20. Visseren FLJ, MacH F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practiceDeveloped by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42(34):3227–3337. doi:10.1093/EURHEARTJ/EHAB484
21. Shimojo GL, da Silva Dias D, Malfitano C, et al. Combined aerobic and resistance exercise training improve hypertension associated with menopause. Front Physiol. 2018;9:1471. doi:10.3389/fphys.2018.01471
22. Conti FF, de Brito JO, Bernardes N, et al. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: autonomic, inflammatory, and oxidative stress evaluations. Am J Physiol Regul Integr Comp Physiol. 2015;309(12):R1532–R1539. doi:10.1152/ajpregu.00076.2015
23. Sanches IC, Conti FF, Bernardes N, et al. Impact of combined exercise training on cardiovascular autonomic control and mortality in diabetic ovariectomized rats. J Appl Physiol. 2015;119(6):656–662. doi:10.1152/japplphysiol.00883.2014
24. Ferreira MJ, Sanches IC, Jorge L, Llesuy SF, Irigoyen MC, de Angelis K. Ovarian status modulates cardiovascular autonomic control and oxidative stress in target organs. Biol Sex Differ. 2020;11(1). doi:10.1186/S13293-020-00290-Y
25. Irigoyen MC, Paulini J, Flores LJF, et al. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats. Hypertension. 2005;46(4):998–1003. doi:10.1161/01.HYP.0000176238.90688.6b
26. Sanches IC, De Oliveira Brito J, Candido GO, et al. Cardiometabolic benefits of exercise training in an experimental model of metabolic syndrome and menopause. Menopause. 2012;19(5):562–568. doi:10.1097/gme.0b013e3182358c9c
27. Brito JO, Ponciano K, Figueroa D, et al. Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Braz J Med Biol Res. 2008;41(9):804–808. doi:10.1590/S0100-879X2008005000030
28. Bonora E, Moghetti P, Zancanaro C, et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68(2):374–378. doi:10.1210/jcem-68-2-374
29. Bertagnolli M, Campos C, Schenkel PC, et al. Baroreflex sensitivity improvement is associated with decreased oxidative stress in trained spontaneously hypertensive rat. J Hypertens. 2006;24(12):2437–2443. doi:10.1097/01.HJH.0000251905.08547.17
30. Montano N, Porta A, Cogliati C, et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev. 2009;33(2):71–80. doi:10.1016/j.neubiorev.2008.07.006
31. Rodrigues B, Figueroa DM, Mostarda CT, Heeren MV, Irigoyen MC, De Angelis K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc Diabetol. 2007;6(1):38. doi:10.1186/1475-2840-6-38
32. Myers J, Gullestad L, Vagelos R, et al. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann Intern Med. 1998;129(4):286–293. doi:10.7326/0003-4819-129-4-199808150-00004
33. Freire Machi J, da Silva Dias D, Freitas S, et al. Impact of aging on cardiac function in a female rat model of menopause: role of autonomic control, inflammation, and oxidative stress. Clin Interv Aging. 2016;11:341. doi:10.2147/CIA.S88441
34. Stunes AK, Erben RG, Schüler C, et al. Skeletal effects of plyometric exercise and metformin in ovariectomized rats. Bone. 2020;132:115193. doi:10.1016/J.BONE.2019.115193
35. Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005;15(6):441449. doi:10.1016/J.NUMECD.2005.06.010
36. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394–405. doi:10.1016/S0140-6736(21)01919-X
37. Véras-Silva A, Mattos K, Gava N, Brum P, Negrão C, Krieger E. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol. 1997;273(6):H2627–31. doi:10.1152/AJPHEART.1997.273.6.H2627
38. Negrão C, Moreira E, Santos M, Farah V, Krieger E. Vagal function impairment after exercise training. J Appl Physiol. 1992;72(5):1749–1753. doi:10.1152/JAPPL.1992.72.5.1749
39. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi:10.1038/NRN1902
40. Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol. 2009;94(9):947–960. doi:10.1113/expphysiol.2009.047449
41. Michelini LC. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clin Exp Pharmacol Physiol. 2007;34(4):369–376. doi:10.1111/j.1440-1681.2007.04589.x
42. Michelini LC, Morris M. Endogenous vasopressin modulates the cardiovascular responses to exercise. In: Annals of the New York Academy of Sciences. Vol. 897. New York Academy of Sciences;1999:198–211. doi:10.1111/j.1749-6632.1999.tb07892.x
43. Santos CR, Ruggeri A, Ceroni A, Michelini LC. Exercise training abrogates age-dependent loss of hypothalamic oxytocinergic circuitry and maintains high parasympathetic activity. J Neuroendocrinol. 2018;30(8):e12601. doi:10.1111/jne.12601
44. Hopf HB, Skyschally A, Heusch G, Peters J. Low-frequency spectral power of heart rate variability is not a specific marker of cardiac sympathetic modulation. Anesthesiology. 1995;82(3):609–619. doi:10.1097/00000542-199503000-00002
45. P B, M RJ, C MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248(1):Pt 2. doi:10.1152/AJPHEART.1985.248.1.H151
46. De Maria B, Bari V, Sgoifo A, et al. Concomitant evaluation of heart period and QT interval variability spectral markers to typify cardiac control in humans and rats. Front Physiol. 2019;10:1478. doi:10.3389/FPHYS.2019.01478/BIBTEX
47. Bernardes N, Da D, Dias S, Dias S. Exercise training improves cardiovascular health in post menopause women; 2008.
48. Quinteiro H, Buzin M, Conti FF, et al. Aerobic exercise training promotes additional cardiac benefits better than resistance exercise training in postmenopausal rats with diabetes. Menopause. 2015;22(5):534–541. doi:10.1097/GME.0000000000000344
49. Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):51–52. doi:10.1152/ajpregu.00806.2000
50. Zaki ME. Effects of whole body vibration and resistance training on bone mineral density and anthropometry in obese postmenopausal women. J Osteoporos. 2014;2014:702589. doi:10.1155/2014/702589
51. Stanisic J, Koricanac G, Kostic M, et al. Low-intensity exercise in the prevention of cardiac insulin resistance-related inflammation and disturbances in NOS and MMP-9 regulation in fructose-fed ovariectomized rats. Appl Physiol Nutr Metab. 2019;44(11):1219–1229. doi:10.1139/apnm-2018-0785
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.