Back to Journals » International Journal of Nanomedicine » Volume 18
Combat Against Gynecological Cancers with Blood Vessels as Entry Point: Anti-Angiogenic Drugs, Clinical Trials and Pre-Clinical Nano-Delivery Platforms
Authors Yang S, Fei W , Zhao Y, Wang F, Ye Y, Wang F
Received 9 March 2023
Accepted for publication 31 May 2023
Published 8 June 2023 Volume 2023:18 Pages 3035—3046
DOI https://doi.org/10.2147/IJN.S411761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Shan Yang,1 Weidong Fei,1 Yunchun Zhao,1 Fengmei Wang,1 Yiqing Ye,1 Fenfen Wang2
1Department of Pharmacy, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, Peoples Republic of China; 2Department of Gynecology Oncology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, Peoples Republic of China
Correspondence: Yiqing Ye; Fenfen Wang, Email [email protected]; [email protected]
Abstract: Angiogenesis is an essential mechanism for the progression of gynecological cancers. Although approved anti-angiogenic drugs have demonstrated clinical efficacy in treating gynecological cancers, the full potential of therapeutic strategies based on tumor blood vessels has not yet been realized. This review summarizes the latest angiogenesis mechanisms involved in the progression of gynecological cancers and discusses the current clinical practice of approved anti-angiogenic drugs and related clinical trials. Given the close relationship between gynecological cancers and blood vessels, we highlight more delicate strategies for regulating tumor vessels, including wise drug combinations and smart nano-delivery platforms to achieve highly efficient drug delivery and overall vessel microenvironment regulation. We also address current challenges and future opportunities in this field. We aim to generate interest in therapeutic strategies that target blood vessels as a key entry point and offer new potential and inspiration for combating gynecological cancers.
Keywords: gynecological cancers, tumor blood vessel, tumor vascularization, anti-angiogenic, clinical trials, nano-delivery platforms, nanoparticles
Introduction
Gynecological cancers, including cervical cancer, ovarian cancer, endometrial cancer, and gestational trophoblastic neoplasia (GTN), pose a significant threat to women’s health. According to the cancer statistics report of 2022,1 ovarian cancer is among the top five leading causes of cancer death in females in the United States. Although the survival rate for most common cancers has improved since the mid-1970s, uterine cervix, and uterine corpus-associated cancers remain excluded. Despite being one of the most preventable cancers, cervical cancer persistently ranks as the second leading cause of cancer death in women aged 20 to 39 years. Furthermore, the mortality rates of endometrial cancer (EC) have been increasing by an average of 1.9% per year, primarily attributed to the increasing incidence of obesity, a known risk factor for the most frequent type of EC.2 Although the incidence of GTN is low, women of childbearing age must undergo adequate periods of cytotoxic chemotherapy.3 In summary, gynecological cancers continue to pose a serious threat to women’s health.
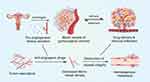
During surgeries, we can clearly see that malignant gynecological tumors are characterized by a high abundance of blood vessels. Angiogenesis is a complex, highly-regulated process that is indispensable for cancer progression. During this process, malignant tumors develop new vasculature to seize the necessary nutrition and oxygen supply.4,5 It is important to note that blood vessels also act as vital channels for delivering therapeutic agents. The over-production of pro-angiogenesis factors, such as vascular endothelial growth factors, gives rise to abnormal angiogenesis in gynecological cancers. Although anti-angiogenic drugs can block this process, the excessive trimming of tumor blood vessels can lead to problems. This is because, in addition to their tight relationship with gynecological cancer progression, blood vessels also play an indispensable role in the transportation of therapeutic agents. Specifically, abnormal intra-tumor vessels tend to be complex, disorganized, irregular, and leaky, resulting in poor delivery of drugs and therapeutic agents to the entire tumor, thereby affecting the chemotherapy response. Destruction of blood vessel integrity after anti-angiogenic treatment may also alter drug penetration,6 offer opportunities for hematogenous metastasis,7 and influence not only drug delivery8 but also immune infiltration (Figure 1).7,9
Therefore, the regulation of tumor vessel microenvironment deserves careful attention, and exploring therapeutic strategies against gynecological cancers with blood vessel regulation as an entry point holds great potential. Although anti-angiogenic drugs have already demonstrated clinical efficacy in gynecological cancers,10 the full potential of tumor blood vessel-associated therapeutic strategies has yet to be fulfilled. Inspiringly, the application of nano-delivery platforms has offered great inspiration for more efficient drug delivery, as well as more delicate tumor vessel regulation and microenvironment rebuild. Nano drugs can spontaneously form a “treatment-delivery” loop to promote therapeutic agents toward deep tumor regions, leading to potent tumor inhibition and overall tumor microenvironment improvements, including tumor vessel microenvironment and immune microenvironment.11
This review collects updated investigations on angiogenesis mechanisms during gynecological cancer progression and current clinical applications of tumor angiogenesis-regulating drugs. Related clinical trials involving tumor vessel regulation and associated combination therapy are also included. Additionally, more delicate tumor blood vessel-regulating strategies under pre-clinical investigation are further discussed, with a focus on cutting-edge nanoplatforms comprehensively regulating the tumor vessel microenvironment. We aim to draw attention to tumor blood vessels as a therapeutic target to develop novel strategies for comprehensive angiogenesis-regulating therapy in gynecological cancers.
Update on Angiogenesis Mechanisms in Gynecological Cancers
The hormonal and metabolic disorder environment often contributes to the angiogenesis and development of gynecological cancers, given the close relationship between hormones and the reproductive system in gynecology. Studies have reported that high levels of estrogen receptor α (ERα) can inhibit tumor growth by modulating angiogenic factors, thereby limiting the blood supply to growing tumors.12,13 Moreover, the estrogen receptor has been found to play a significant role in mediating cervical cancer invasion and progression.14 Apart from the well-studied angiogenic growth factor, vascular endothelial growth factor (VEGF), ovarian and uterine cells can also overexpress other molecules that act as angiogenic factors, such as neurotrophins and their receptors.15 However, the specific relationship between gynecological cancer progression and blood vessels varies among different gynecological cancer types and thus requires further exploration and comprehensive understanding. In this section, we present an update on the investigations of the angiogenesis mechanisms of the four main gynecological cancer types.
Cervical Cancer
Angiogenesis is among the hallmarks that contribute to the development of precancerous lesions and progression in many cancer types, including cervical cancer and its precursor lesions.16 Due to the strong causative association with human papillomavirus (HPV), the mechanism of angiogenesis regulation is thought to be unique in cervical cancer. It has been reported that the HPV18 E6 oncoprotein could contribute to tumor angiogenesis by inducing vascular endothelial growth factor (VEGF) transcription from the promoter in a p53-independent manner. Specifically, the production of E6 protein leads to dysregulation of p53 induction and ubiquitination, resulting in p53 protein degradation and consequently, upregulation of VEGF.17 Additionally, the E7 protein can displace histone deacetylases HDAC1, HDAC4, and HDAC7, leading to upregulation of hypoxia-inducible factor 1α and consequently increasing VEGF production.18 All of the aforementioned HPV-associated mechanisms contribute to the angiogenesis of cervical cancer.
Ovarian Cancer
Ovarian cancer is widely accepted as highly vascularized, and 4D bi-directional power Doppler has vividly displayed statistically significant vascularization differences between normal and malignant ovaries.19 Several studies have also found significantly higher levels of vascular endothelial growth factor in tissues and biological fluids of women with ovarian cancer compared to healthy control.20 Vascular endothelial growth factor signaling can activate several downstream effectors, including the Notch pathway, which plays an essential role in vascular homeostasis and integrity in the normal ovary. The Notch pathway is frequently altered in ovarian cancers, resulting in over-angiogenesis.21 A high degree of tumor angiogenesis has been shown to correlate with poor survival in ovarian cancer patients.10,22 Thus, it is crucial to regulate tumor angiogenesis, including both angiogenesis-related key factors and downstream effectors, to rebuild the tumor vessel microenvironment, which can facilitate tumor growth and influence treatment.
Endometrial Cancer
The formation of new blood vessels is vital for blastocyst implantation and the growth of the placenta in obstetrics.23 Therefore, human endometrial cells have strong angiogenic potential. Studies have reported that micro-vessel density significantly increases in endometrial cancer compared to normal endometrium. Micro-vessel density is a common indicator for evaluating tumor angiogenesis and has also been shown to correlate with endometrial cancer survival, International Federation of Gynecology and Obstetrics (FIGO) stage, as well as other histologic and clinicopathologic parameters of endometrial cancer.24 Estrogen has been reported to drive angiogenesis in the endometrium directly through increased expression of VEGF and β-FGF via the NF-κB signaling pathway,25 as well as indirectly through HIF-1α and PI3K/Akt activation.26
Gestational Trophoblastic Neoplasia
Maternal vascular adaptation to pregnancy is critical to expanding the capacity for blood flow through the uteroplacental unit to meet the needs of the developing fetus. During pregnancy, a variety of vessel-associated growth factors and cytokines are present.27 There is a delicate balance between the normal physiologic degradation of maternal decidual vasculature and the concurrent angiogenesis of the growing embryo. When such balance is disturbed, gestational trophoblastic diseases characterized by vascular abnormalities of the trophoblast may occur.28 Angiogenin and matrix metalloproteinase-2, two important molecules during the angiogenic process, were significantly elevated in the placental tissues and maternal serum of patients with gestational trophoblastic diseases. A high level of placental growth factor has been reported to promote the development of blood vessels in choriocarcinomas.29 High expression of VEGF and its receptor has also been found in gestational trophoblastic diseases.30
Clinical Practice Involving Vessel Regulation
Approved Drugs
There are already approved vessel-regulating drugs for the treatment of gynecological cancers, and some are still under clinical trials. Bevacizumab, the recombinant humanized monoclonal antibody against VEGF and the most well-recognized blood vessel-regulating drug, has been approved for use in numerous indications, including advanced-stage epithelial ovarian and cervical cancers. Unfortunately, there are currently no validated biomarkers to select patients who will benefit from bevacizumab, and further investigation is required. Another small molecular angiogenesis inhibitor, lenvatinib, has also obtained approval, in combination with pembrozliumab, for subsequent-line treatment of advanced endometrial cancer.10 Table 1 presents more information about currently-approved drugs and their indications/clinical applications in gynecological cancers.
 |
Table 1 Approved Vessel-Regulating Drugs for Gynecological Cancers |
Clinical Trials
Current clinical application mainly focuses on anti-angiogenic therapy, yet there are still various problems, including disappointing outcomes and worrying adverse effects.39 Moreover, rapid adaptive resistance might occur within several months after initiating bevacizumab treatment, hindering the achievement of any overall survival benefit.40 Thus, a more comprehensive regulation of tumor blood vessels, along with the corresponding tumor microenvironment, needs to be considered. To meet this goal, a smart combination of drugs, as well as novel active pharmaceutical ingredients, are under clinical investigation.
Ovarian Cancer
Bevacizumab was first approved by the European Union (EU) for the first-line treatment of ovarian cancer in 2011 and subsequently approved by the EU for platinum-sensitive recurrent ovarian cancer in 2012. In 2014, it was further approved for platinum-resistant recurrent ovarian cancer.41 Bevacizumab-mediated apoptosis of tumor endothelial cells and a decrease in interstitial fluid pressure within the tumors allow greater capacity for chemotherapeutic drugs to reach targeted sites. Therefore, Bevacizumab has also demonstrated significant therapeutic benefits in combination with standard chemotherapy.42 The combination of blood vessel-regulating drugs (not limited to Bevacizumab) and other drugs has attracted interest in the management of tricky ovarian cancers. Cediranib, an oral multi-targeted tyrosine kinase inhibitor (TKI), has demonstrated clinical efficacy both as antiangiogenic monotherapy and in combination with other drugs, such as platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer and olaparib in recurrent platinum-resistant ovarian cancer patients without germline BRCA1/2 mutation.43,44 Apatinib is another small-molecule antiangiogenic agent that selectively inhibits vascular endothelial growth factor receptor 2 (VEGFR-2) and mildly inhibits c-Kit and c-Src tyrosine kinases.45 The efficacy and safety of the combination therapy of apatinib and oral etoposide were assessed in patients with platinum-resistant or platinum-refractory ovarian cancer, considering the potential advantage of home administration without hospital admission.46 Ovarian cancer has been the subject of extensive clinical research compared to other gynecological cancers. Table 2 presents more examples.
 |
Table 2 Clinical Trials of Combination Strategies/Single Active Pharmaceutical Ingredients Involving Vessel-Regulating for Gynecological Cancers |
Endometrial Cancer
Clinical trials have been conducted to evaluate the effectiveness of anti-angiogenic therapy in endometrial cancer, with mixed results.57 Bevacizumab was found to have a clinical benefit rate of 19% in pretreated patients with advanced endometrial cancer.58 While lenvatinib, a multitargeted tyrosine kinase inhibitor that targets both vascular endothelial growth factor and placental growth factor, showed limited efficacy as a second-line treatment for recurrent endometrial cancer, combining lenvatinib with the immune-regulating drug pembrolizumab led to significantly longer progression-free survival and overall survival than chemotherapy among patients with advanced endometrial cancer.59 Based on these results, lenvatinib has been approved for use in endometrial cancer in combination with pembrozliumab. In addition to drug combinations, single-agent cediranib, and brivanib were also evaluated in recurrent/persistent endometrial cancer. Other multi-targeted tyrosine kinase inhibitors are listed in Table 2.
Cervical Cancer
A retrospective study was conducted to evaluate the efficacy and safety of anlotinib in patients with persistent, metastatic, or recurrent cervical cancer who had failed first-line therapy.60 Interestingly, the study compared the efficacy of anlotinib (targeting vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFR), fibroblast growth factor receptors (FGFR), etc.), and apatinib (targeting only VEGFR2). The study reported that anlotinib showed superior efficacy and safety over apatinib. A Cochrane review assessed the benefits and harms of VEGF-targeting agents in the management of persistent, recurrent, or metastatic cervical cancer.61 Low-certainty evidence was found in favor of using bevacizumab plus chemotherapy, despite an increased risk of adverse events such as gastrointestinal perforations or fistulae, thromboembolic events, and hypertension. However, the use of cediranib plus chemotherapy, apatinib plus chemotherapy, or pazopanib monotherapy was all disappointing, and some results have not been published (Table 2).
Gestational Trophoblastic Neoplasia
Most gestational trophoblastic diseases can be successfully treated with standard chemotherapy regimens, and anti-angiogenic drugs are seldom used. However, treatment options for patients with high-risk chemo-refractory or relapsed GTN are scarce. A case of refractory choriocarcinoma treated with bevacizumab was reported to achieve satisfactory clinical efficacy. After failing to respond to several lines of chemotherapy treatment, the patient was treated with bevacizumab (10mg/kg) and anti-endoglin monoclonal antibody (TRC105), and her HCG levels decreased to normal after four courses.62 Furthermore, a single-arm, open-label, Phase 2 trial was conducted to evaluate the anti-tumor activity and safety of camrelizumab (an immune checkpoint inhibitor) plus apatinib (a VEGFR inhibitor) in patients with high-risk chemo-refractory or relapsed GTN, and the results were promising (Table 2).24
Pre-Clinical Nano-Delivery Platforms Involving Vessel Regulation
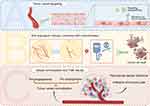
The use of nano-delivery platforms sheds light on the application of vascular modulation as an adjuvant therapy in combination with other treatments, as well as global remodeling of the vascular microenvironment.63 In addition to direct tumor inhibition, the nanoplatform construction can improve drug distribution in vivo, reduce adverse effects, and combat drug resistance.64,65 Therapeutic strategies assisted by nano-delivery platforms are gaining increasing attention and showing great potential in gynecological cancers (Figure 2).
Tumor Vessel-Targeted Nanoplatforms
Although standard chemotherapies theoretically target highly proliferating cancer cells, they have little effect on the slowly dividing endothelial cells. The F3 peptide has also shown the capability of binding to nucleolin protein expressed on the surface of tumor endothelial cells as well as on the surface of tumor cells. Winer et al used the F3 peptide to modify cisplatin-loaded polyacrylamide (PAA) nanoparticles to target tumors in both murine and human ovarian cancer models.66 Their data strongly supported the efficacy of vascular-targeted nanoparticle therapy in ovarian cancer, which was also the first to show chemo-nanoparticle binding to human tumor vessels in vivo. In their model, tumor vascular cells were derived from human embryonic stem cells, and the binding of F3-modified nanoparticles to human CD31+ tumor vessels was vividly observed. This treatment was effective in both platinum-sensitive and platinum-resistant cell lines, showing great potential for overcoming ovarian cancer chemoresistance by directly targeting nanoplatforms to the tumor neovasculature. Apart from the F3 peptide, RGD (arginine-glycine-aspartic acid) motif-containing peptides have also been utilized to modify nanoplatforms, which can bind to integrin molecules that are up-regulated on tumor vessels.67
In addition to the consideration that tumor vascularization depends on the proliferation of endothelial cells and the sprouting of new capillaries, vasculogenic mimicry (VM) of tumor cells cannot be ignored. VM is an important supplement to tumor angiogenesis and deserves special attention. Wang et al designed cRGD (cyclic Arg-Gly-Asp) peptide-containing nanoparticles to inhibit not only endothelium-dependent vessels (EDV) but also VM formation in ovarian cancer cells.68 The classical cRGD peptide can specifically bind with integrin αvβ3 and α5β1, exhibiting a specific inhibitory effect on EDV. They found that cRGD could also suppress VM formation in ovarian cancer cells by regulating the VM essential pathway (MMP2/Laminin 5c2) and reversing epithelial-mesenchymal transition (EMT). In their work, the tumor-specific ligands folate and cRGD were conjugated with the nanocarrier heparin to self-assemble into heparin-folate-cRGD-nanoparticles (cRGD-NPs). These versatile anti-angiogenic nanoplatforms achieved significant cytotoxicity and superior anti-tumor effects in SKOV3 (a human ovarian cancer cell line) xenografts.
Antiangiogenic and Chemotherapy Co-Loading Nanoplatforms
Ebeid et al reported that polymeric nanoparticles (NP) co-loaded with the angiogenesis molecular inhibitor BIBF 1120 and paclitaxel had significant selective cytotoxicity against serous uterine cancer with a loss-of-function p53 mutation.69 The NPs also inhibited tumor progression but also improved survival in an endometrial cancer xenograft model. PTX-loaded nanoparticles were found to be superior to PTX in solution, displaying the advantage of the nano-delivery formulation. Moreover, the combination of PTX-loaded NPs with antiangiogenic regulation showed the most promising effects.
Particle replication in non-wetting templates (PRINT®), a soft lithography process, is a recently developed method of fabricating nanocarriers for the efficient delivery of therapeutic moieties. Docetaxel incorporated in poly (lactic acid-co-glycolic acid) (PLGA)-PRINT nanoparticles displayed higher plasma exposure and greater intratumor accumulation than docetaxel alone. In addition to PLGA-PRINT nanoparticles containing docetaxel, another antiangiogenic component with mEZH2 siRNA incorporated into chitosan nanoparticles was designed.70 Endothelial cells were found to be quite sensitive to low doses of docetaxel therapy in a metronomic schedule, and PLGA-PRINT displayed strong anti-proliferative, anti-apoptotic, and anti-angiogenic effects in ovarian cancers. Combining PLGA-PRINT with antiangiogenic mEZH2 siRNA proved to be the most efficacious in reducing tumor burden.
Vessel Normalization and TME Rebuilding Nanoplatforms
The vascular dysfunction of ovarian cancer is known to contribute to chemotherapeutic resistance. Targeted delivery of miR-484 via RGD-modified exosomes was reported to improve vascular normalization, sensitize cancer to chemotherapy, and prolong the survival time of tumor-bearing mice, providing a promising avenue for the clinical management of chemotherapy resistance.71 Vascular maturity and functionality are associated with chemo-sensitization, which restore the physiological perfusion and oxygenation of tumor vasculature and improve the delivery of chemotherapy drugs. Targeted drug delivery assisted with nanoplatforms further enhances this strategy.
Lipid nanoparticles (LNP) have been proven effective in the delivery of siRNAs to the liver and tumors in animals. Scientists initiated a trial of the nanoplatform named ALN-VSP, an LNP formulation of siRNAs targeting VEGF and kinesin spindle protein, to examine the activity and safety of LNP-formulated siRNAs in humans. Biweekly intravenous administration of ALN-VSP was shown to be safe and well-tolerated. Moreover, the detection of drugs in tumor biopsies, siRNA-mediated mRNA cleavage in the liver, pharmacodynamics suggestive of target downregulation, and anti-tumor activity, including complete regression of liver metastases in endometrial cancer, were demonstrated.72 This nanoplatform suggests that more delicate tumor vessel regulation assisted with nano-delivery strategy is favorable for overall tumor microenvironment rebuild, including both tumor vessel microenvironment and immune microenvironment.
With deep insights into the tumor microenvironment (TME) and intratumor barriers that hinder drug delivery, attention has shifted towards regulating the TME (not just tumor vessel microenvironment) to augment tumor targeting and therapeutic efficacy. One study utilized sequential modulations of the tumor vasculature and stromal barriers to augment the active targeting efficacy of an antibody-modified nano-photosensitizer in desmoplastic ovarian cancer. The researchers revealed that while tumor vasculature normalization by thalidomide enhanced the tumor accumulation of nanoparticles, they may still get sequestered in the stromal bed of the tumor mass. However, photoablation of stromal cells located in perivascular regions significantly improved the accessibility of the antibody-modified nano-photosensitizer to receptor-overexpressed cancer cells. Hence, comprehensive regulation and rebuilding of the TME could help enhance the antitumor efficacy through synergistic enhancements of tumor accumulation and cancer cell accessibility of therapeutic nanoparticles.73
Challenges and Opportunities
Toxicology concerns regarding therapeutic agents, including the combination of anti-angiogenic drugs and chemotherapy drugs, as well as nano-delivery platforms, always exist. However, associated clinical trials have been carried out to evaluate their safety. For instance, a phase Ib study evaluated the safety and efficiency of paclitaxel combined with navicixizumab (a bispecific anti-angiogenic antibody against vascular endothelial growth factor and delta-like ligand 4) in platinum-resistant ovarian cancer.74 The study found that navicixizumab plus paclitaxel demonstrated promising clinical activity with manageable toxicity. Another Phase I study found that intraperitoneal administration of nanoparticles in combination with standard neoadjuvant chemotherapy in patients newly diagnosed with epithelial ovarian, fallopian tube, or primary peritoneal cancer was safe and had an impact on the tumor microenvironment.75 Here, the IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer was utilized, indicating the great potential of nano-delivery platforms and their bright future in clinical transformation.
In addition to cancer treatment, vessel-related investigations are also favorable for risk assessment and diagnosis. Scientists found that a high level of serum vascular endothelial growth factor has prognostic significance for identifying ovarian cancer patients with a higher risk of death and/or recurrence.76 Similarly, across the molecular spectrum of endometrial cancer, elevated levels of pro-angiogenic factors such as VEGF, platelet-derived endothelial cell growth factor, and fibroblast growth factor are prognostic for survival.77 FK506 binding protein-like (FKBPL), a member of the immunophilin family with anti-angiogenic effects capable of inhibiting the migration of endothelial cells and blood vessel formation, demonstrated a significant decrease in endometrioid endometrial carcinoma (in comparison to benign endometrial hyperplasia). Thus, the loss of FKBPL expression displays a high predictive value for malignancy.78
Assisted by high-resolution photoacoustic microscopy and a professional quantification algorithm, researchers have developed an efficient method for analyzing the vascular features of ovarian and fallopian tube specimens, including the mean vessel diameter, vascular density, global vascular directionality, local vascular definition, and local vascular tortuosity/branchedness.79 Microscopic vascular analysis of a larger set of samples in the future could potentially contribute to the current understanding of how ovarian malignancies develop at an early stage. Today, scientists can model the diffusive flow of individual drug molecules through the three-dimensional network of blood vessels that vascularize the tumor and surrounding tissues, using molecular mechanics techniques, which might help us better understand drug transportation in complex vascular networks in the future.80 Moreover, the development of nanoparticles with a unique capacity for encapsulating or conjugating therapeutic and imaging agents has created a great revolution in all aspects of cancer diagnosis, prevention, and treatment.81
Conclusion
This review provides an updated investigation of angiogenesis mechanisms during gynecological cancer progression and the current clinical applications of tumor angiogenesis-regulating drugs. However, we have found that we are still far from effectively tailoring nano-delivery platforms, and much work remains to be accomplished to push forward into clinical practice for each patient. With the gradual deepening of our understanding of the mechanism of cancer progression and the accumulation of both clinical and pre-clinical investigation data, we are on a meaningful path to develop novel strategies for comprehensive angiogenesis-regulating therapy. Considering the close connection between gynecological cancers and blood vessels, more efficient therapeutic strategies with tumor blood vessels as the entry point, such as wise clinical drug combinations and smart nano-delivery platforms, are expected to broaden the therapeutic landscape of gynecological cancers.
Acknowledgment
This study was supported by the Natural Science Foundation of Zhejiang Province (LYY22H300002).We thank Figdraw (www.figdraw.com) for the assistance in creating figures in this work.
Disclosure
The authors declare no conflict of interest.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Oaknin A, Bosse TJ, Creutzberg CL, et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(9):860–877. doi:10.1016/j.annonc.2022.05.009
3. Tidy J, Seckl M, Hancock BW. Management of gestational trophoblastic disease: green-top guideline No. 38 - June 2020. BJOG. 2021;128(3):e1–e27. doi:10.1111/1471-0528.16266
4. Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy, Nature clinical practice. Oncology. 2008;5(4):194–204. doi:10.1038/ncponc1051
5. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
6. McMullen M, Madariaga A, Lheureux S. New approaches for targeting platinum-resistant ovarian cancer. Semin Cancer Biol. 2021;77:167–181. doi:10.1016/j.semcancer.2020.08.013
7. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi:10.2353/ajpath.2010.100105
8. Nusrat O, Belotte J, Fletcher NM, et al. The role of angiogenesis in the persistence of chemoresistance in epithelial ovarian cancer. Reproduct Sci. 2016;23(11):1484–1492. doi:10.1177/1933719116645191
9. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi:10.3389/fimmu.2018.00978
10. Kasherman L, Liu SL, Karakasis K, Lheureux S. Angiogenesis: a pivotal therapeutic target in the drug development of gynecologic cancers. Cancers. 2022;14(5):1122. doi:10.3390/cancers14051122
11. Xu C, Yang S, Jiang Z, et al. Self-propelled gemini-like LMWH-scaffold nanodrugs for overall tumor microenvironment manipulation via macrophage reprogramming and vessel normalization. Nano Lett. 2020;20(1):372–383. doi:10.1021/acs.nanolett.9b04024
12. Ali SH, O’Donnell AL, Mohamed S, Mousa S, Dandona P. Overexpression of estrogen receptor-alpha in the endometrial carcinoma cell line Ishikawa: inhibition of growth and angiogenic factors. Gynecol Oncol. 2004;95(3):637–645. doi:10.1016/j.ygyno.2004.08.034
13. Ali SH, O’Donnell AL, Balu D, et al. Estrogen receptor-alpha in the inhibition of cancer growth and angiogenesis. Cancer Res. 2000;60(24):7094–7098.
14. Zhai Y, Bommer GT, Feng Y, Wiese AB, Fearon ER, Cho KR. Loss of estrogen receptor 1 enhances cervical cancer invasion. Am J Pathol. 2010;177(2):884–895. doi:10.2353/ajpath.2010.091166
15. Garrido MP, Torres I, Vega M, Romero C. Angiogenesis in gynecological cancers: role of neurotrophins. Front Oncol. 2019;9:913. doi:10.3389/fonc.2019.00913
16. Vici P, Mariani L, Pizzuti L, et al. Emerging biological treatments for uterine cervical carcinoma. J Cancer. 2014;5(2):86–97. doi:10.7150/jca.7963
17. Clere N, Bermont L, Fauconnet S, et al. The human papillomavirus type 18 E6 oncoprotein induces Vascular Endothelial Growth Factor 121 (VEGF121) transcription from the promoter through a p53-independent mechanism. Exp Cell Res. 2007;313(15):3239–3250. doi:10.1016/j.yexcr.2007.06.029
18. Bodily JM, Mehta KP, Laimins LA. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Res. 2011;71(3):1187–1195. doi:10.1158/0008-5472.CAN-10-2626
19. Kudla MJ, Zikan M, Fischerova D, Stolecki M, Alcazar JL. 4D Doppler ultrasound in high grade serous ovarian cancer vascularity evaluation-preliminary study. Diagnostics. 2021;11(4):582. doi:10.3390/diagnostics11040582
20. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85(1):178–187. doi:10.1002/(SICI)1097-0142(19990101)85:1<178::AID-CNCR25>3.0.CO;2-7
21. Perez-Fidalgo JA, Ortega B, Simon S, Samartzis EP, Boussios S. NOTCH signalling in ovarian cancer angiogenesis. Ann Translat Med. 2020;8(24):1705. doi:10.21037/atm-20-4497
22. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research, Nature reviews. Cancer. 2013;13(4):273–282.
23. Boss AL, Chamley LW, James JL. Placental formation in early pregnancy: how is the centre of the placenta made? Hum Reprod Update. 2018;24(6):750–760. doi:10.1093/humupd/dmy030
24. Berger AA, Dao F, Levine DA. Angiogenesis in endometrial carcinoma: therapies and biomarkers, current options, and future perspectives. Gynecol Oncol. 2021;160(3):844–850. doi:10.1016/j.ygyno.2020.12.016
25. Zhang J, Song H, Lu Y, Chen H, Jiang S, Li L. Effects of estradiol on VEGF and bFGF by Akt in endometrial cancer cells are mediated through the NF-κB pathway. Oncol Rep. 2016;36(2):705–714. doi:10.3892/or.2016.4888
26. Kazi AA, Koos RD. Estrogen-induced activation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology. 2007;148(5):2363–2374. doi:10.1210/en.2006-1394
27. Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1):R27–R44. doi:10.1530/JOE-16-0340
28. Weng D, Han T, Dong J, et al. Angiogenin and MMP-2 as potential biomarkers in the differential diagnosis of gestational trophoblastic diseases. Medicine. 2022;101(5):e28768. doi:10.1097/MD.0000000000028768
29. Bagley RG, Ren Y, Kurtzberg L, et al. Human choriocarcinomas: placental growth factor-dependent preclinical tumor models. Int J Oncol. 2012;40(2):479–486. doi:10.3892/ijo.2011.1257
30. Chen S, Li T, Meng L, Liu K. Advances in immunotherapy and molecular targeted therapy of gestational trophoblastic tumor: current practice and future perspectives. Am J Cancer Res. 2022;12(6):2422–2432.
31. Food and Drug Administration. Highlights of prescribing information for ALYMSYS® (bevacizumab-maly) injection, for intravenous use. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761231s000lbl.pdf.
32. Food and Drug Administration. Highlights of prescribing information for LENVIMA® (lenvatinib) capsules, for oral use. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/206947s025lbl.pdf.
33. Vergote I, Powell MA, Teneriello MG, et al. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol. 2020;156(3):575–582. doi:10.1016/j.ygyno.2019.12.039
34. Campos SM, Penson RT, Matulonis U, et al. A Phase II trial of Sunitinib malate in recurrent and refractory ovarian, fallopian tube and peritoneal carcinoma. Gynecol Oncol. 2013;128(2):215–220. doi:10.1016/j.ygyno.2012.07.126
35. Castonguay V, Lheureux S, Welch S. et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: a study of the Princess Margaret. Chicago California Consortia. 2014;134(2):274–280.
36. Mackay HJ, Tinker A, Winquist E, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116(2):163–167. doi:10.1016/j.ygyno.2009.08.012
37. Matei D, Sill MW, Lankes HA, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol. 2011;29(1):69–75. doi:10.1200/JCO.2009.26.7856
38. Dizon DS, Sill MW, Schilder JM, et al. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer. NRG Oncol Gynecol Oncol Group Study. 2014;135(3):441–445.
39. Garcia YG, Ferré A, Mendiola C, Barretina-Ginesta MP, Martin AG. Efficacy and safety results from GEICO 1205, a randomized phase II trial of neoadjuvant chemotherapy with or without bevacizumab for advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2019;29(6):1050–1056. doi:10.1136/ijgc-2019-000256
40. Pisarsky L, Bill R, Fagiani E, et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 2016;15(6):1161–1174. doi:10.1016/j.celrep.2016.04.028
41. Jin C, Yuan M, Bu H, Jin C, Tomao F. Antiangiogenic strategies in epithelial ovarian cancer: mechanism, resistance, and combination therapy. J Oncol. 2022;2022:4880355. doi:10.1155/2022/4880355
42. Mao CL, Seow KM, Chen KH. The utilization of bevacizumab in patients with advanced ovarian cancer: a systematic review of the mechanisms and effects. Int J Mol Sci. 2022;23(13):6911. doi:10.3390/ijms23136911
43. Lee JM, Moore RG, Ghamande S, et al. Cediranib in combination with olaparib in patients without a germline BRCA1/2 mutation and with recurrent platinum-resistant ovarian cancer: phase IIb CONCERTO trial. Clin Cancer Res. 2022;28(19):4186–4193. doi:10.1158/1078-0432.CCR-21-1733
44. Liu JF, Brady MF, Matulonis UA, et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, Phase III trial. J Clin Oncol. 2022;40(19):2138–2147. doi:10.1200/JCO.21.02011
45. Zhang M, Tian Z, Sun Y. Successful treatment of ovarian cancer with apatinib combined with chemotherapy: a case report. Medicine. 2017;96(45):e8570. doi:10.1097/MD.0000000000008570
46. Lan CY, Wang Y, Xiong Y, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 2018;19(9):1239–1246. doi:10.1016/S1470-2045(18)30349-8
47. Morgan RD, Banerjee S, Hall M, et al. Pazopanib and Fosbretabulin in recurrent ovarian cancer (PAZOFOS): a multi-centre, phase 1b and open-label, randomised phase 2 trial. Gynecol Oncol. 2020;156(3):545–551. doi:10.1016/j.ygyno.2020.01.005
48. Monk BJ, Poveda A, Vergote I, et al. Final results of a Phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): long-term survival, impact of ascites, and progression-free survival-2. Gynecol Oncol. 2016;143(1):27–34. doi:10.1016/j.ygyno.2016.07.112
49. Liu JF, Herold C, Gray KP, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a Phase 2 clinical trial. JAMA Oncol. 2019;5(12):1731–1738. doi:10.1001/jamaoncol.2019.3343
50. Chase DM, Chaplin DJ, Monk BJ. The development and use of vascular targeted therapy in ovarian cancer. Gynecol Oncol. 2017;145(2):393–406. doi:10.1016/j.ygyno.2017.01.031
51. Wang T, Tang J, Yang H, et al. Effect of apatinib plus pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin alone on platinum-resistant recurrent ovarian cancer: the APPROVE randomized clinical trial. JAMA Oncol. 2022;8(8):1169–1176. doi:10.1001/jamaoncol.2022.2253
52. Joly F, Fabbro M, Berton D, et al. Paclitaxel with or without pazopanib for ovarian cancer relapsing during bevacizumab maintenance therapy: the GINECO randomized phase II TAPAZ study. Gynecol Oncol. 2022;166(3):389–396. doi:10.1016/j.ygyno.2022.06.022
53. Bender D, Sill MW, Lankes HA, et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;138(3):507–512. doi:10.1016/j.ygyno.2015.07.018
54. Powell MA, Sill MW, Goodfellow PJ, et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135(1):38–43. doi:10.1016/j.ygyno.2014.07.083
55. Monk BJ, Lopez LM, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28(22):3562–3569. doi:10.1200/JCO.2009.26.9571
56. Zhang L, Chen L, Yu H. Phase II study of apatinib, a novel tyrosine kinase inhibitor targeting tumor angiogenesis, as second-line treatment for recurrent or advanced cervical cancer patients. Invest New Drugs. 2020;38(4):1186–1191. doi:10.1007/s10637-019-00858-5
57. Rubinstein MM, Dickinson S, Narayan P, et al. Bevacizumab in advanced endometrial cancer. Gynecol Oncol. 2021;161(3):720–726. doi:10.1016/j.ygyno.2021.04.016
58. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–448. doi:10.1056/NEJMoa2108330
59. Yang H, Sun S, Mei Z, et al. A retrospective cohort study evaluates clinical value of anlotinib in persistent, metastatic, or recurrent cervical cancer after failure of first-line therapy. Drug Des Devel Ther. 2021;15:4665–4674. doi:10.2147/DDDT.S335870
60. Chuai Y, Rizzuto I, Zhang X, et al. Vascular endothelial growth factor (VEGF) targeting therapy for persistent, recurrent, or metastatic cervical cancer. Cochrane Database Syst Rev. 2021;3(3):CD013348.
61. Worley MJ, Elias KM, Horowitz NS, Quade BJ, Berkowitz RS. Durable remission for a woman with refractory choriocarcinoma treated with anti-endoglin monoclonal antibody and bevacizumab: a case from the New England Trophoblastic Disease Center, Brigham and Women’s Hospital and Dana-Farber Cancer Institute. Gynecol Oncol. 2018;148(1):5–11. doi:10.1016/j.ygyno.2017.11.029
62. Cheng H, Zong L, Kong Y, et al. Camrelizumab plus apatinib in patients with high-risk chemorefractory or relapsed gestational trophoblastic neoplasia (CAP 01): a single-arm, open-label, phase 2 trial. Lancet Oncol. 2021;22(11):1609–1617. doi:10.1016/S1470-2045(21)00460-5
63. Huang N, Liu Y, Fang Y, et al. Gold nanoparticles induce tumor vessel normalization and impair metastasis by inhibiting endothelial Smad2/3 signaling. ACS nano. 2020;14(7):7940–7958. doi:10.1021/acsnano.9b08460
64. Miller EM, Samec TM, Alexander-Bryant AA. Biology, medicine, nanoparticle delivery systems to combat drug resistance in ovarian cancer. Nanomedicine. 2020;31:102309. doi:10.1016/j.nano.2020.102309
65. Xiong X, Arvizo RR, Saha S, et al. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget. 2014;5(15):6453–6465. doi:10.18632/oncotarget.2203
66. Winer I, Wang S, Lee YE, et al. F3-targeted cisplatin-hydrogel nanoparticles as an effective therapeutic that targets both murine and human ovarian tumor endothelial cells in vivo. Cancer Res. 2010;70(21):8674–8683. doi:10.1158/0008-5472.CAN-10-1917
67. Temming K, Schiffelers RM, Molema G, Kok RJ. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updates. 2005;8(6):381–402. doi:10.1016/j.drup.2005.10.002
68. Wang Y, Tong L, Wang J, et al. cRGD-functionalized nanoparticles for combination therapy of anti-endothelium dependent vessels and anti-vasculogenic mimicry to inhibit the proliferation of ovarian cancer. Acta biomaterialia. 2019;94:495–504. doi:10.1016/j.actbio.2019.06.039
69. Ebeid K, Meng X, Thiel KW, et al. Synthetically lethal nanoparticles for treatment of endometrial cancer. Nat Nanotechnol. 2018;13(1):72–81. doi:10.1038/s41565-017-0009-7
70. Gharpure KM, Chu KS, Bowerman CJ, et al. Metronomic docetaxel in PRINT nanoparticles and EZH2 silencing have synergistic antitumor effect in ovarian cancer. Mol Cancer Ther. 2014;13(7):1750–1757. doi:10.1158/1535-7163.MCT-13-0930
71. Zhao Z, Shuang T, Gao Y, et al. Targeted delivery of exosomal miR-484 reprograms tumor vasculature for chemotherapy sensitization. Cancer Lett. 2022;530:45–58. doi:10.1016/j.canlet.2022.01.011
72. Tabernero J, Shapiro GI, LoRusso PM. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi:10.1158/2159-8290.CD-12-0429
73. Yan Y, Chen B, Wang Z, et al. Sequential modulations of tumor vasculature and stromal barriers augment the active targeting efficacy of antibody-modified nanophotosensitizer in desmoplastic ovarian carcinoma. Adv Sci. 2021;8(3):2002253. doi:10.1002/advs.202002253
74. Fu S, Corr BR, Culm-Merdek K, et al. Phase Ib study of navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2022;40(23):2568–2577. doi:10.1200/JCO.21.01801
75. Thaker PH, Bradley WH, Leath CA, et al. GEN-1 in combination with neoadjuvant chemotherapy for patients with advanced epithelial ovarian cancer: a Phase I dose-escalation study. Clin Cancer Res. 2021;27(20):5536–5545. doi:10.1158/1078-0432.CCR-21-0360
76. Bandiera E, Franceschini R, Specchia C, et al. Prognostic significance of vascular endothelial growth factor serum determination in women with ovarian cancer. ISRN Obstet Gynecol. 2012;2012:245756. doi:10.5402/2012/245756
77. Papa A, Zaccarelli E, Caruso D, et al. Targeting angiogenesis in endometrial cancer - new agents for tailored treatments. Expert Opin Investig Drugs. 2016;25(1):31–49. doi:10.1517/13543784.2016.1116517
78. Obradović DD, Milić NM, Miladinović N, McClements L, Oprić DM. Loss of expression of antiangiogenic protein FKBPL in endometrioid endometrial carcinoma: implications for clinical practice. Medicina. 2022;58(10):1330. doi:10.3390/medicina58101330
79. Leng X, Kou S, Lin Y, et al. Quantification of ovarian lesion and fallopian tube vasculature using optical-resolution photoacoustic microscopy. Sci Rep. 2022;12(1):15850. doi:10.1038/s41598-022-19778-1
80. Troendle EP, Khan A, Searson PC, Ulmschneider MB. Predicting drug delivery efficiency into tumor tissues through molecular simulation of transport in complex vascular networks. J Control Release. 2018;292:221–234. doi:10.1016/j.jconrel.2018.11.010
81. Seyyednia E, Oroojalian F, Baradaran B, Mojarrad JS, Mokhtarzadeh A, Valizadeh H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J Control Release. 2021;338:367–393. doi:10.1016/j.jconrel.2021.08.044
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.