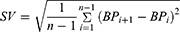Back to Journals » Clinical Interventions in Aging » Volume 18
Collateral Status Modification of the Association Between Blood Pressure Variation Within 72 Hours After Endovascular Treatment and Clinical Outcome in Acute Ischemic Stroke: A Retrospective Cohort Study
Authors Jiang X, Gao L, Wang J, Bao J, Fang J, He L
Received 4 June 2023
Accepted for publication 3 September 2023
Published 11 September 2023 Volume 2023:18 Pages 1491—1499
DOI https://doi.org/10.2147/CIA.S424347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Xin Jiang,* Lijie Gao,* Jian Wang, Jiajia Bao, Jinghuan Fang, Li He
Department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinghuan Fang; Li He, Department of Neurology, West China Hospital, Sichuan University, No. 37 Guoxue Lane, Chengdu, Sichuan Province, 610041, People’s Republic of China, Email [email protected]; [email protected]
Background: Blood pressure variation and collateral status have been reported to be associated with clinical outcome in patients with acute ischemic stroke who received endovascular treatment; however, the relationship between blood pressure variation within 72 hours after EVT and clinical outcome in different collateral status remains unclear.
Methods: Acute ischemic stroke patients due to large vessel occlusion with EVT were retrospectively enrolled. We classified participants into poor collateral (ASITN/SIR grade < 2) and good collateral subgroups (ASITN/SIR grade ≥ 2). The primary outcome was unfavorable neurological outcome defined as a 3-month modified Rankin Scale (mRS) score ≥ 2. The interactive effect was tested to determine the influence of collateral status on the association between BP variation and clinical outcome.
Results: A total of 545 patients were included. The poor collateral subgroup was detected in 198 patients with an average age of 70.2 years. The association between BP variation and primary outcome did not differ under different collateral status (P for interaction > 0.05). However, the association between the mean and coefficient of variation (CV) values of DBP and 3-month mortality was significantly discrepant under different collateral status (P for interaction < 0.05). In the good collateral subgroup, higher mean DBP was associated with a lower risk of 3-month mortality (OR 0.95, 95% CI 0.91– 1, P = 0.033) compared with the poor subgroup (OR 1.04, 95% CI 0.97– 1.1, P = 0.286). In addition, a higher CV of DBP was associated with a higher risk of 3-month mortality (OR 1.24, 95% CI 1.13– 1.36, P < 0.01) compared with poor status (OR 1.08, 95% CI 0.94– 1.23, P=0.275).
Conclusion: For patients who received EVT with good collateral status, increased CV of DBP was significantly associated with higher 3-month mortality, while higher mean DBP within 72 h after EVT was associated with a decrease in 3-month mortality.
Keywords: acute ischemic stroke, blood pressure variability, clinical outcome, collateral status, endovascular treatment
Introduction
Endovascular treatment (EVT) is an approved therapy for patients with acute ischemic stroke due to large vessel occlusion (LVO).1–3 Although 75–80% of patients could obtain complete recanalization, patients with poor baseline collateral status have a higher risk of increased infarct volume and a worse clinical outcome, regardless of recanalization status.4–8 The potential mechanism may be that collateral circulation could maintain the perfusion of the ischemic penumbra, thus reducing ischemic necrosis of brain tissue.4–7,9 Previous studies have shown that blood pressure (BP) metrics within 24 h after EVT were dissimilarly associated with clinical outcomes in different collateral status.10–12 In patients with poor collateral status, higher systolic blood pressure (SBP) and larger SBP and DBP variations within 24 hours after EVT were associated with worse neurological outcomes.10 Further studies revealed that in patients with poor collateral status, a mean SBP value of more than 140 mmHg within 24 hours after EVT was related to poor neurological function, and no significant correlation was observed in patients with good collateral status.11 However, available studies mainly focused on the relationship between blood pressure variation (BPV) and clinical outcome within 24 hours after EVT in patients with LVO, and the relationship between BPV and clinical outcome within 72 hours after EVT remains unclear. Therefore, this study intended to retrospectively collect LVO patients who received EVT and aimed to investigate the relationship between blood pressure variation within 72 hours after EVT and clinical outcome under different collateral status.
Materials and Methods
Study Participants
Patients with acute ischemic stroke due to large vessel occlusion who received EVT from January 2016 to December 2021 in West China Hospital were retrospectively reviewed. The inclusion criteria in our study were as follows: (1) aged 18 years or over; (2) time from stroke onset to groin puncture within 24 hours; (3) occlusion of the carotid artery, middle cerebral artery (M1 to M3 segment), anterior cerebral artery, posterior cerebral artery, vertebral artery or basilar artery; and (4) received EVT. We excluded patients who had any 1 or more of the following: (1) a known prestroke mRS ≥2; (2) patients with baseline collateral circulation unavailable by digital subtraction angiography (DSA) assessment; (3) unavailable post-EVT BP data; (4) insufficient BP data; and (5) lost to follow-up at 3 months. This study was performed in accordance with the ethical principles of the 1964 Declaration of Helsinki and its later amendments and approved by the Ethics Committee of West China Hospital [No. 2020(69)]. The need to obtain written informed consent was waived because of the retrospective and observational nature of the study.
Data Collection
BP was measured by a noninvasive BP monitoring device on the nonparalytic arm on a regular basis. BP data obtained in routine clinical practice during hospitalization were recorded into the electronic health record system, and all BP values within the first 72 hours after EVT were collected. The frequency of BP collection was determined by the clinical care. Blood pressure parameters were routinely measured and recorded every 15 minutes for 2 hours, every 30 minutes at 2 to 6 hours, and every 2 hours within 72 hours after EVT. Data that deviated from the mean blood pressure value by more than 30 mmHg were checked for errors, and queries were resolved with the principal investigator.
Collateral status was measured by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) standard from digital subtraction angiography before EVT.13 ASITN/SIR <2 was defined as a poor collateral status, and ≥2 was defined as a good collateral status.
Other covariates included age, sex, body mass index (BMI), current smoking status, disease history (previous stroke, hypertension, diabetes, atrial fibrillation and coronary artery heart disease), TOAST classification, baseline NIHSS score, Alberta Stroke Program Early Score (ASPECTS), time from onset to thrombectomy, anesthesia method, occlusion vessels, successful recanalization after thrombectomy and laboratory examination (serum triglycerides and cholesterol). Previous history was obtained by self-report from patients or patients’ families. The recanalization status of the diseased vessels after thrombectomy was evaluated by the Modified Thrombolysis in Cerebral Infarction (mTICI).14
Outcome Measures
The primary outcome was unfavorable neurological outcome at 3 months (modified Rankin Scale (mRS) score ≥2). The 3-month mRS score was collected prospectively through a structured telephone interview by investigators. Secondary outcomes included 90-day mortality, hemorrhagic transformation, and early neurological deterioration, which was defined as an increase of four points in the National Institutes of Health Stroke Score (NIHSS) between admission and 24 h after EVT.15
Blood Pressure Parameters
Blood pressure variability indicators included the mean value of blood pressure, blood pressure variation range, mean standard deviation (SD), coefficient of continuous variation (SV), coefficient of variation (CV) and absolute coefficient of variation (ARV).16–19 The average value of BP was calculated by dividing the sum of BP records within 72 hours after EVT by the number of measurements. The range of blood pressure changes referred to the difference between the maximum and minimum values in BP records within 72 hours after EVT. CV was the standard deviation of BP within 72 hours after EVT divided by the mean value of blood pressure. The ARV was calculated as follows:
n refers to the number of BP records.
Statistical Analysis
We presented the mean ± standard deviation (SD) for continuous variables and frequencies (percentages) for categorical variables. Student’s t-tests for continuous variables and chi-square tests for categorical variables were applied to assess differences in clinical characteristics. To evaluate the independent effect of BP on outcomes, we further adjusted the analyses for age, sex, smoking, BMI, TOAST, baseline NIHSS, disease history (diabetes, hypertension, coronary heart disease, atrial fibrillation and stroke), thrombolysis therapy, anesthesia, time from onset to puncture, successful recanalization, total cholesterol, and triglyceride. The correlation coefficients (β/OR) and 95% CIs between BP metrics and clinical outcomes across varying collateral status were evaluated with linear/logistic regression. Interaction effects were further conducted to assess any significant different associations of BP metrics and outcomes in varying collateral status. A P value <0.05 was considered to be statistically significant in all analyses. The data were analyzed using R software (version 4.0.1; Vienna, Austria; https://www.R-project.org/).
Results
Basic Characteristics of Enrolled Participants
Participant enrollment is shown in Figure 1. A total of 664 LVO patients who received EVT were eligible for preliminary screening. As a result, 545 patients were enrolled in the final analysis. The baseline characteristics of enrolled patients with different collateral status are presented in Table 1. Among the 545 patients, 56.3% (n = 307) were male, with an average age of 67.4 years. There were 347 patients with good collateral status, 56.5% (n = 196) of whom were male. The average age of patients with good collateral status was 65.9 years, which was significantly lower than that of patients with poor collateral status (70.2 years, P < 0.05). In addition, patients with good collateral status had a significantly lower mean baseline NIHSS score of 15.3 than those with poor collateral status (16.9) (P < 0.05).
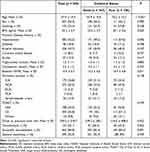 |
Table 1 Basic Characteristics of Enrolled Participants |
 |
Figure 1 Flow chart of patient enrollment. |
BPV and Outcomes in Different Collateral Status
The BPV within 72 hours after EVT in different collateral status is shown in Table 2. The median number of blood pressure measurements within 72 hours after thrombectomy was 58. There were significant differences in the distribution of SD, CV, ARV and SV of SBP within 72 hours after EVT in different collateral status groups (P < 0.05). The SD of the poor collateral status subgroup was 14.8, which was significantly higher than that of the good collateral status subgroup (13.8, p = 0.01). The CV of the poor collateral status subgroup was 12.1, which was also significantly higher than that of the good collateral status subgroup (11.2, p = 0.007). The ARV in different collateral status was significantly different (11.2 in poor collateral status vs 10.6 in good collateral status, p = 0.018). The SV of the poor collateral status subgroup (15.1) was higher than that of the good collateral status subgroup (14.2, p = 0.031).
 |
Table 2 Blood Pressure Variation Within 72 Hours After Endovascular Treatment |
Collateral Status Modification of the Association Between BPV and Outcomes
Outcomes of different collateral status are shown in Supplementary Table 1. Our results showed that collateral status did not modify the association between BPV and unfavorable neurological outcome (Figure 2), END events (Supplementary Figure 1), and hemorrhagic transformation (Supplementary Figure 2) within 72 h after EVT. However, the association between mean DBP and 3-month mortality significantly differed among collateral status (P for interaction = 0.047, Figure 3). Mean DBP within 72 h after EVT was negatively associated with 3-month mortality in a borderline way (OR 0.95, 95% CI 0.91–1) in the good collateral subgroup. In the poor collateral subgroup, mean DBP was not significantly associated with 3-month mortality (OR 1.04, 95% CI 0.97–1.1). For CV, the association between DBP CV and 3-month mortality was also significantly different among collateral status (P for interaction = 0.022, Figure 3). CV of DBP within 72 h after EVT significantly increased 3-month mortality in the good collateral subgroup (OR 1.24, 95% CI 1.13–1.36) but not in the poor collateral subgroup (OR 1.08, 95% CI 0.94–1.23). The association between SBP variation within 72 h after EVT and 3-month mortality did not significantly differ among collateral subgroups.
 |
Figure 2 Collateral status modification on the association between BPV within 72 hours post endovascular treatment and unfavorable neurological outcome. |
 |
Figure 3 Collateral status modification on the association between BPV within 72 hours after endovascular treatment and 3-month mortality. |
Discussion
We performed this retrospective cohort study to investigate whether the association between blood pressure variations within 72 hours after EVT and outcomes differed under different collateral status. We found that collateral status did not modify the association between SBP variations within 72 hours and outcomes after EVT. However, the association between mean DBP, CV within 72 hours and 3-month mortality was significantly discrepant among different collateral status.
Previous studies have revealed that blood pressure variations within 24 h after EVT were associated with clinical outcomes.20–26 Studies have also reported some factors that influenced the association between BPV after endovascular treatment and clinical outcomes, such as recanalization status, thrombolysis rate, and baseline collateral status.11,27,28 Chang et al reported that higher SBP and DBP variations within 24 h after EVT were associated with unfavorable outcomes but were observed only in patients with poor collateral status, which was consistent with the results of Liu et al.10,11 In our study, we found that collateral status did not modify the association between SBP variations within 72 hours after EVT and outcomes, and a significant correlation was observed only in patients with good collateral status. The reasons for this difference may be as follows. First, we defined the primary outcome as mRS ≥2 in our study, as opposed to mRS >2 in previous studies. Moreover, previous studies mainly included patients with occlusion of the anterior circulation and successful recanalization after EVT, while some of our included patients had occlusion of the posterior circulation, and some did not achieve recanalization. This may reflect an increased tolerance to moderate blood pressure fluctuations over time after endovascular treatment.10,29 In addition, previous studies only investigated BPV within 24 h after EVT, while our study included blood pressure variations during the first 72 hours after EVT.
Poor collateral status not only correlates with larger baseline infarct volume and faster infarct growth but also correlates with worse neurological outcomes after EVT.10,30 In our study, the association between DBP variations within 72 h after EVT and 3-month mortality was significantly observed in the good collateral subgroup but not in the poor collateral subgroup. This may be because peripheral vascular resistance was greater in patients with poor collateral status than in those with good collateral circulation.30 For the good collateral subgroup, higher DBP was associated with lower 3-month mortality, but this association was not significant. This might be limited by the sample size in this study. The reason for the difference in previous studies may be that the mean DBP in our included patients with good collateral status was 69.8 mmHg within 72 hours after EVT, which was obviously lower than that in previous studies.12 A relatively high level of DBP may, to some extent, be protective to clinical outcomes.
This study indicated a 72-hour attention on blood management in different collateral status after EVT. We added evidence on BP management in the first 72 hours after EVT and information on the impact of DBP on EVT prognosis. However, limitations of this study should also be acknowledged. First, patients with missing collateral circulation status, blood pressure and follow-up information were excluded, which caused inevitable selection bias. Second, this study is a single-center and retrospective study with a single antihypertensive treatment plan, and no further analysis of the antihypertensive drug subgroup was carried out. Based on the local cerebral tissue perfusion pressure, the relationship between blood pressure after EVT and outcome events under different collateral circulation conditions can be further explored. Finally, the median number of BP tests was 58, with the first quantile of 4 and the third quantile of 91. There was no unified plan for continuous blood pressure measurement, which might influence our results.
Conclusion
The association between BP variations within 72 hours after EVT and clinical outcome differed under different collateral status. For patients with good collateral status, increased CV of DBP was significantly associated with higher 3-month mortality, while higher mean DBP within 72 h after EVT was associated with a decrease in 3-month mortality. The BP management of patients with LVO stroke who receive EVT needs to be individualized considering the preoperative collateral circulation status.
Abbreviations
AIS, Acute ischemic stroke; BPV, Blood pressure variability; EVT, Endovascular treatment; LVO, Large vessel occlusion; mRS, Modified Rankin Scale; mTICI, Modified Thrombolysis in Cerebral Infarction; NIHSS, National Institute of Health Stroke Scale; OR, Odds ratio; SBP, Systolic blood pressure; SICH, Symptomatic intracerebral hemorrhage; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author on reasonable request. Supplemental Material for this article is available online.
Institutional Review Board Statement
This study was performed in accordance with the ethical principles of the 1964 Declaration of Helsinki and approved by the Ethics Committee of West China Hospital [No. 2020(69)].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 82101395), China’s Postdoctoral Science Foundation (No. 2021M692294) and Fundamental Research Funds for the Central Universities (No. 2021SCU12012, the postdoctoral foundation of Sichuan University).
Disclosure
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313(14):1451–1462. doi:10.1001/jama.2015.3058
2. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
3. Jadhav AP, Desai SM, Jovin TG. Indications for mechanical thrombectomy for acute ischemic stroke: current guidelines and beyond. Neurology. 2021;97(20 Suppl 2):S126–S136. doi:10.1212/WNL.0000000000012801
4. Jung S, Gilgen M, Slotboom J, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136(Pt 12):3554–3560. doi:10.1093/brain/awt246
5. Lima FO, Furie KL, Silva GS, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41(10):2316–2322. doi:10.1161/STROKEAHA.110.592303
6. Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132(Pt 8):2231–2238. doi:10.1093/brain/awp155
7. Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42(3):693–699. doi:10.1161/STROKEAHA.110.595256
8. Anadani M, Finitsis S, Clarençon F, et al. Collateral status reperfusion and outcomes after endovascular therapy: insight from the Endovascular Treatment in Ischemic Stroke (ETIS) Registry. J Neurointerv Surg. 2022;14(6):551–557. doi:10.1136/neurintsurg-2021-017553
9. Kim BJ, Singh N, Menon BK. Hemodynamics of Leptomeningeal Collaterals after Large Vessel Occlusion and Blood Pressure Management with Endovascular Treatment. J Stroke. 2021;23(3):343–357. doi:10.5853/jos.2021.02446
10. Chang JY, Jeon SB, Jung C, Gwak DS, Han MK. Postreperfusion blood pressure variability after endovascular thrombectomy affects outcomes in acute ischemic stroke patients with poor collateral circulation. Front Neurol. 2019;10:346. doi:10.3389/fneur.2019.00346
11. Liu D, Nie X, Pan Y, et al. Adverse outcomes associated with higher mean blood pressure and greater blood pressure variability immediately after successful embolectomy in those with acute ischemic stroke, and the influence of pretreatment collateral circulation status. J Am Heart Assoc. 2021;10(5):e019350. doi:10.1161/JAHA.120.019350
12. Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci. 2014;339(1–2):164–168. doi:10.1016/j.jns.2014.02.007
13. Kim SJ, Noh HJ, Yoon CW, et al. Multiphasic perfusion computed tomography as a predictor of collateral flow in acute ischemic stroke: comparison with digital subtraction angiography. Eur Neurol. 2012;67(4):252–255. doi:10.1159/000334867
14. Behme D, Tsogkas I, Colla R, et al. Validation of the extended thrombolysis in cerebral infarction score in a real world cohort. PLoS One. 2019;14(1):e0210334. doi:10.1371/journal.pone.0210334
15. Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86(1):87–94. doi:10.1136/jnnp-2014-308327
16. Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23(3):505–511. doi:10.1097/01.hjh.0000160205.81652.5a
17. Lijarcio MA, Yuste EA, Senso AA, et al. Use of the median for the evaluation of blood pressure self-measurement (BPSM). Eur J Intern Med. 2007;18(1):31–34. doi:10.1016/j.ejim.2006.07.023
18. Muntner P, Joyce C, Levitan EB, et al. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J Hypertens. 2011;29(12):2332–2338. doi:10.1097/HJH.0b013e32834cf213
19. Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Relationships between metrics of visit-to-visit variability of blood pressure. J Hum Hypertens. 2013;27(10):589–593. doi:10.1038/jhh.2013.19
20. Schönenberger S, Uhlmann L, Ungerer M, et al. Association of Blood Pressure With Short- and Long-Term Functional Outcome After Stroke Thrombectomy: post Hoc Analysis of the SIESTA Trial. Stroke. 2018;49(6):1451–1456. doi:10.1161/STROKEAHA.117.019709
21. Goyal N, Tsivgoulis G, Pandhi A, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. 2017;89(6):540–547. doi:10.1212/WNL.0000000000004184
22. Mistry EA, Mistry AM, Nakawah MO, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc. 2017;6(5):e006167. doi:10.1161/JAHA.117.006167
23. Maier IL, Tsogkas I, Behme D, et al. High Systolic blood pressure after successful endovascular treatment affects early functional outcome in acute ischemic stroke. Cerebrovasc Dis. 2018;45(1–2):18–25. doi:10.1159/000484720
24. Anadani M, Orabi Y, Alawieh A, et al. Blood pressure and outcome post mechanical thrombectomy. J Clin Neurosci. 2019;62:94–99. doi:10.1016/j.jocn.2018.12.011
25. Ding X, Xu C, Zhong W, et al. Association of maximal systolic blood pressure with poor outcome in patients with hyperattenuated lesions on immediate NCCT after mechanical thrombectomy. J Neurointerv Surg. 2020;12(2):127–131. doi:10.1136/neurintsurg-2019-014846
26. Katsanos AH, Malhotra K, Ahmed N, et al. Blood pressure after endovascular thrombectomy and outcomes in patients with acute ischemic stroke: an individual patient data meta-analysis. Neurology. 2022;98(3):e291–e301. doi:10.1212/WNL.0000000000013049
27. Maïer B, Dargazanli C, Bourcier R, et al. Effect of steady and dynamic blood pressure parameters during thrombectomy according to the collateral status. Stroke. 2020;51(4):1199–1206. doi:10.1161/STROKEAHA.119.026769
28. Qin J, Zhang Z. Prognostic significance of early systolic blood pressure variability after endovascular thrombectomy and intravenous thrombolysis in acute ischemic stroke: a systematic review and meta-analysis. Brain Behav. 2020;10(12):e01898. doi:10.1002/brb3.1898
29. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55(11):1475–1482. doi:10.1001/archneur.55.11.1475
30. Nambiar V, Sohn SI, Almekhlafi MA, et al. CTA collateral status and response to recanalization in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2014;35(5):884–890. doi:10.3174/ajnr.A3817
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.