Back to Journals » Clinical Epidemiology » Volume 10
Colonoscopy-related complications in a nationwide immunochemical fecal occult blood test-based colorectal cancer screening program
Authors Mikkelsen EM, Thomsen MK , Tybjerg J , Friis-Hansen L, Andersen B , Jørgensen JC, Baatrup G , Njor SH, Mehnert F, Rasmussen M
Received 23 July 2018
Accepted for publication 11 September 2018
Published 13 November 2018 Volume 2018:10 Pages 1649—1655
DOI https://doi.org/10.2147/CLEP.S181204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Irene Petersen
Ellen M Mikkelsen,1 Mette Kielsholm Thomsen,1 Julie Tybjerg,2 Lennart Friis-Hansen,3 Berit Andersen,4,5 Jens Christian Riis Jørgensen,6 Gunnar Baatrup,7,8 Sisse H Njor,2,4,5 Frank Mehnert,1 Morten Rasmussen9
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 2RKKP, The Danish Clinical Registries, A National Quality Improvement Programme, Aarhus, Denmark; 3Department of Clinical Biochemistry, Nordsjællands Hospital, Hillerød, Denmark; 4Department of Public Health Programmes, Randers Regional Hospital, The Central Denmark Region, Randers, Denmark; 5Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; 6Department of Surgery, Vejle Hospital, Vejle, Denmark; 7Department of Surgery, Odense University Hospital, Odense, Denmark; 8Department of Clinical Research, University of Southern Denmark, Odense, Denmark; 9Department of Digestive Diseases K, Bispebjerg Hospital, Copenhagen, Denmark
Background: The Danish national screening program for colorectal cancer (CRC) consists of an immunochemical fecal occult blood test (iFOBT) followed by colonoscopy. The Danish Colorectal Cancer Screening Database (DCCSD) records data on the incidence of hospital-registered complications after colonoscopy. However, the validity of these data is unknown, and the incidence of complications is potentially underreported.
Objective: To evaluate the validity of the colonoscopy complications registered in the DCCSD by using medical records as the reference. Further, to evaluate the incidence of complications leading to hospital contact.
Methods: Among 14,671 individuals with a positive iFOBT result and a colonoscopy procedure performed from March 3, 2014 to December 31, 2014, we selected 295 individuals for medical record review. We calculated sensitivity as the proportion of true complications registered in the DCCSD out of all complications found in the medical records, and the positive predictive value (PPV) as the number of true complications in the DCCSD out of all DCCSD-registered complications. On the basis of the medical record data, we calculated the incidence proportion of hospital-registered complications overall and by subtype.
Results: In total, we reviewed 286 records and found 102 individuals with at least one complication. The sensitivity of the DCCSD for any complication was 29.4% (95% CI: 20.8–39.3) and the PPV was 88.2% (95% CI: 72.6–96.7). On the basis of the medical record data, the incidence proportion of any complication after colonoscopy was 0.70% (95% CI: 0.57–0.84) and that of perforation or lesion was 0.10% (95% CI: 0.06–0.17); bleeding, 0.41% (95% CI: 0.31–0.53); post-polypectomy syndrome, 0.16% (95% CI: 0.10–0.24); and other medical complications, 0.04 (95% CI: 0.02–0.09).
Conclusion: The DCCSD has low sensitivity for complications, and improvements in data registration are warranted. The incidence proportion of any hospital-treated post-colonoscopy complication was 0.70% in 2014, which was the first year of the Danish national CRC screening program. This is within the range of complications reported by other studies.
Keywords: prevention, public health, harms
Background
Population-based screening for colorectal cancer (CRC) has the potential to reduce incidence and mortality of CRC by discovering the disease in its early latent stage and enabling premalignant lesions to be removed.1–3 In 2003, the European Union recommended that men and women aged 50 years or more should participate in CRC screening,4 and by 2015, mass screening programs had been implemented in a large number of Western countries, including Denmark.5
In Denmark, the CRC screening program consists of an immunochemical fecal occult blood test (iFOBT) followed by colonoscopy for participants who tested positive for occult blood.6 Mass screening targets apparently healthy individuals. Thus, it is crucial that the involved procedures cause minimal harm. Harms range in severity from overdiagnosis, psychosocial distress, pain and discomfort related to the bowel cleansing process, bleeding and perforations, and death.7,8 A systematic review of CRC screening-related morbidity and mortality estimated a pooled risk per 1,000 colonoscopies of 0.07 (95% CI: 0.006–0.17) for perforation and 0.8 (95% CI: 0.18–1.63) for major bleeding. However, the single estimates for the 39 included studies varied greatly depending on the definition of complications and the primary screening method (colonoscopy vs fecal test). None of the included studies reported any mortality.7
As recommended by the European guidelines,4 the quality of the Danish CRC screening program is monitored, and data for this purpose are assembled in the Danish Colorectal Cancer Screening Database (DCCSD).6 In the first annual report of the DCCSD, the overall incidence of hospital-registered complications (perforation, bleeding, medical complications during colonoscopy, and post-polypectomy syndrome) after screening-related colonoscopy was 3.0 per 1,000. However, the validity of these data is unknown, and the incidence of complications is potentially underreported as they may occur with a delay, that is, hours or days after the individual has been discharged from the colonoscopy department. Follow-up treatment might be provided at a department or emergency clinic unrelated to the colonoscopy department. Therefore, the patient might be diagnosed and treated correctly, but inadvertently not registered with a screening-related complication. In addition, along with the establishment of the screening program, novel codes for registration of complications were introduced, which likely prompted underreporting, especially in the starting period.6
In this study, we aimed primarily to evaluate the validity of data for screening-related colonoscopy complications registered in the DCCSD by using hospital records as the gold standard. In addition, we aimed to evaluate the overall incidence and severity of complications leading to a hospital contact.
Methods
Setting
The national Danish CRC screening program was initiated in March 2014 and targets all residents aged 50–74 years. Screening is free of charge and offered biennially, but for logistic reasons, the first round was implemented over a 4-year period. Individuals are invited according to a randomly assigned sequence of birth months, although those who turned 50 or 74 years within the initial 4-year screening round received their first invitation no later than 1 month before that particular birthday. Invitation letters, including home sampling kits, are administrated on the national level and supported by the information technology system “The Invitation and Administration Module” (IAM). Submitted stool samples are analyzed at five laboratories (one in each of the five Danish geographical regions), and follow-up colonoscopies are performed at 19 departments throughout the country.9 The IAM contains the name and civil personal registration (CPR) number of all individuals invited, dates on the logistics (invitations, reminders, iFOBT analyses, and referral to colonoscopy), region of residence, and results of iFOBTs (positive result ≥100 ng hemoglobin/mL buffer ≈20 µg hemoglobin/g feces) obtained using the OC-Sensor (Eiken Chemical Co. Ltd, Tokyo, Japan).9
The DCCSD was established to monitor the screening program and consists of data from existing data sources: the IAM, the Danish National Patient Registry (DNPR), and the National Pathology Registry.6 The DNPR is a national registry that collects data on all in- and outpatient somatic admissions including CPR number, dates of admission and discharge, and codes for diagnoses (using the ICD-10) and procedures (Health Care Service Classification System).10 Thus, all clinical data, including screening-related diagnoses (eg, type of lesion and complication) and procedures (eg, number of polyps observed and completion of colonoscopy), are reported to DNPR from the departments or emergency rooms providing the colonoscopy or follow-up treatment, and subsequently assembled in the DCCSD.
Study population
The source population for this study was identified in the DCCSD and included 14,671 individuals who were invited to CRC screening, had a positive iFOBT result, and underwent a colonoscopy procedure performed in the period from March 3, 2014 to December 31, 2014. We aimed to evaluate the validity of data pertaining to the first year because of considerable research interest in the program and the need for valid data to monitor the program from the very beginning. Because complications are relatively rare, it is not feasible to identify individuals for medical record review by random sampling of individuals who had undergone a screening-related colonoscopy. Instead, we identified individuals for medical record review (the review group) by using a four-step strategy: First, we reviewed all diagnosis and procedure codes registered in the DNPR for those individuals in the source population who had a hospital contact within 30 days after a colonoscopy (including up to five colonoscopies per person within 3 months of their positive iFOBT). Three medical doctors specialized in the field reviewed all the codes and selected 101 codes that could potentially be related to complications after colonoscopy. Secondly, we identified all individuals in the source population who had a hospital contact with at least one of the selected 101 codes within 14 days for surgical-related diagnoses and 2 days for nonsurgical-related diagnoses after a screening colonoscopy (n=233). To capture as many complications as possible, we used a 14-day time window as bleeding after polypectomy can occur immediately or up to 14 days after the procedure.11,12 Thirdly, we used data from the Danish Civil Registration System (CRS) to identify individuals in the source population who died within 90 days of a complete colonoscopy (n=41). The CRS contains data on all individuals residing in Denmark and is updated daily with information on migration and vital status.13 Finally, we identified individuals in the DCCSD registered with any complication (n=34) including the following four categories: 1) perforation or lesion during colonoscopy (DT812G1), 2) post-colonoscopy bleeding that required treatment or prompted the patient to contact a hospital for medical evaluation (DT810J1), 3) other medical complications related to colonoscopy or sedation (eg, hypotension, pain, vomiting, and respiratory complications) that prevented completion of the colonoscopy or required medical treatment (DT888U1), and 4) post-polypectomy syndrome defined as fever and abdominal pain without symptoms of perforation or pneumoperitoneum (DT888L). Thus, within the source population, in total, we identified 295 individuals with a potential complication and thus were relevant for medical record review. Of the 295 individuals, 233 individuals were based on one of the 101 selected diagnosis and procedure codes, 41 were based on the date of death (3 of these overlapped with the 101 codes), and 34 were based on the four specific complication codes (10 of these overlapped with the 101 codes).
Hospital record review
Hospital records were reviewed according to a standardized electronic protocol developed by members of the DCCSD steering committee. The protocol was pilot tested by a noncommittee member on five patients within the target population. The final protocol consisted of eight main items and 71 subitems.
The DCCSD was accepted as a clinical quality database in October 2014 by the Danish Health Authority and registered by the Danish Data Protection Agency (2007-58-0014); thus, the study complies with Danish regulations.
Statistics
We described the demographic characteristics (age, gender, and region of residence) of the review group and the nonreview group (the source population minus the review group) using means, medians, and proportions. The medical record data for each patient in the review group were used as the reference for the comparison with the DCCSD data. We calculated the sensitivity of the DCCSD to evaluate its ability to identify all true complications. Sensitivity was calculated as the proportion of true complications in the DCCSD out of all complications found in the medical records. Further, we calculated the positive predictive value (PPV) as the number of true complications in the DCCSD out of all complications registered in the DCCSD. The PPV indicates the probability that a patient coded with a complication in the DCCSD actually had a complication according to the medical records. By using the reference data from the medical records, we calculated the incidence proportion of complications overall and by subtypes. In addition, we stratified any complication according to yes vs no for “performed polypectomy”.
By using the medical record data, the severity of each complication was ranked using the Clavien–Dindo classification: grade I (no need for treatment), II (pharmacological treatment), IIIa (surgical, endoscopic, or radiologic treatment without general anesthesia), IIIb (surgical, endoscopic, or radiologic treatment under general anesthesia), IVa (single organ dysfunction), IVb (multiorgan dysfunction), and V (death).14 Finally, we reported proportions of treatment provided to the patients according to each type of complication. Exact 95% CI for the binomial distribution were calculated for the estimates. We used statistical software SAS 9.4 (SAS Institute Inc, Cary, NC, USA) for all analyses.
Results
Within the source population (14,671), we identified 295 individuals who potentially could have experienced complications and were thus eligible for medical record review (Figure 1). Six records were not available for review for practical reasons, and three patients had not been screened or had no information about a screening-related colonoscopy in the medical records. Thus, in total, 286 records were reviewed. The mean age was 64.9 years in the review group and 63.8 years in the nonreview group (Table 1). In the review group, 62.9% were males, and in the nonreview group, 55.7% were males. With regard to the geographical region, the largest proportions of individuals were residents of the Capital Region of Denmark both in the review group (30.4%) and in the nonreview group (27.4%). Of the 286 individuals in the review group, 102 had one or more complications and 184 had no complication according to the medical records. For any complication, the number of true positive complications in the DCCSD was 30 and the number of false positives was four. Hence, the sensitivity of the DCCSD for any complication was 29.4% (95% CI: 20.8–39.3) and the PPV was 88.2% (95% CI: 72.6–96.7) (Table 2).
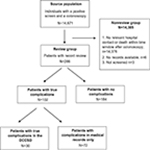  | Figure 1 Flowchart of study population for medical record review. Abbreviation: DCCSD, Danish Colorectal Cancer Screening Database. |
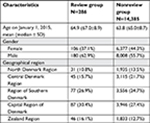  | Table 1 Characteristics of the review group and the nonreview group, N=14,671 |
  | Table 2 Validity of complication codes in the DCCSD compared with hospital records Abbreviations: DCCSD, Danish Colorectal Cancer Screening Database; PPV, positive predictive value. |
On the basis of data from the medical records, the incidence proportion of any complication after colonoscopy was 0.70% (95% CI: 0.57–0.84) per person (N=14,671) and 0.61% (95% CI: 0.50–0.74) per colonoscopy (N=14,712). In total, 8,057 individuals in the source population had a polypectomy, and among these, the incidence proportion of complications was 1.15% (95% CI: 0.93–1.41) vs 0.14% (95% CI: 0.06–0.26) for patients with no such procedure. Of the 102 individuals with complications, we identified 15 individuals (0.10% [95% CI: 0.6–0.17]) with perforation or lesion, 60 (0.41% [95% CI: 0.31–0.53]) with bleeding, 6 (0.04% [95% CI: 0.02–0.09]) with other medical complications, and 24 (0.16% [95% CI: 0.10–0.24]) with post-polypectomy syndrome. Three patients had two types of complications. In total, eleven patients in the review group died, but according to the medical records and the DCCSD, none of them died from a complication due to a screening-related colonoscopy. However, one of these patients had a severe chronic disease, and we cannot rule out the possibility that the bowel cleansing procedure may have contributed to his death.
By using the Clavien–Dindo classification, 45 (46%) of the 99 graded complications were classified in the category of lowest severity (grade I) (Table 3). Approximately 15% of the complications were graded as level II, IIIa, and IIIb, and 2% (two complications) were graded as level IVa. None were classified as the most severe levels (IVb and V). Among the 15 patients with a perforation or lesion, 10 (66.7%) were treated with surgery and 8 (53.3%) had an abdominal computerized tomography (CT) scan. Of the 60 patients with bleeding, intravenous fluid therapy (55.0%), observation (48.3%), and re-colonoscopy (38.3%) were the most frequent treatments (Table 4). Of the 24 individuals with post-polypectomy syndrome, 75% had an abdominal CT scan.
  | Table 4 Examinations and treatments for colonoscopy-related complications, N=105 |
Discussion
We report the validity of complications assembled in the DCCSD, which aims to monitor the quality of the Danish national CRC screening program. In addition, we report the incidence of complications as reported in medical records. Overall, the sensitivity of the DCCSD for complications was 29% and the PPV was 88%. The incidence of any complication was 0.70 per 100 colonoscopies. No deaths related to colonoscopy were identified, and the incidence of the most severe complications, perforation and bleeding, was 0.1% and 0.4%, respectively.
Compared with registration of, for example, clean colon (69.0%) and CRC (72.7%) as assessed previously in the DCCSD, the sensitivity of complications was poor (29%) for any complication.6 In contrast, the PPVs were similar for clean colon (96.1%), CRC (88.9%), and any complication (88.3%).6 The poor sensitivity leads to an underestimation of the incidence of complications in the CRC Danish screening program when based on the DCCSD data. As the database was established recently and the data pertain to the first year of the national CRC screening program, the sensitivity of the DCCSD may have improved over time.
Data on morbidity attributed to primary fecal occult blood test and colonoscopy screening have been reported widely.7,15 However, few data exist on complications attributed to colonoscopy in an iFOBT-based screening program.16–19 In a Spanish study that included 675 individuals having a colonoscopy after a positive iFOBT, the incidence of major complications was 1.5% (1.2% bleedings and 0.2% hypotension/bradycardia).16 In the first round of the national CRC program in Slovenia, 13,919 individuals had a colonoscopy after a positive iFOBT, and the overall incidence of serious complications was 0.08% (0.05% perforations and 0.03% bleedings).17 From the Basque Country, Arana-Arri et al19 reported a 1.0% overall complication rate based on an iFOBT screening program with 39,254 colonoscopies. Thus, our estimate of any complication (0.7%) is within the range of those reported from programs in Spain, Slovenia, and the Basque Country. Similar to the Basque study, we included all hospital-registered complications, whereas the Spanish and the Slovenian studies included only the more severe complications (perforations and bleedings) and excluded post-polypectomy syndrome. A study of a regional population-based guaiac-based faecal occult blood test screening program in France in 2003–2010, including 10,277 colonoscopies, identified a much higher incidence (6.3%) of any complication, including all adverse events occurring within 30 days of colonoscopy.20 The lower incidence of complications in the Danish and Basque populations may be explained by technical improvement of the equipment used for colonoscopy over the past 10 years. Overall, comparing the reported frequencies of complications attributed to CRC, screening is hampered by various definitions of complications and small number of complications.
When CRC screening was implemented in Denmark, it was expected that the incidence of complications would constitute between 0.07% and 0.2% for perforation and between 0.07% and 0.4% for significant bleeding.21 Our estimate for perforation is in line with the expectations, and our estimate for bleeding is very close. In addition, we have included all hospital-registered bleedings, and not restricted our results to significant bleedings.
To identify complications attributed to screening, beyond those registered with a specific complication code, we selected 101 hospital diagnosis and procedure codes that potentially could hide a complication. We evaluated all patients who had one of the selected surgical diagnoses or procedures within 14 days after colonoscopy or a nonsurgical-related diagnosis within 2 days after colonoscopy, and patients who died within 90 days after colonoscopy. Using this procedure, we may have underestimated the incidence of complications because some complications possibly were not included in the 101 selected codes or appeared outside our time window. In addition, we have focused on colonoscopy-related complications, which are monitored by the DCCSD. Thus, we may have overlooked other hospital-treated complications, for example, complications related to the bowel cleansing procedure. Finally, less severe complications such as discomfort related to bowel preparation and post-colonoscopy abdominal complaints are not identified, as they do not lead to a hospital contact.
According to the Clavien–Dindo categorization, the majority of complications were of low severity. However, all patients with one or more complications had a hospital contact, which by itself indicates a somewhat serious medical problem.14 Further, among 15 patients with a perforation, 67% had a surgical procedure and 38% of the 60 patients with bleeding had a new colonoscopy, indicating quite serious complications.
In conclusion, the DCCSD has low sensitivity for complications, and improvements in data registration are essential for surveillance of complications and for research purposes. The incidence of any hospital-treated post-colonoscopy complication was 0.7% in the first year of the Danish national screening program. This is within the range of complications reported in other studies and in accordance with the expectations expressed before implementation of the CRC screening program in Denmark.
Acknowledgments
We thank Niels de Haas for pilot testing the online questionnaire and for data extraction. We also thank the following individuals for assistance with hospital record review: Inge Bernstein, Flemming Knudsen, Kasper Jarlhelt Andersen, Peter Nerstrøm, Niels Hald, Einar Pahle, Mirjana Komljen, Hans Rahr, Henrik Møller, Mona Skarbye, Claus Juul, Jakob Hendel, Per Vadgaard Andersen, Svend Schulze, Mogens Jepsen, Nina Brander, and Merete Skovmode. Finally, we thank Henriette Kristoffersen for developing the online version of the data extraction manual.
Disclosure
The authors report no conflicts of interest in this work.
References
Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681–691. | ||
Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. | ||
Welch HG, Robertson DJ. Colorectal Cancer on the Decline--Why Screening Can’t Explain It All. N Engl J Med. 2016;374(17):1605–1607. | ||
Segnan N, Patnick J, von Karsa L. European Guidelines for Quality Assurance in Colorectal Cancer Screening an Diagnosis. 1st ed. Luxembourg: Publications Office of the European Union; 2010. | ||
Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. | ||
Thomsen MK, Njor SH, Rasmussen M, et al. Validity of data in the Danish Colorectal Cancer Screening Database. Clin Epidemiol. 2017;9:105–111. | ||
Vermeer NC, Snijders HS, Holman FA, et al. Colorectal cancer screening: Systematic review of screen-related morbidity and mortality. Cancer Treat Rev. 2017;54:87–98. | ||
Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135(6):1899–1906. | ||
Manual for implementering og drift af tværregional tarmkræftscreening [Manual for implementation and operation of the national colorectal cancer screening programme]. 2014. Available from: http://www.regionshospitalet-randers.dk/siteassets/afdelinger/afdeling-for-folkeundersogelser/pdf-episerver/retningslinjer/2014_09_25-manual-for-implementeirng-og-drift-af-tvarregional-tarmkraftscreening version-1.pdf. Accessed April 19, 2018. | ||
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. | ||
Sawhney MS, Salfiti N, Nelson DB, Lederle FA, Bond JH. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40(2):115–119. | ||
Watabe H, Yamaji Y, Okamoto M, et al. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc. 2006;64(1):73–78. | ||
Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. | ||
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. | ||
Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–886. | ||
Castells A, Quintero E. Programmatic screening for colorectal cancer: the COLONPREV study. Dig Dis Sci. 2015;60(3):672–680. | ||
Tepeš B, Bracko M, Novak Mlakar D, et al. Results of the FIT-based National Colorectal Cancer Screening Program in Slovenia. J Clin Gastroenterol. 2017;51(6):e52–e59. | ||
Denters MJ, Deutekom M, Bossuyt PM, Fockens P, Dekker E. Patient burden of colonoscopy after positive fecal immunochemical testing for colorectal cancer screening. Endoscopy. 2013;45(5):342–349. | ||
Arana-Arri E, Imaz-Ayo N, Fernández MJ, et al. Screening colonoscopy and risk of adverse events among individuals undergoing fecal immunochemical testing in a population-based program: A nested case-control study. United European Gastroenterol J. 2018;6(5):755–764. | ||
Denis B, Gendre I, Sauleau EA, Lacroute J, Perrin P. Harms of colonoscopy in a colorectal cancer screening programme with faecal occult blood test: a population-based cohort study. Dig Liver Dis. 2013;45(6):474–480. | ||
Anbefalinger vedrørende screening for tyk og endetarmskræft [Recommendations for colorectal cancer screening]. 2010. Available from: https://www.sst.dk/da/udgivelser/2012/~/media/1327A2433DDD454C86D031D50FE6D9D6.ashx. Accessed June 13, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

