Back to Journals » Journal of Inflammation Research » Volume 16
Combination of NK and Other Immune Markers at Early Phase Stratify the Risk of Sepsis Patients: A Retrospective Study
Authors Hu Z , Dong D, Peng F, Zhou X, Sun Q, Chen H, Chang W, Gu Q, Xie J, Yang Y
Received 20 June 2023
Accepted for publication 10 October 2023
Published 18 October 2023 Volume 2023:16 Pages 4725—4732
DOI https://doi.org/10.2147/JIR.S426828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Zihan Hu,1,* Danjiang Dong,2,* Fei Peng,1,* Xing Zhou,1 Qin Sun,1 Hui Chen,1,3 Wei Chang,1 Qin Gu,2,* Jianfeng Xie,1,* Yi Yang1,*
1Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, 210009, People’s Republic of China; 2Department of Critical Care Medicine, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China; 3Department of Critical Care Medicine, The First Affiliated Hospital of Soochow University, Soochow University, Suzhou, 215000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianfeng Xie; Qin Gu, Department of Critical Care Medicine, Zhongda Hospital, School of medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, People’s Republic of China, Tel/Fax +00862583262500, Email [email protected]; [email protected]
Purpose: Immune dysfunction plays a pivotal role in sepsis pathogenesis. Previous studies have revealed the crucial role of T cells and human leukocyte antigen-DR (HLA-DR) in sepsis. However, the function of natural killer (NK) cells remains unclear. This study aimed to investigate whether NK cells are associated with sepsis prognosis. In addition, we aimed to explore the interrelation and influence between NK and other immunological features in patients with sepsis.
Patients and Methods: This retrospective, observational study included patients with sepsis from two hospitals in mainland China. The clinical characteristics and immune results during the early phase were collected. Patients were classified according to the level of immune cells to analyze the relationship between immunological features and 28-day mortality.
Results: A total of 984 patients were included in this study. Non-survivors were older and had lower levels of lymphocytes, monocytes, NK cells, HLA-DR, and T cells. Patients were classified into eight groups according to their levels of NK cells, HLA-DR, and T cells. Only patients with decreased NK and T cell counts showed a significant increase in 28-day mortality. An increase in CD8+ T cells was correlated with the alleviation of 28-day mortality only among patients with high NK cell levels.
Conclusion: This study provides novel insights into the association between NK cells and 28-day mortality as well as the interrelation between NK cells and other immune cells in sepsis. The relationship between CD8+ T cells and 28-day mortality in sepsis is dependent on NK cell count.
Keywords: sepsis, immune dysfunction, natural killer cell, HLA-DR, T cell
Introduction
Sepsis is a life-threatening organ dysfunction caused by dysregulated host responses to infection.1 It is estimated that 48.9 million people present with sepsis and 11.0 million people die due to sepsis per year worldwide.2 Recent study carried out in mainland China reported an incidence of 328.25 (95% CI 315.41–341.09), 359.26 (95% CI 345.4–373.12) and 421.85 (95% CI 406.65–437.05) sepsis cases per million people in 2017, 2018 and 2019 respectively.3 A previous study revealed a 20.6% prevalence of sepsis and a 90-day mortality rate of 35.5% in patients admitted to intensive care units (ICUs) in mainland China.4
Immune dysfunction is a pivotal pathophysiological feature of sepsis that involves different types of immune cells. This alarming case fatality has led to intense research efforts, but with no exciting results.5–7 Immune cell apoptosis is commonly observed in sepsis, especially in T cells, B cells, natural killer (NK) cells, and dendritic cells.6 The number of T cells, B cells, and NK cells decreased over time in patients with sepsis. Moreover, a decrease in T cells, especially CD8+ T cells, is closely associated with the severity and mortality of sepsis patients.8–10 Diminished expression of human leukocyte antigen-DR (HLA-DR) in blood monocytes is a well-established marker of immunosuppression in sepsis and correlates with poor outcome.11 Patients with delayed or no improvement in HLA-DR or a decline in HLA-DR expression had a higher risk of 28-day mortality.12 A multicenter study illustrated that the combination of regulatory T cells, HLA-DR, and CD88 had a stronger correlation with mortality in patients with sepsis than with each marker alone.13 Though much attention on T cells and HLA-DR, less is about the role innate immune cells play in sepsis, which is the first line of defense against infection. NK cells are distinctive components of the innate immune system. It is a cytotoxic lymphocyte of the innate immune system that is capable of killing targeted cells directly.14,15 However, the relationship between NK cells and sepsis outcome remains unclear. In this study, we combined NK cells, HLA-DR, and T cells, which are components of innate immunity, antigen presentation, and adaptive immunity, respectively, to represent different immunological features. Our aim was to investigate the relationship between NK cell levels and 28-day mortality in sepsis, as well as the interrelation and influence between NK and other immunological features in sepsis patients.
Materials and Methods
Patients
This retrospective study was conducted at two tertiary teaching hospitals in China (Zhongda Hospital affiliated with Southeast University and Drum Tower Hospital in Nanjing) between December 2016 and December 2021. Patients diagnosed with sepsis according to Sepsis-3 criteria were included.1 Exclusion criteria included patients aged less than 18 years or > 85 years, readmitted to the ICU, pregnant, diagnosed with immune disorders, cancer, agranulocytosis or AIDS, taking immunosuppressant drugs, long-term or high-dose steroid use, or lack of immune cell testing.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Zhongda Hospital, Southeast University.
Data Collection
Clinical characteristics, including age, sex, sequential organ failure assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation (APACHE II) score, and 28-day mortality, were retrospectively collected from medical records. The first laboratory test recorded during hospitalization included CD3+ T cells, CD4+ T cells, CD8+ T cells, neutrophils, lymphocytes, monocytes, natural killer (NK) cells, and human leukocyte antigen (HLA)- DR.
Statistical Analysis
Variables were presented as mean values and standard deviations or median and interquartile range (IQR), depending on their normality. Categorical variables were presented as percentages. Normality distribution for quantitative variables was tested using the Kolmogorov–Smirnov test (P > 0.10).
The receiver operating characteristic curve and Youden index were used to determine the cutoff values for immune cells. Immune cell levels were then classified using cutoff values. We categorized patients into eight groups according to the level of immune cells as follows: group I, high levels of NK cells, HLA-DR, and T cells; group II, high NK cells, high HLA-DR, and low T cells; group III, high NK cells, low HLA-DR, and high T cells; group IV, high NK cells, low HLA-DR, and low T cells; group V, low NK cells, high HLA-DR, and high T cells; group VI, low NK cells, high HLA-DR, and low T cells; group VII, low NK cells, low HLA-DR, and high T cells; and group VIII, low levels of NK cells, HLA-DR, and T cells (Figure 1). Groups were compared using the Student’s t-test, rank-sum test, or one-way analysis of variance, as appropriate. Kaplan-Meier curves, Log rank tests, and univariate and multivariate Cox regression analyses were used for survival analysis. All tests were two-sided, and statistical significance was set at P <0.05. Statistical analyses were performed using the Stata 15.0 software (version 15.0; StataCorp, College Station, TX, USA). Graphs were prepared using GraphPad Prism (version 7.0; GraphPad Software, La Jolla, CA, USA).
 |
Figure 1 Classification of patients according to the level of immune cells. Abbreviations: NK cell, natural killer cell; HLA-DR, human leukocyte antigen-DR. |
Results
Baseline Characteristics
A total of 984 patients between December 2016 and December 2021 were included in the analysis. The median age of the patients was 64 (52, 75) years, and 322 (32.9%) were female. The median SOFA score was 9 (6, 12) and the median APACHE II score was 19 (14, 25). The numbers of lymphocytes, monocytes, natural killer cells, HLA-DR, total T cells, CD4+T cells, and CD8+T cells were lower in non-survivors than in survivors (P < 0.001). In total, 225 patients (22.9%) died within 28 days (Table 1).
 |
Table 1 Comparisons of Clinical and Biochemical Variables According to Clinical Outcome |
The Level of NK Cells Was Associated with 28-Day Mortality
The patients were divided into two groups according to their NK cell levels. Compared to patients exhibiting high levels of NK cells, those with low levels of NK cells showed significantly higher 28-day mortality (Figure S1). After adjusting for age, sex, and SOFA score, this association remained statistically significant (Table S1).
Different Immune Feature Was Associated with 28-Day Mortality
The baseline clinical and biochemical characteristics of each group, stratified by NK cell count, HLA-DR, and T cells, are presented in Table S2. SOFA and APACHE II scores were significantly higher in group VIII than in the other groups. The impact of different immune features on the 28-day mortality is shown in Figure 2 and Table 2. Group I had the lowest mortality rate, whereas group VIII had the highest mortality rate during the 28-day follow-up period. In the unadjusted Cox regression model, all groups exhibited higher mortality rates than group I, except for group V (P < 0.001). After adjusting for age, sex, and SOFA score, only groups VI (HR 2.31; 95% CI 1.43 to 3.71, P = 0.001) and VIII (HR 3.31; 95% CI 2.07 to 5.29, P < 0.001) showed higher 28-day mortality than group I.
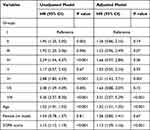 |
Table 2 Independent Predictors of 28-Day Mortality by Cox Regression Analysis |
 |
Figure 2 Survival curve of different groups. |
The Level of NK Cell Impacts the Association of T Cells with 28-Day Mortality
The patients were classified into low- and high-NK cell groups. As shown in Figure S2 and Table 3, in the high NK cell group, an increase in CD8+ T cells was associated with significantly lower mortality after adjusting for age, sex, and SOFA score (HR, 0.62; 95% CI 0.42 to 0.92; P = 0.016). However, in the low NK cell group, the association did not reach statistical significance after adjustment (HR, 0.68; 95% CI 0.45 to 1.03, P = 0.07; Figure S3 and Table S3). Interestingly, the increase in CD4+ T cells was not related to 28-day mortality in either the high or low NK cell group (Figure S4, Figure S5, Table S4, and Table S5).
 |
Table 3 Cox Regression Analysis for 28-Day Mortality According to Level of CD8+ T Cells in Patients with High NK Cells Counts |
Discussion
This study used a multiparametric immunophenotype to determine immune dysfunction in patients with sepsis. Through this study, we confirmed the relationship between NK cells and 28-day mortality, as well as the interrelation and influence between NK cells and other immunological features in patients with sepsis. Although each marker alone was related to 28-day mortality of sepsis, the combination of the three markers showed a more interesting immunological feature associated with sepsis. Impairment of any of the three markers alone did not affect the outcome of sepsis. Only when dysfunction of NK cells and T cells occurred concurrently (with or without a decrease in HLA-DR) would the 28-day mortality show a statistically significant increase. Moreover, NK cells can influence the relationship between CD8+T cells but not CD4+T cells and the outcome of sepsis.
Compared with survivors, non-survivors of sepsis presented with an immunosuppressive phenotype. In addition to older age and higher severity scores, non-survivors had significantly lower levels of lymphocytes, monocytes, NK cells, HLA-DR, total T cells, CD4+T cells, and CD8+T cells. Many immune cells undergo apoptosis during sepsis, except neutrophils, which display delayed apoptosis.16 Our results showed no significant difference in neutrophil counts between survivors and non-survivors. This is consistent with previous studies that showed that neutrophils were higher in sepsis patients than in healthy volunteers but exhibited no difference between survivors and non-survivors.17,18 During sepsis, T-cells exhibit elevated levels of apoptosis and exhaustion. The apoptosis rate of T cells shows an obvious increase in sepsis, and inhibition of apoptosis alleviates the severity of sepsis.19,20 The exhaustion of T cells is also closely related to the mortality of sepsis patients.10,21,22 HLA-DR is a class II major histocompatibility complex (MHC) protein that mediates antigen-specific recognition by CD4+ helper T lymphocytes.23 Decrease of HLA-DR lead to diminished antigen presentation and reduced adaptive immune activation, leading to immunosuppression and poor outcome.23–25 NK cells were discovered in the early 1970s as a group of cytotoxic lymphocytes that regulate both innate and adaptive immunity.26,27 Some studies have reported the beneficial effects of NK cells in sepsis, and inhibition of these effects increase mortality.28,29 Other studies have shown that NK cells act as a risk factor for sepsis.30–32 Another study reported no differences between non-survivors and survivors.17 This difference may be explained by the heterogeneity of patients, including the infection site, course of disease, and organism of infection.
Diverse immunological features have been associated with different clinical outcomes. In our study, patients were divided into several groups according to their levels of NK cells, HLA-DR, and T cells. Although each marker alone had a predictive ability, in combination they showed a more interesting phenomenon. Compared with patients with no deficiency in NK cells, HLA-DR, or T cells, only a decrease in both NK cells and T cells (with or without a decrease in HLA-DR) was an independent risk factor for 28-day mortality. Many studies have established the pivotal role of T cells and HLA-DR in the prognosis of sepsis.9,11,12,23,33 Monocytic HLA-DR has been used for risk stratification in clinical trials (NCT 02361528). However, our study showed that, as long as there was no deficiency in NK cells, neither a decrease in T cells nor HLA-DR was associated with 28-day mortality in patients with sepsis. Our findings indicate that the relationship between T cells or HLA-DR and mortality from sepsis is influenced by NK cells and that innate immune cells also play a crucial role in sepsis.
To gain further knowledge about the impact of NK cells on T cells, we analyzed the correlation between CD4+ T cells, CD8+ T cells, and the prognosis of sepsis. Interestingly, an increase in CD8+ T cells was significantly associated with a decrease in 28-day mortality only when NK cell counts were high. This phenomenon was not observed in the CD4+ T cells. In defense against tumors, NK cells have been shown to enhance the function of CD8+ T cells through a T-bet/Eomes-dependent pathway.34 Further studies are required to verify this phenomenon and its specific mechanism of action.
This study provides novel insights into the role of immune cells and their interactions in sepsis. But there are still some limitations. First, as this was a retrospective study, statistical bias was inevitable and causality could not be assessed. The patients were recruited from two hospitals in China; therefore, our results may not be applicable to other regions of the world. Second, tests were performed in peripheral blood samples, which may differ from the results obtained in tissues. In addition, we focused on patients aged between 18 and 85 years; therefore, the results do not reflect the phenomenon occurring in children and older adults. Besides, parameters including source of infection, pathogenesis, mechanical ventilation, presence of shock and use of vasoactive drugs was lacking in our study, which may influence the prognosis. Furthermore, the study collected laboratory results during the early phase of sepsis, which could be different from those during the late phase. Lastly, we did not analyze the dynamic results of this study. It would be meaningful to investigate dynamic immune markers in the future.
Conclusion
In conclusion, our study confirmed an association between different immunological features and 28-day mortality in patients with sepsis. The combination of immune cells allows for a more nuanced assessment of mortality due to sepsis, with important implications for supporting future immunomodulatory therapies in patients. As innate cytotoxic lymphocytes, NK cell is closely related to the prognosis of sepsis. In addition, the level of NK cells correlates with the relationship between CD8+ T cells and sepsis prognosis.
Abbreviations
ICU, intensive care unit; NK cell, natural killer cell; HLA-DR, human leukocyte antigen-DR; SOFA, sequential organ failure assessment; APACHE, Acute Physiology and Chronic Health Evaluation.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Zhongda Hospital, Southeast University.
Consent for Publication
The informed consent was waived for the following reasons: 1) The study uses medical record data obtained from previous clinical consultations, and the risk to the subject is not greater than the minimum risk, and the exemption of informed consent will not adversely affect the rights and health of the subject; 2) The information collected by the Institute ensures that the privacy and identity of the subjects is confidential and protected; 3) The study does not utilize medical records and specimens that the patient/subject has previously expressly refused to use. Waiver of informed consent statement was shown in the file named “Waiver of informed consent statement”.
Acknowledgments
We acknowledge that all the participants participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Key Research and Development Program (2021YFC2500804), National Natural Science Foundation of China (82072155), National Natural Science Foundation of China (82272210), Jiangsu Provincial Key Research and Development Program (BE2022854), Jiangsu Provincial Medical Innovation Center (CXZX202221), Jiangsu Provincial Medical Key Discipline (Laboratory) (ZDXYS202205), and National Natural Science Foundation of China (81901945).
Disclosure
The authors report no conflict of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
3. Weng L, Xu Y, Yin P, et al. National incidence and mortality of hospitalized sepsis in China. Crit Care. 2023;27(1):84. doi:10.1186/s13054-023-04385-x
4. Xie J, Wang H, Kang Y, et al. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit Care Med. 2020;48(3):e209–e218. doi:10.1097/CCM.0000000000004155
5. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi:10.1038/nri3552
6. van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450–2464. doi:10.1016/j.immuni.2021.10.012
7. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi:10.1038/nri.2017.36
8. Venet F, Davin F, Guignant C, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34(4):358–363. doi:10.1097/SHK.0b013e3181dc0977
9. Hohlstein P, Gussen H, Bartneck M, et al. Prognostic relevance of altered lymphocyte subpopulations in critical illness and sepsis. J Clin Med. 2019;8(3):353. doi:10.3390/jcm8030353
10. Yan L, Chen Y, Han Y, Tong C. Role of CD8+ T cell exhaustion in the progression and prognosis of acute respiratory distress syndrome induced by sepsis: a prospective observational study. BMC Emerg Med. 2022;22(1):182. doi:10.1186/s12873-022-00733-2
11. Landelle C, Lepape A, Voirin N, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36(11):1859–1866. doi:10.1007/s00134-010-1962-x
12. Leijte GP, Rimmelé T, Kox M, et al. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit Care. 2020;24(1):110. doi:10.1186/s13054-020-2830-x
13. Conway Morris A, Datta D, Shankar-Hari M, et al. Cell-surface signatures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med. 2018;44(5):627–635. doi:10.1007/s00134-018-5247-0
14. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100. doi:10.1038/s41571-020-0426-7
15. Guo Y, Patil NK, Luan L, Bohannon JK, Sherwood ER. The biology of natural killer cells during sepsis. Immunology. 2018;153(2):190–202. doi:10.1111/imm.12854
16. Wang JF, Wang YP, Xie J, et al. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood. 2021;138(9):806–810. doi:10.1182/blood.2020009417
17. Tang H, Qin S, Li Z, Gao W, Tang M, Dong X. Early immune system alterations in patients with septic shock. Front Immunol. 2023;14:1126874. doi:10.3389/fimmu.2023.1126874
18. Lei W, Ren Z, Su J, et al. Immunological risk factors for sepsis-associated delirium and mortality in ICU patients. Front Immunol. 2022;13:940779. doi:10.3389/fimmu.2022.940779
19. Reizine F, Grégoire M, Lesouhaitier M, et al. Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2022;119(8):e2115139119. doi:10.1073/pnas.2115139119
20. Ma Y, Cheng Z, Zheng Y, et al. Low dose of Esmolol attenuates sepsis-induced immunosuppression via modulating T-lymphocyte apoptosis and differentiation. Shock. 2023;59(5):771–778. doi:10.1097/SHK.0000000000002104
21. Chen W, Liu J, Ge F, et al. Long noncoding RNA HOTAIRM1 promotes immunosuppression in sepsis by inducing T cell exhaustion. J Immunol. 2022;208(3):618–632. doi:10.4049/jimmunol.2100709
22. He W, Xiao K, Xu J, et al. Recurrent sepsis exacerbates CD4+ T cell exhaustion and decreases antiviral immune responses. Front Immunol. 2021;12:627435. doi:10.3389/fimmu.2021.627435
23. Joshi I, Carney WP, Rock EP. Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis. Front Immunol. 2023;14:1130214. doi:10.3389/fimmu.2023.1130214
24. Pfortmueller CA, Meisel C, Fux M, Schefold JC. Assessment of immune organ dysfunction in critical illness: utility of innate immune response markers. Intensive Care Med Exp. 2017;5(1):49. doi:10.1186/s40635-017-0163-0
25. Huang C, Xiong H, Li W, et al. T cell activation profiles can distinguish gram negative/positive bacterial sepsis and are associated with ICU discharge. Front Immunol. 2022;13:1058606. doi:10.3389/fimmu.2022.1058606
26. Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5(2):117–121. doi:10.1002/eji.1830050209
27. Hf P, J M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975;21(2):11–15
28. Jensen IJ, McGonagill PW, Butler NS, Harty JT, Griffith TS, Badovinac VP. NK cell-derived IL-10 supports host survival during sepsis. J Immunol. 2021;206(6):1171–1180. doi:10.4049/jimmunol.2001131
29. Garzón-Tituaña M, Sierra-Monzón JL, Comas L, et al. Granzyme A inhibition reduces inflammation and increases survival during abdominal sepsis. Theranostics. 2021;11(8):3781–3795. doi:10.7150/thno.49288
30. de Roquetaillade C, Mansouri S, Brumpt C, et al. Comparison of circulating immune cells profiles and kinetics between coronavirus disease 2019 and bacterial sepsis. Crit Care Med. 2021;49(10):1717–1725. doi:10.1097/CCM.0000000000005088
31. Zhang Y, Wang J, Hu L, et al. Predictive value of immune cell subsets for mortality risk in patients with sepsis. Clin Appl Thromb Hemost. 2021;27:10760296211059498. doi:10.1177/10760296211059498
32. Li D, Zhang J, Bai G, Chen J, Cheng W, Cui N. Lymphocyte and NK cell counts can predict sepsis-associated delirium in elderly patients. Front Aging Neurosci. 2020;12:621298. doi:10.3389/fnagi.2020.621298
33. Schefold JC. Measurement of monocytic HLA-DR (mHLA-DR) expression in patients with severe sepsis and septic shock: assessment of immune organ failure. Intensive Care Med. 2010;36(11):1810–1812. doi:10.1007/s00134-010-1965-7
34. Bi J, Jin X, Zheng C, et al. Checkpoint TIPE2 Limits the Helper Functions of NK Cells in Supporting Antitumor CD8 + T Cells. Adv Sci. 2023;10(12):e2207499. doi:10.1002/advs.202207499
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
