Back to Journals » Infection and Drug Resistance » Volume 17
Combination of rRT-PCR and Clinical Features to Predict Coronavirus Disease 2019 for Nosocomial Infection Control
Authors Yamaguchi F , Suzuki A , Hashiguchi M, Kondo E, Maeda A, Yokoe T, Sasaki J , Shikama Y , Hayashi M, Kobayashi S, Suzuki H
Received 23 August 2023
Accepted for publication 29 December 2023
Published 18 January 2024 Volume 2024:17 Pages 161—170
DOI https://doi.org/10.2147/IDR.S432198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Fumihiro Yamaguchi,1 Ayako Suzuki,2 Miyuki Hashiguchi,3 Emiko Kondo,3 Atsuo Maeda,4 Takuya Yokoe,1 Jun Sasaki,4 Yusuke Shikama,1 Munetaka Hayashi,4 Sei Kobayashi,5 Hiroshi Suzuki6
1Department of Respiratory Medicine, Showa University Fujigaoka Hospital, Yokohama, Japan; 2Department of Pharmacy, Showa University Fujigaoka Hospital, Yokohama, Japan; 3Department of Infection Control, Showa University Fujigaoka Hospital, Yokohama, Japan; 4Department of Emergency and Critical Care Medicine, Showa University Fujigaoka Hospital, Yokohama, Japan; 5Department of Otolaryngology, Showa University Fujigaoka Hospital, Yokohama, Japan; 6Department of Cardiology, Showa University Fujigaoka Hospital, Yokohama, Japan
Correspondence: Fumihiro Yamaguchi, Department of Respiratory Medicine, Showa University Fujigaoka Hospital, 1-30 Fujigaoka, Aoba-ku, Yokohama, 227-8501, Japan, Email [email protected]
Background: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), immediately became a pandemic. Therefore, nosocomial infection control is necessary to screen for patients with possible COVID-19.
Objective: This study aimed to investigate commonly measured clinical variables to predict COVID-19.
Methods: This cross-sectional study enrolled 1087 patients in the isolation ward of a university hospital. Conferences were organized to differentiate COVID-19 from non-COVID-19 cases, and multiple nucleic acid tests were mandatory when COVID-19 could not be excluded. Multivariate logistic regression models were employed to determine the clinical factors associated with COVID-19 at the time of hospitalization.
Results: Overall, 352 (32.4%) patients were diagnosed with COVID-19. The majority of the non-COVID-19 cases were predominantly caused by bacterial infections. Multivariate analysis indicated that COVID-19 was significantly associated with age, sex, body mass index, lactate dehydrogenase, C-reactive protein, and malignancy.
Conclusion: Some clinical factors are useful to predict patients with COVID-19 among those with symptoms similar to COVID-19. This study suggests that at least two real-time reverse-transcription polymerase chain reactions of SARS-CoV-2 are recommended to exclude COVID-19.
Keywords: severe acute respiratory syndrome coronavirus-2, coronavirus disease 2019, nosocomial infection control, nucleic acid test, obesity, cross sectional study, Japan
Introduction
The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) first emerged at the end of 2019. Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 subsequently became a pandemic and remains a burden to humans. Because COVID-19 has no characteristic symptoms,1,2 nucleic acid or antigen tests to detect the N gene or N protein of SARS-CoV-2 are necessary for the diagnosis; however, false-negative results are a critical medical problem.3,4 In November 2020, we experienced a large cluster of COVID-19 in our institute because a few patients with COVID-19 were missed. At that time, a single nucleic acid test was used to determine whether a patient had COVID-19. Thereafter, significant changes in nosocomial infection control of COVID-19 were established in our institute. More specifically, we organized conferences of respiratory physicians to differentiate COVID-19 from non-COVID-19 cases and decided to transfer patients without COVID-19 from the isolation ward to the general ward. In addition, multiple nucleic acid tests should be performed if COVID-19 could not be excluded based on physical and imaging findings even if a nucleic acid test was negative. This strategy may be effective in future pandemic events.
It is necessary to screen patients with possible COVID-19 for nosocomial infection control. Previous studies have mostly focused on mortality-related factors of COVID-19.5–9 However, only a few studies have analyzed the risk of COVID-19. Since November 2021, omicron variants have been reported worldwide, and there is growing concern about its infectivity, transmissibility, and antigenicity.10,11 Most notably, BA.5, an omicron variant that has been endemic in Japan since July 2022, has a predominance of increased infection and immune escape, compared with previous lineages.12 Meanwhile, the previous lineages had a higher rate of severe illness and mortality than BA.5, making it crucial to prevent horizontal nosocomial transmission by strict isolation. In this study, we aimed to investigate the efficacy of clinical factors to predict patients with COVID-19 in the isolation ward before the emergence of the BA.5 lineage.
Subjects and Methods
Population and Data Collection
In this cross-sectional study, the clinical factors of COVID-19 at the time of hospitalization in routine practice at the Showa University Fujigaoka Hospital, from December 2020 to March 2022, were investigated. The line charts of COVID-19 diagnosis, the bars of the estimated variants of SARS-CoV-2, and the number of SARS-CoV-2 vaccinations during the same period in Japan are shown in Figures 1 and 2, respectively. The criteria for admission to the isolation ward were as follows: body temperature ≥37 degrees C, percutaneous oxygen saturation <96%, history of contact with patients diagnosed with COVID-19, or a ground-glass pattern on chest computed tomography images. All clinical data were collected from patients’ medical records on the day of admission. The estimated glomerular filtration rate (eGFR) of each patient was calculated.13 Chronic kidney disease (CKD) was defined as an eGFR < 60 mL/min/1.73 m2. The study protocol was approved by the Institutional Ethics Committee of Showa University (approved no. 22–131-B). The requirement to obtain informed consent from the patients was waived with the approval of the Ethics Committee due to the retrospective nature of this study. This study was conducted in accordance with the Declaration of Helsinki guidelines.
Method for the Diagnosis of COVID-19 and Determining Severity
COVID-19 was diagnosed based on nucleic acid tests to detect SARS-CoV-2 RNA using samples derived from the nasopharynx. Nasopharyngeal swabs were collected from patients with suspected COVID-19 and tested by real-time reverse-transcription polymerase chain reaction (rRT-PCR) performed using a SARS-CoV-2 Detection Kit Multi (TOYOBO, Osaka, Japan) to amplify two regions (N1 and N2) in the N gene of SARS-CoV-2 RNA, according to the manufacturer’s protocol. Cycle threshold (Ct) values were observed in a SARS-CoV-2 rRT-PCR-positive case (Ct ≤40). Severity was defined as follows: (i) mild (percutaneous oxygen saturation (SpO2) ≥96%), (ii) moderate I (93% < SpO2 <96%), (iii) moderate II (SpO2 ≤93%), and (iv) severe (patients receiving intensive care during the acute COVID-19 period).14
Procedure to Release Patients with Suspected COVID-19 from the Isolation Ward
As shown in Figure 3, all patients meeting at least one of the criteria were admitted to the isolation ward. A SARS-CoV-2 rRT-PCR was performed for each patient. If the rRT-PCR test was negative, conferences to differentiate COVID-19 from non-COVID-19 cases were organized to decide on release of such patients from the isolation ward. The decision was established by two independent physicians in the Department of Respiratory Medicine, including at least one specialist, based on the clinical course and imaging studies. If COVID-19 was ruled out, patients were transferred to the general ward. Conversely, if COVID-19 could not be excluded, repeat rRT-PCR testing was required and conferences were reviewed to identify potential COVID-19 cases. When patients with COVID-19 diagnosed as rRT-PCR-positive were presumed to have decreased infectivity with time since illness onset, repeat SARS-CoV-2 rRT-PCRs were performed to compare the Ct values with those at the time of diagnosis, followed by conferences to decide whether such patients should be released.
Statistical Analysis
All data were expressed as mean ± standard deviation for continuous variables or as percentages for categorical variables. Group mean values were compared using the Mann–Whitney rank-sum test. Pearson’s chi-squared test or Fisher’s exact test was used for the univariate analysis of the association between two categorical variables. The adjusted effects of multiple variables were evaluated using a logistic regression model, and the findings were presented as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was set at P < 0.05. All statistical analyses were performed using JMP software version 16.0 (SAS Institute, Cary, NC).
Results
Patient Characteristics and COVID-19 Diagnosis
During this period, 1237 patients were admitted to the isolation ward. Of these patients, 38 with fever during hospitalization, 49 with re-admission cases, and 63 from cluster outbreaks were excluded. Eventually, 1087 patients were statistically analyzed. Patient characteristics are shown in Table 1. Of the patients, 669 (61.5%) were male and 418 (38.5%) were female, with ages ranging from 5 to 101 years. The mean values of age, body mass index (BMI), serum albumin, lactate dehydrogenase (LDH), and C-reactive protein (CRP) were 71.4 ± 18.1 years, 21.7 ± 4.4, 3.4 ± 0.7 g/dL, 302.4 ± 244.4 U/L, and 8.3 ± 8.1 mg/dL, respectively. Overall, 30 (2.8%) patients were receiving corticosteroids (at least 10 mg/day of prednisolone) for various indications, and 249 (22.9%) had diabetes mellitus. Moreover, 283 (26%) patients had various malignancies (Supplementary Table 1), including 93 diagnosed within 1 year and 48 receiving chemotherapy within 3 months. In addition, 443 (40.8%) patients had CKD and 53 (4.9%) had autoimmune diseases (Supplementary Table 2). Of the 1087 patients, 352 (32.4%) were diagnosed with COVID-19 based on rRT-PCR, which included 67 patients with mild, 55 with moderate I, 200 with moderate II, and 30 with severe diseases at the time of admission.
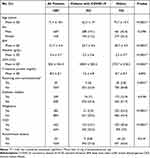 |
Table 1 Association of Each Variable with COVID-19 |
Cases of Suspected COVID-19
Table 2 indicates the details of the suspected COVID-19 cases. The majority of cases were bacterial infections, including respiratory, intra-abdominal, and urinary tract infections, which accounted for approximately 69.4% (510 of 735) of all suspected COVID-19 cases. Among non-infectious diseases, interstitial pneumonia, ground-glass opacities of unknown etiology, and cardiogenic pulmonary edema were frequently observed.
 |
Table 2 Cases of Suspected COVID-19 |
Statistical Analysis of COVID-19 and Clinical Features
In the univariate analyses, COVID-19 was found to be significantly associated with age, BMI, serum albumin, LDH, oral corticosteroid use, malignancy, CKD, and autoimmune disease. However, no significant association was found between COVID-19 and sex, CRP, and diabetes mellitus.
Multivariate analyses were employed to control for the potential confounding effects of these variables. Because of significant bias in the distribution chart, thresholds were established for age, LDH, and CRP (Supplementary Figure 1). Table 3 presents the logistic regression models of factors associated with COVID-19. The frequency of COVID-19 increased significantly with BMI (OR, 1.1148; 95% CI, 1.0699–1.1615). COVID-19 was independently associated with age ≥65 (OR, 0.2659; 95% CI, 0.1843–0.3837), female sex (OR, 1.6902; 95% CI, 1.2205–2.3406), LDH ≥300 (OR, 3.4544; 95% CI, 2.4916–4.7894), CRP≥10 (OR, 0.5261; 95% CI, 0.3645–0.7594), and malignancy (OR, 0.2302; 95% CI, 0.1479–0.3582). Serum albumin, corticosteroid use, diabetes mellitus, CKD, and autoimmune diseases were not significantly associated with COVID-19.
 |
Table 3 Logistic Regression Models of COVID-19 |
Outcome of Patients in the Isolation Ward
The details of the outcomes in the isolation ward are shown in Table 4. Significant differences were found between the COVID-19 group and the non-COVID-19 group in terms of transfer to the general ward or intensive care unit (ICU), leaving the hospital, transferring to another hospital, but not in terms of death. The frequency of SARS-CoV-2 rRT-PCR was significantly lower in the COVID-19 group than in the non-COVID-19 group, while the COVID-19 group spent significantly more time in the isolation ward than the non-COVID-19 group. Approximately two rRT-PCR assays were required to exclude COVID-19. Table 5 indicates the details of the deaths. The majority of deaths in the COVID-19 group were attributed to pneumonia caused by COVID-19. The top three causes of death in the non-COVID-19 group were bacterial pneumonia, interstitial pneumonia, and malignancy.
 |
Table 4 Outcome of Patients in the Isolation Ward |
 |
Table 5 Details of the Death |
Discussion
In this study, the correlation between COVID-19 and commonly measured clinical variables was analyzed in patients who met at least one of the criteria shown in Figure 3. Notably, clinical factors were found to be predictive of patients with COVID-19 among those with symptoms similar to COVID-19. Of the 1087 patients, 352 (32.4%) were diagnosed with COVID-19. Multivariate analysis indicated that the likelihood of COVID-19 at the time of hospitalization was significantly associated with age, sex, BMI, LDH, CRP, and malignancy. Malnutrition, corticosteroid use, diabetes mellitus, CKD, and autoimmune diseases were not more frequent in the COVID-19 group than in the non-COVID-19 group.
This study indicated that COVID-19 was more common in patients aged < 65 years and that females were more likely to be infected with COVID-19 than males. Previous reports on the relationship between COVID-19 infection and age or sex are inconsistent.15–17 This may be related to race, ethnicity, age distribution of the population, lifestyle, or health care system. This study excluded the occurrence of clusters including nursing homes and was limited to community-acquired infections. In the case of community-acquired infections, individuals who engaged in physical activities could encounter SARS-CoV-2 more frequently than those with sedentary lifestyle. Indeed, several studies have indicated that COVID-19 relatively rarely occurs in older people.18,19 In this study, although the absolute number of COVID-19 was lower in females than in males, females were more likely to suffer from COVID-19 than males after adjustment for relevant confounders. Male sex has been reported to be an important independent predictor of SARS-CoV-2 infection,17 whereas the male dominance for the risk of COVID-19 may not be as great as previously estimated. Additionally, this study period coincided exactly with the completion of the second SARS-CoV-2 vaccination drive. In Japan, the rate of SARS-CoV-2 vaccine evasion was higher among females than among males,20 whereas the rates of first and second vaccination were higher among males than among females.21 Therefore, the sex difference in this study may have been influenced by SARS-CoV-2 vaccination.
Obesity has been reported to be associated with severe COVID-19.7,8 In overweight and obese individuals, detrimental adipocytokine production, including tumor necrosis factor-α, monocyte chemotactic protein-1, and interleukin-6 (IL-6), is derived from the enlarged adipocytes.22 This cytokine production in adipose tissue has been reported to be associated with BMI.23 Indeed, the anti-IL-6 antibody tocilizumab was found to be beneficial in patients with COVID-19 and mild obesity.24 However, whether obesity is a risk factor for susceptibility to SARS-CoV-2 is uncertain. Obesity is characterized by a chronic state of low inflammation. In a mouse model, a high-fat diet leads to an increase in Toll-like receptor 7 (TLR7) expression, followed by an increase in inflammatory cytokines.25 TLR7 is an innate immune receptor that recognizes single-stranded RNA viruses, including SARS-CoV-2, and plays a key role in antimicrobial host defense. Chronic activation of TLR7 induced by obesity would lead to the development of TLR tolerance26 and may fail to prevent SARS-CoV-2 infection. Indeed, several studies have indicated that obesity is associated with an increased risk of a positive SARS-CoV-2 test.18,27
Autopsy findings indicate that SARS-CoV-2 is detected in type II alveolar epithelial cells, and alveolar inflammation induced by viral infection is considered the major cause of COVID-19-related lung disease.28 Diffuse alveolar damage is characteristic of severe cases as well as acute respiratory distress syndrome or acute exacerbation of interstitial pneumonia.29 Thus, LDH should be elevated in COVID-19 due to lung parenchymal damage. CRP is an acute-phase inflammatory protein that plays a role in biological defense mechanisms. CRP binds to phosphorylcholine in the cytoplasmic membrane of bacteria and activates the complement pathway of innate immunity.30 Bacterial infection is a potent stimulus for marked CRP elevation, whereas the level of CRP is not very high in viral infection.31,32 In the present study, approximately 70% of the cases with suspected COVID-19 had bacterial infections. Therefore, the cut-off value of 10 mg/dL could differentiate between bacterial infection and COVID-19.
Patients with any malignancy had a significantly increased risk of COVID-19.27 Especially, within the first year after diagnosis of malignancy, the risk of contracting COVID-19 should be even higher.33 However, in this study, patients with malignancy had lower ORs for COVID-19, and the rates of COVID-19 in patients with malignancy diagnosed within 1 year were comparable to those of other patients with malignancy (data not shown). One of the possible reasons is that patients with malignancy performed normal activities of daily living while being more cautious about developing infections than other patients. Reducing activities of daily living, such as staying at home, may reduce the risk of contracting COVID-19, and this finding is consistent with the low likelihood of COVID-19 in elderly patients in this study. Regarding antineoplastic therapy, patients receiving antineoplastic agents that can decrease the expression of angiotensin-converting enzyme 2 (ACE2) expression, including tyrosine kinase inhibitors or antimetabolites, may have a lower rate of contracting COVID-19 than patients receiving other antineoplastic agents34 because SARS-CoV-2 infects human cells by attaching its spike glycoproteins to ACE2.
As shown in Table 4, significant differences in outcomes were found between the COVID-19 and the non-COVID-19 groups, except for mortality. These data suggest that mortality in the elderly is not extremely increased by COVID-19. Patients with COVID-19 were basically discharged to home. In addition, they were encouraged to be transferred to another hospital as much as possible in accordance with administrative policy, even if the discharge criteria were not satisfied.35 Thus, few patients with COVID-19 were transferred to the general ward. Compared with the non-COVID-19 group, more patients in the COVID-19 group, who were initially considered to have a mild or moderate disease, subsequently developed severe disease and were transferred to the ICU; even patients with mild COVID-19 should be carefully monitored during the course of the disease. In this study, no new outbreaks have occurred since the new strategy was implemented, and at least two rRT-PCRs of SARS-CoV-2 were recommended to rule out COVID-19. Together, multiple SARS-CoV-2 rRT-PCRs are needed for nosocomial infection control.
This study had several limitations. First, the study was conducted in a single facility, which might have reduced the generalizability of the findings. However, the nosocomial infection control measures are unique to our institute and cannot be easily copied by other facilities. Second, COVID-19 cases in this study were mostly mild or moderate; therefore, the presented nosocomial infection control measures may not be applicable to facilities with more severe cases. Third, as mentioned repeatedly, this study was conducted before the emergence of the omicron variant BA.5 and may not be applicable to the current situation.
Conclusion
This study demonstrates the prediction of patients with COVID-19 using clinical findings in routine practice. Although not perfect, the data from the current study suggest that rRT-PCR of SARS-CoV-2 should be performed at least twice to exclude COVID-19. Multiple rRT-PCR of SARS-CoV-2 may be useful to prevent nosocomial infection. Clinical analysis following the emergence of omicron variant BA.5 is warranted.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to Hitoshi Kobayashi, Kento Hirata, Kenta Miyo, Chika Kondo, Mamiko Kanzaki, Kazusawa Tei, Takashi Abe, Shunsuke Sakakura, Haruka Kitano, Mina Hayashi, Yo Shiratori, Shota Onozaki, Mari Nakamoto, Saori Kawamura, Miku Kosuge, Kenji Atarashi, Hidekazu Cho, and Shohei Shimizu for their assistance in the interpretation of the results and critical review of the manuscript. We would like to thank Maruzen (https://kw.maruzen.co.jp) for English language editing.
Funding
There is no funding to report.
Disclosure
We declare that we have no conflict of interest in connection with this paper, and we received no payment or services from a third party in relation to this study.
References
1. Locquet M, Diep AN, Beaudart C, et al. A systematic review of prediction models to diagnose COVID-19 in adults admitted to healthcare centers. Arch Public Health. 2021;79(1):105. doi:10.1186/s13690-021-00630-3
2. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi:10.1136/bmj.m1328
3. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. doi:10.1371/journal.pone.0242958
4. Sun Z, Guo Y, He W, et al. Development of clinical risk scores for detection of COVID-19 in suspected patients during a local outbreak in China: a retrospective cohort study. Int J Public Health. 2022;67:1604794. doi:10.3389/ijph.2022.1604794
5. Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH, Kretzschmar MEE. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. doi:10.1371/journal.pmed.1003321
6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
7. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi:10.1038/s41586-020-2521-4
8. Jin J, Agarwala N, Kundu P, et al. Individual and community-level risk for COVID-19 mortality in the United States. Nat Med. 2021;27(2):264–269. doi:10.1038/s41591-020-01191-8
9. Estiri H, Strasser ZH, Klann JG, Naseri P, Wagholikar KB, Murphy SN. Predicting COVID-19 mortality with electronic medical records. NPJ Digit Med. 2021;4(1):15. doi:10.1038/s41746-021-00383-x
10. Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602. doi:10.1038/s41586-022-04980-y
11. Cao Y, Song W, Wang L, et al. Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75. Cell Host Microbe. 2022;30(11):1527–39.e5. doi:10.1016/j.chom.2022.09.018
12. National Institute of Infectious Diseases. Coronavirus disease (COVID-19). Available from: https://www.niid.go.jp/niid/ja/2019-ncov/2551-cepr/11469-sars-cov-2-20.html.
13. Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi:10.1053/j.ajkd.2008.12.034
14. Ministry of Health, Labour and Welfare. 新型コロナウイルス感染症 COVID-19 診療の手引き 第10.0版 [COVID-19 Clinical Practice Guideline, Version 10.0]. Available from: https://www.mhlw.go.jp/content/000904136.pdf.
15. Hobbs CV, Drobeniuc J, Kittle T, et al. Estimated SARS-CoV-2 seroprevalence among persons aged <18 years - Mississippi, May-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):312–315. doi:10.15585/mmwr.mm7009a4
16. Smith BK, Janowski AB, Danis JE, et al. Seroprevalence of SARS-CoV-2 antibodies in children and adults in St. Louis, Missouri, USA. mSphere. 2021;6(1):e01207–e012020. doi:10.1128/mSphere.01207-20
17. Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11(1):e044640. doi:10.1136/bmjopen-2020-044640
18. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–1042. doi:10.1016/S1473-3099(20)30371-6
19. Lewis NM, Chu VT, Ye D, et al. Household transmission of severe acute respiratory syndrome Coronavirus-2 in the United States. Clin Infect Dis. 2021;73(7):1805–1813. doi:10.1093/cid/ciaa1166
20. National Center of Neurology and Psychiatry. Home page. Available from: https://www.ncnp.go.jp/topics/2021/20210625p.html.
21. Digital Agency Vaccination Record System (VRS). 新型コロナワクチンの接種状況 [Severe Acute Respiratory Syndrome Coronavirus-2 Vaccination Status]. Available from: https://info.vrs.digital.go.jp/dashboard/#overview.
22. Lumeng CN. Innate immune activation in obesity. Mol Aspects Med. 2013;34(1):12–29. doi:10.1016/j.mam.2012.10.002
23. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332–341. doi:10.5551/jat.3939
24. Abe T, Yamaguchi F, Sakakura S, Yamazaki Y, Shikama Y. Effect of tocilizumab treatment in mildly-obese patients with coronavirus disease 2019: a case series. Ann Transl Med. 2022;10(23):1263. doi:10.21037/atm-2022-49
25. Hanna Kazazian N, Wang Y, Roussel-Queval A, et al. Lupus autoimmunity and metabolic parameters are exacerbated upon high fat diet-induced obesity due to TLR7 signaling. Front Immunol. 2019;10:2015. doi:10.3389/fimmu.2019.02015
26. Englmeier L, Subburayalu J. What’s happening where when SARS-CoV-2 infects: are TLR7 and MAFB sufficient to explain patient vulnerability? Immun Ageing. 2022;19(1):6. doi:10.1186/s12979-022-00262-3
27. Meister T, Pisarev H, Kolde R, et al. Clinical characteristics and risk factors for COVID-19 infection and disease severity: a nationwide observational study in Estonia. PLoS One. 2022;17(6):e0270192. doi:10.1371/journal.pone.0270192
28. Carcaterra M, Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: a physio-pathological theory. Med Hypotheses. 2021;146:110412. doi:10.1016/j.mehy.2020.110412
29. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi:10.1016/S2213-2600(20)30076-X
30. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
31. Nakabayashi M, Adachi Y, Itazawa T, et al. MxA-based recognition of viral illness in febrile children by a whole blood assay. Pediatr Res. 2006;60(6):70–74. doi:10.1203/01.pdr.0000246098.65888.5b
32. Sambursky R, Shapiro N. Evaluation of a combined MxA and CRP point-of-care immunoassay to identify viral and/or bacterial immune response in patients with acute febrile respiratory infection. Eur Clin Respir J. 2015;2(1):28245. doi:10.3402/ecrj.v2.28245
33. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227. doi:10.1001/jamaoncol.2020.6178
34. Foote MB, White JR, Jee J, et al. Association of antineoplastic therapy with decreased SARS-CoV-2 infection rates in patients with cancer. JAMA Oncol. 2021;7(11):1686–1691. doi:10.1001/jamaoncol.2021.3585
35. Ministry of Health, Labour and Welfare. 新型コロナウイルス感染症から回復した患者の転院を受け入れる後方支援医療機関の確保について [Logistical support for medical facilities to receive patients recovering from COVID-19]. Available from: https://www.mhlw.go.jp/content/000778332.pdf.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



