Back to Journals » Infection and Drug Resistance » Volume 17
Coinfection with Leprosy and Tuberculosis: A Case Series in Malagasy Patients
Authors Rakotoarisaona MF , Razafimaharo TI, Sendrasoa FA , Andrianarison M, Razanakoto NH, Ratovonjanahary VT, Raharolahy O , Ranaivo IM , Ramarozatovo LS, Rapelanoro Rabenja F
Received 9 February 2024
Accepted for publication 11 April 2024
Published 15 April 2024 Volume 2024:17 Pages 1507—1513
DOI https://doi.org/10.2147/IDR.S458888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Video abstract presented by Rakotoarisaona.
Views: 93
Mendrika Fifaliana Rakotoarisaona,1,* Tsiory Iarintsoa Razafimaharo,2,* Fandresena Arilala Sendrasoa,2,* Malalaniaina Andrianarison,1,* Naina Harinjara Razanakoto,3,* Volatantely Tobiniaina Ratovonjanahary,2,* Onivola Raharolahy,2,* Irina Mamisoa Ranaivo,4 Lala Soavina Ramarozatovo,1 Fahafahantsoa Rapelanoro Rabenja1
1Department of Dermatology, University Hospital of Analakely, Antananarivo, Madagascar; 2Department of Dermatology, University of Befelatanana, Antananarivo, Madagascar; 3Department of Dermatology, University Hospital of Mahavoky Antsimo, Mahajanga, Madagascar; 4Department of Dermatology, University Hospital of Morafeno, Tamatavy, Madagascar
*These authors contributed equally to this work
Correspondence: Mendrika Fifaliana Rakotoarisaona, Department of Dermatology, University Hospital of Analakely, Antananarivo, 101, Madagascar, Tel +261 34 61 947 34, Email [email protected]
Background: Leprosy and tuberculosis are two of the oldest and most common mycobacterial infections, caused by Mycobacterium leprae and Mycobacteium lepramatosis for leprosy and Mycobacterium tuberculosis for tuberculosis. Dual infections have been known since ancient times; however, cases remain rarely reported in the literature, even in countries where both diseases are endemic, such as Madagascar.
Purpose: We report a case series of simultaneous occurrence of leprosy and tuberculosis.
Patients and Methods: In this retrospective study, we reviewed the medical records of patients with leprosy registered at the Department of Dermatology, University Hospital Befelatanana, Antananarivo, Madagascar, between January 2012 and June 2021. Patients with leprosy and diagnosed as coinfected by tuberculosis were included in the study.
Results: Of the 120 leprosy cases observed during the study period, coinfection with leprosy and tuberculosis was found in five patients. The mean age was 43.4 (SD 13.2) ranging, 21– 59 years. Male gender was predominant (4/5). Four patients presented with lepromatous leprosy, and one with borderline lepromatous leprosy. Three patients experienced leprosy reaction. Four cases of pulmonary tuberculosis and one case of multifocal tuberculosis were observed. The diagnosis of leprosy preceded tuberculosis in four cases, and a coinfection diagnosis was made simultaneously in one case. The average time to develop tuberculosis was 38.8 (SD 10.2) months. HIV infection, malnutrition, alcohol consumption, and long-term corticosteroid therapy were the immunosuppressive factors reported in our patients. Three patients received concomitant multidrug therapy for leprosy and tuberculosis.
Conclusion: Dermatologists should be aware of the importance of screening patients affected by leprosy for latent or active tuberculosis to prevent morbidity and mortality due to coinfection and to reduce the risk of acquired resistance to rifampicin, which is the greatest risk of this association.
Keywords: Madagascar, Mycobacterium leprae, rifampicin
A Letter to the Editor has been published for this article.
Introduction
Leprosy and tuberculosis are two of the oldest and most common mycobacterial infections caused by gram-positive aerobic acid-fast bacilli (AFB), Mycobacterium leprae and Mycobacteium lepramatosis for leprosy and Mycobacterium tuberculosis for tuberculosis.1,2 Both diseases are characterized by granulomatous inflammatory reactions and their clinical forms depend on immunity.3 Leprosy involves mostly the skin and peripheral nerves, while tuberculosis is a multisystem disease that primarily affects the lungs but can also involve extrapulmonary sites. Dual infections have been known since ancient times and were first reported in 1954 by Relvich et al. However, cases remain rarely reported in the literature, even in a country where both diseases are endemic, such as Madagascar.4 A comprehensive recent review on leprosy co-infections including tuberculosis highlighted the importance of understanding leprosy coinfections as they may impact the disease impact and treatment.5 Coinfections occur when two or more genetically distinct pathogens are present in the same host.5 Hereby, we report a case series of simultaneous occurrence of leprosy and tuberculosis.
Materials and Methods
In this retrospective study, we reviewed the medical records of patients with leprosy registered at the Department of Dermatology, University Hospital Befelatanana, Antananarivo, Madagascar, between January 2012 and June 2021. Patients affected by leprosy diagnosed clinically, bacteriologically, histologically, and co-infected with tuberculosis confirmed bacteriologically and radiologically were included, and those with incomplete medical records were excluded. Demographic, clinical, and bacteriological data were collected from the records and evaluated using simple descriptive analysis.
This study was approved by the Ethics Commission of the University Hospital of Antananarivo, and was conducted after obtaining written informed consent from the patients. Our study complies with the Declaration of Helsinki. Statistical analysis was performed using the Epi Info software (version 7.2.6).
Results
Of the 120 leprosy cases observed during the study period, coinfection with leprosy and tuberculosis was found in six patients; of these, one case was excluded because of incomplete medical data, and five cases were eligible for this study. In this study, the frequency of this association was 4.16%. The mean age was 43.4 (SD 13.2) and the range, 21–59 years. Three patients lived in the capital city Antananarivo and two lived in a rural region.
Two patients received Bacillus Calmette-Guérin (BCG), and the vaccinal status was unknown in three patients. None of the patients had a history of leprosy or tuberculosis contact.
Lepromatous leprosy (LL) was the most common clinical form of leprosy (n=4), and only one patient presented with a borderline lepromatous (BL) form. All the patients with leprosy had skin and nerve involvement (Figure 1a and b). Leprosy reactions were documented in three patients, type 2 reaction was seen in two patients, while type 1 reaction occurred in one patient (Figure 2a and b). Renal amyloidosis was documented in one patient with a type 2 reaction.
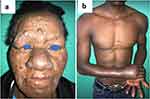 |
Figure 1 (a) Lepromatous leprosy with diffused papulonodular skin-colored lesions on the face; (b) neuritis. |
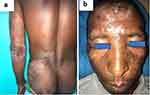 |
Figure 2 (a) Type 1 reaction with erythematous and swelling lesions; (b) type 2 reaction with erythema multiforme-like bullous lesions. |
Of the five leprosy and tuberculosis coinfections, leprosy diagnosis preceded tuberculosis diagnosis in four. The average time to develop tuberculosis was 38.8 (SD 10.2) months, ranging between 12 and 96 months. In one patient, tuberculosis occurred during the type 2 reaction, four months after the end of treatment with multibacillary multidrug therapy (MDT) for leprosy. Only one patient presented with simultaneous clinical symptoms of leprosy and tuberculosis on the first visit and was diagnosed with co-infection by both diseases at the same time. In terms of the clinical form of tuberculosis, pulmonary tuberculosis was predominant (n=4) and extrafocal tuberculosis (pulmonary then miliary tuberculosis) was less common (n=1). Table 1 summarizes the epidemiological and clinical profiles of leprosy and tuberculosis co-infections. The diagnosis of all leprosy cases was based on clinical aspects and was confirmed by bacterial or histological investigations. In all patients, the bacillary index (BI) of the slit skin smear was positive, with a mean BI value of 3.4, and the polymerase chain reaction (PCR) of skin biopsies was positive for Mycobacterium leprae. Only two patients underwent histological investigation, which showed granulomatous reactions surrounding vessels and nerves without central caseous. Ziehl-Neelsen stain for acid-fast bacilli was negative. Regarding the diagnosis of tuberculosis, sputum smear microscopy revealed positive acid-fast bacilli in all cases. Molecular diagnosis of tuberculosis confirmed infection by Mycobacterium tuberculosis and no resistance to rifampicin was detected in GeneXpert MTB/RIF. Chest radiography revealed radiological evidence of tuberculosis in three patients (Figure 3). Chest tomography was performed in one patient and showed alveolar-interstitial opacities in the left lower lobe (Figure 4). In the present series, the Mantoux and QuantiFERON Gold tests were not performed because no patient could afford to pay the high cost of these tests. HIV testing returned positive in one patient. Risk factors for tuberculosis included long-term corticosteroid therapy as a treatment for leprosy (n=3), HIV infection (n=1), alcohol consumption (n=1), and malnutrition (n=2).
 |
Table 1 Summary of Epidemiological and Clinical Profile of Leprosy and Tuberculosis Coinfection |
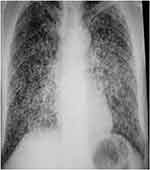 |
Figure 3 Chest X-ray showing numerous small nodular opacities scattered throughout the lungs. |
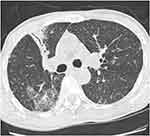 |
Figure 4 Chest tomography showing alveolar-interstitial opacities in the left lower lobe. |
According to the type of multidrug therapy (MDT), all patients with leprosy were treated with a multibacillary (MDT-MB) regimen of the World Health Organization (WHO) standard regimen and received clofazimine (300 mg/month and 50 mg/day) and dapsone (100 mg/day). Three patients received concomitant MDT for leprosy and tuberculosis. Rifampicin was administered daily and included in the antitubercular therapy according to the WHO guidelines. One patient was prescribed antiretroviral therapy (ART) at the same time. The treatment of choice for leprosy reactions is prednisolone associated with clofazimine or doxycycline even for type 2 reaction since thalidomide is not available in Madagascar.
In terms of outcome, improvement in the clinical symptoms of both leprosy and tuberculosis was seen in two patients. Two patients were lost to follow up. One patient presenting with leprosy, tuberculosis, and HIV discontinued medications and died a few months after diagnosis.
Discussion
Leprosy and tuberculosis have been prevalent worldwide for over a century. They constitute a global health issue despite advancement of medical research and the success of MDT by the WHO.6 Coinfection with Mycobacterium leprae and Mycobacterium tuberculosis is rarely reported and varies from 2.5 to 7.7% in India.7,8 Coinfection of leprosy and tuberculosis are not uncommon, though infrequently reported.5 In the recent literature, the new cases detection of the coinfection was 0.02 per 100,000 inhabitant per year worldwide, and was estimated to 2–6 new cases per 100, 000 in Madagascar.9,10 Both leprosy and tuberculosis remain endemic in Madagascar, and previous study reported 8 per 100 000 new leprosy cases and 233 per 100 000 new tuberculosis cases per year. However, there are few reports on dual mycobacterial infections in Madagascar. Sendrasoa et al reported in 2015, the first and only documented case of association of pulmonary tuberculosis and lepromatous leprosy in Madagascar.10 There is a worldwide decline in the number of co-infections of leprosy and tuberculosis in the modern era, which may be explained by the high prevalence of BCG vaccination coverage.11
Mycobacterium leprae is a weakly acid-resistant bacillus that is unable to grow in vitro as an obligate intracellular pathogen, whereas Mycobacterium tuberculosis is a strongly acid-resistant bacillus and an extra or intracellular pathogen in macrophages that is cultivated in vitro.12,13 Despite these specific characteristics, Mycobacterium leprae and Mycobacterium tuberculosis present more than 95% homology in amino acid sequences. This reason has been put forth against the occurrence of concomitant leprosy and tuberculosis.14 It is evidenced by the fact that BCG vaccination confers protection against leprosy and tuberculosis and by the conversion of lepromin intradermal tests after the administration of BCG.11,14 Furthermore, positive Mantoux and QuantiFERON Gold tests and the presence of acid-fast bacilli in sputum are misleading, as the sputum of patients with leprosy (mainly lepromatous leprosy) may stain positive for acid-fast bacilli, creating a diagnostic dilemma with sputum-positive pulmonary tuberculosis.1,15 Therefore, chest X-ray evidence of active tuberculosis and positive tuberculosis cultures are the gold standard for the diagnosis of active tuberculosis infection in a patient with leprosy.1
There are conflicting explanations in the literature regarding the association between leprosy and tuberculosis in the same patient. The interaction of the illness remains unclarified and debatable.16–18 Some investigators suggested two theories that may explain this rare association including cross-immunity and coinfection.5 It has been suggested that an impaired cell-mediated response in the anergic lepromatous leprosy form would predispose to tuberculosis. On the other hand, other investigations reported an antagonism between the two diseases, suggesting that patients with acquired immunity against M. leprae will be protected against tuberculosis.19–21 However, risk factors for leprosy and tuberculosis coinfection include poor socioeconomic status, malnutrition, immunocompromised status (immunosuppression induced by HIV infection or chemotherapy), and diabetes.1,22 In the present case series, the use of steroids in the treatment of leprosy was one of the most frequent factors that may increase the susceptibility to developing tuberculosis. HIV coinfection was in one case.
The most common clinical form of leprosy associated with tuberculosis is lepromatous leprosy, followed by borderline lepromatous leprosy, in concordance with the findings of our case series. Only a few cases of tuberculoid leprosy were reported.23–25 Moreover, and in patients affected by leprosy, pulmonary tuberculosis and severe cases of tuberculosis are frequent.26
Tuberculosis can occur throughout the spectrum of leprosy, and some patients had leprosy for a very long time.4 The gap duration between the infection varies from 1 month for Trindade et al to 10–15 years for Nigam et al19,26 In our study, the symptoms of leprosy preceded the symptoms of tuberculosis in most cases, with a mean duration of 38.8 months. It was 32 months for Dioussé et al, which is similar to our finding.27 In only one patient, the diagnoses of leprosy and tuberculosis were made simultaneously. The most common non-viral leprosy coinfections include tuberculosis, leishmaniasis, chromoblastomycosis and helminthic infections. The majority of the patients in this study (3/5) experienced leprosy reaction. In leprosy patient, a secondary infection is considered to increase the risk of leprosy reactions.5 However, Fróes et al found that coinfection with tuberculosis and leishmaniasis appeared to reduce leprosy reactions.5
There are no specific guidelines regarding the treatment of the association between leprosy and tuberculosis. Verma et al reported that management of leprosy and coinfection does not change, with the same WHO treatment categorization, whereas other authors suggested the use of rifampicin only daily in their study.6 Since Rifampicin, a frontline antitubercular drug, is also used in the treatment of leprosy. The use of rifampicin only monthly in leprosy patients with concomitant tuberculosis could induce the emergence of rifampicin-resistant tuberculosis.15,26,28 However, Rawson et al did not observe Mycobacterium tuberculosis resistance after rifampicin was used for leprosy, which is similar to our finding.9
Conclusion
In conclusion, we reported six cases of leprosy and tuberculosis coinfection, an association rarely reported in the literature. Dermatologists should be aware of the importance of screening leprosy patients for latent or active tuberculosis, especially when they present immunosuppressive factors, such as long-term corticosteroid therapy or HIV, to prevent morbidity and mortality due to coinfection and to reduce the risk of acquired resistance to rifampicin, which is the greatest risk factor for this association.
Informed Consent
Written informed consent for publication of case details and images was obtained from patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Keragala BSDP, Herath HMMTB, Janapriya GHDC, et al. Coexistence of mycobacterial infections - Mycobacterium tuberculosis and Mycobacterium leprae - in Sri Lanka: a case series. J Med Case Rep. 2020;14(1):101. doi:10.1186/s13256-020-02413-w
2. Ruthramoorthy P, Jose JA, Revendran J, et al. Co-infection of Mycobacterium tuberculosis and Mycobacterium leprae complicated by pulmonary embolism: a rare case report. Int J Mycobacteriol. 2023;12(4):513–515. doi:10.4103/ijmy.ijmy_186_23
3. Ghunawat S, Bansal S, Sahoo B, et al. Borderline tuberculoid leprosy with scrofuloderma: an uncommon association. Indian J Dermatol Venereol Leprol. 2014;80(4):381. doi:10.4103/0378-6323.136995
4. Relvich AL. The treatment of tuberculosis in leprosy patients. Lepr Rev. 1954;25(4):179–186. doi:10.5935/0305-7518.19540025
5. Fróes LAR, Toma TS, Jachiet M, et al. Bacterial, fungal and parasitic co-infections in leprosy: a scoping review. PLos Negl Trop Dis. 2023;17(5):e0011334. doi:10.1371/journal.pntd.0011334
6. Verma AK, Singh A, Prakash V, et al. Coexistence of leprosy and pulmonary tuberculosis: an uncommon entity. Med J DY Patil Univ. 2015;8(5):675–677. doi:10.4103/0975-2870.164954
7. Ravindra K, Sugareddy RT, Ramachander T. Coexistence of borderline tuberculoid Hansen’s disease with tuberculosis verrucosa cutis in a child--a rare case. Indian J Lepr. 2010;82(2):91–93.
8. Rao GR, Sandhya S, Sridevi M, et al. Lupus vulgaris and borderline tuberculoid leprosy: an interesting co-occurrence. Indian J Dermatol Venereol Leprol. 2011;77(1):111. doi:10.4103/0378-6323.74997
9. Rawson TM, Anjum V, Hodgson J, et al. Leprosy and tuberculosis concomitant infection: a poorly understood, age-old relationship. Lepr Rev. 2014;85(4):288–295. doi:10.47276/lr.85.4.288
10. Sendrasoa FA, Ranaivo IM, Raharolahy O, et al. Pulmonary tuberculosis and lepromatous leprosy coinfection. Case Rep Dermatol Med. 2015;2015:898410. doi:10.1155/2015/898410
11. Sami CA, Hassan SS, Khan AH, et al. A young female with borderline lepromatous leprosy and tuberculous lymphadenitis: a rare coinfection. Cureus. 2022;14(4):e23892.
12. Mattos KA, Lara FA, Oliveira VG, et al. Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell Microbiol. 2011;13(2):259–273. doi:10.1111/j.1462-5822.2010.01533.x
13. Huang Z, Luo Q, Guo Y, et al. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas in vitro. PLoS One. 2015;10(6):e0129744.
14. Rajagopala S, Devaraj U, D’Souza G, et al. Co-infection with M. tuberculosis and M. leprae-case report and systematic review. J Mycobac Dis. 2012;2:118.
15. Shetty S, Umakanth S, Manandhar B, et al. Coinfection of leprosy and tuberculosis. BMJ Case Rep. 2018;2018:bcr2017222352. doi:10.1136/bcr-2017-222352
16. Viel B, Dallien H. The relationship between leprosy and tuberculosis. Int J Lepr. 1960;28:483.
17. Glaziou P, Cartel JL, Moulia-Pelat JP, et al. Tuberculosis in leprosy patients detected between 1902 and 1991 in French Polynesia. Int J Lepr Other Mycobact Dis. 1993;61(2):199–204.
18. Chaussinand R. Tuberculosis and leprosy, antagonist diseases. Prevention of leprosy by tuberculosis. Int J Lepr. 1948;16:431–438.
19. Trindade MÂ, Miyamoto D, Benard G, et al. Leprosy and tuberculosis co-infection: clinical and immunological report of two cases and review of the literature. Am J Trop Med Hyg. 2013;88(2):236–240. doi:10.4269/ajtmh.2012.12-0433
20. Hasan Z, Jamil B, Zaidi I, et al. Elevated serum CCL2 concomitant with a reduced mycobacterium -induced response leads to disease dissemination in leprosy. Scand J Immunol. 2006;63(3):241–247. doi:10.1111/j.1365-3083.2006.01733.x
21. Ohara N, Matsuoka M, Nomaguchi H, et al. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by recombinant Bacillus Calmette-Guérin (BCG). Vaccine. 2000;31(14):1294–1297. doi:10.1016/S0264-410X(99)00420-X
22. Farhana-Quyum M-H, Ahmed Z, Ahmed Z. A case of lepromatous leprosy with co-existing tuberculosis verrucosa cutis (TVC). Lepr Rev. 2015;86(2):176–179. doi:10.47276/lr.86.2.176
23. Gupta MC, Prasad M. Associated infection of pulmonary tuberculosis and leprosy. Indian J Med Sci. 1971;25(3):183–185.
24. Gajwani BW, Verma BS, Marwaha RK, et al. Simultaneous infection with M. tuberculosis and M. leprae. J Assoc Physicians India. 1968;16(8):563–564.
25. Grace M, Shameemurahman R. Coinfection of two age old diseases. Indian J Community Med. 2011;36(3):228–230. doi:10.4103/0970-0218.86526
26. Nigam P, Dubey AL, Dayal SG, Goyal BM, Saxena HN, Samuel KC. The association of leprosy and pulmonary tuberculosis. Lepr India. 1979;51(1):65–73.
27. Dioussé P, Fall L, Lawson ATD, et al. Comorbidity leprosy and tuberculosis: with reference to six cases. Rev Mali Infect Microbiol. 2018;12:40–44.
28. Cavalcante Trindade L, Da Silveira Mendes M, Conceição Martins L, et al. Co-infection leprosy and tuberculosis: a systematic review. J Infect Dev Ctries. 2021;15(11):1569–1577. doi:10.3855/jidc.14308
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
