Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Cognitive Dysfunction in Hospitalized Patient with Moderate-to-Severe COVID-19: A 1-Year Prospective Observational Study
Authors Vasile MC , Vasile CI , Arbune AA , Nechifor A , Arbune M
Received 24 August 2023
Accepted for publication 16 October 2023
Published 8 November 2023 Volume 2023:16 Pages 3367—3378
DOI https://doi.org/10.2147/JMDH.S432969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mihaela-Camelia Vasile,1,2,* Claudiu-Ionut Vasile,2,3,* Anca-Adriana Arbune,4,5,* Alexandru Nechifor,1,* Manuela Arbune1,6,*
1Clinical Medical Department, Medicine and Pharmacy Faculty, “Dunărea de Jos” University, Galati, Romania; 2Infectious Diseases Department II, Clinic Hospital for Infectious Diseases, Galati, Romania; 3Psychiatry Department I, Clinic Psychiatry Hospital, Galati, Romania; 4Neurology Department, Fundeni Clinical Institute, Bucharest, Romania; 5Multidisciplinary Integrated Center of Dermatological Interface Research (MIC-DIR), “Dunărea de Jos” University, Galați, Romania; 6Infectious Diseases Department I, Clinic Hospital for Infectious Diseases, Galati, Romania
*These authors contributed equally to this work
Correspondence: Claudiu-Ionut Vasile, Email [email protected]
Purpose: To screen the neurocognitive impairment persistent post-COVID-19.
Patients and Methods: We assessed the neuropsychiatric disorders associated with COVID-19 in a prospective study, by “Mini-Mental State Examination” (MMSE) and Montreal Cognitive Assessment (MoCA) questionnaires, applied in the discharge to COVID-19 hospitalized patients for moderate and severe forms of disease. They were followed-up in 6 and 12 months.
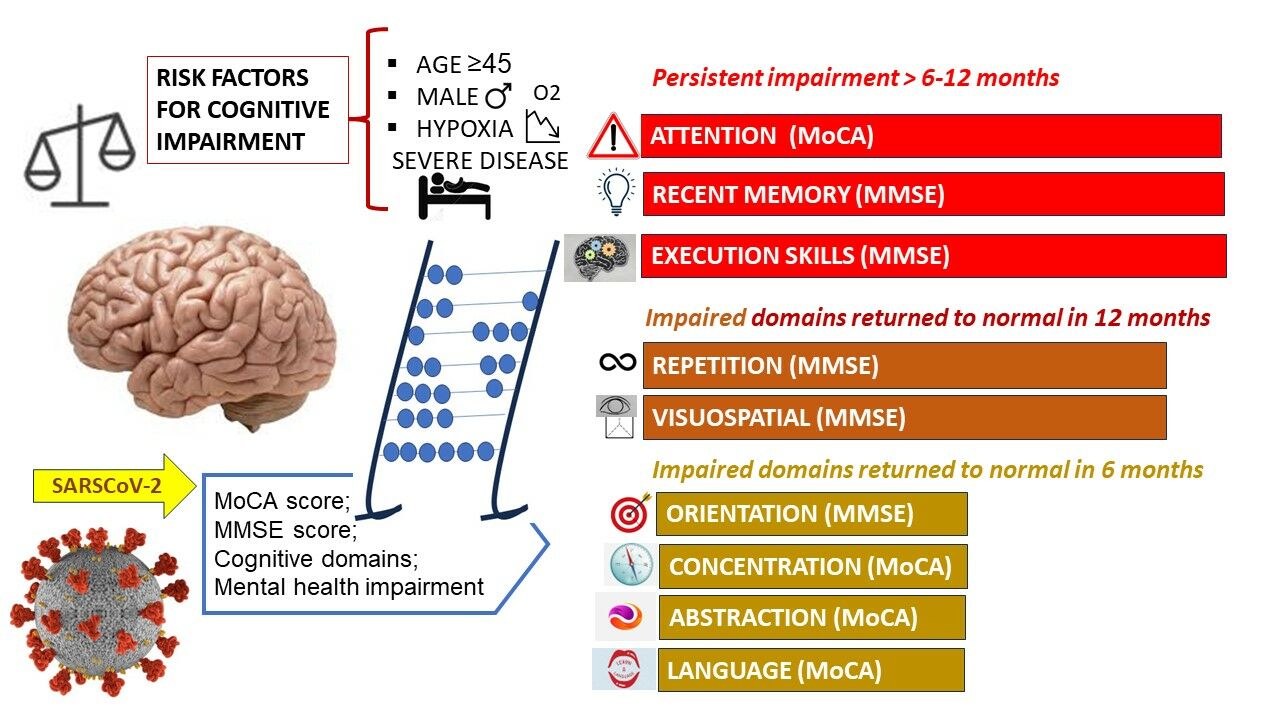
Results: The tests were performed in the baseline and were reevaluated after 6 and 12 months. Baseline cognitive dysfunction was found in 12.4% of patients, according to the MMSE test and in 19.7% by the MoCA scores. Overall cognitive dysfunction in COVID-19 was returned to normal after 6 months, although some tasks are more severe and persistently impaired, such as attention, concentration, short memory, and execution skills. The male gender and the degree of hypoxia, related to the severity of COVID-19 infection, were related to cognitive dysfunction in the study group.
Conclusion: Cognitive domain impairments related to COVID-19 could persist over 6 months post-acute infectious episode requiring systematic screening for early diagnosis of progressive brain pathologies and rehabilitation.
Keywords: mental health, MoCA test, MMSE test, cognitive domains, COVID-19
Graphical Abstract:
Introduction
Previous influenza pandemics, as well as those caused by SARS-1 and MERS coronaviruses, have been associated with long-term neuropsychiatric outcomes in affected populations. Recent studies support the hypothesis that COVID-19 infection, caused by SARS-CoV2, may also be associated with neurological and psychiatric manifestations, even from the onset.1 In the context of the pandemic, especially in people who had COVID-19 and were healthy before infection showed mild or moderate symptoms associated with deficits in the domains of working memory (WM), set-shifting, divided attention, and processing speed.2
Several prolonged or even permanent neurocognitive disorders may be sequelae of hypoxia, encephalitis, or strokes occurring during acute infection, requiring long-term monitoring.2–4
A systematic review conducted by Tavares-Junior JWL, on COVID-19 patients confirmed by serology or PCR, who developed new cognitive impairment or worsened from previous cognitive impairment after infection, included cohort, cross-sectional, and case–control studies from 10 countries.5 It comprises patients during hospitalization, discharged or outpatients, regardless of severe, moderate, or mild form of disease, either in acute or 12 weeks after infection. The frequency of COVID-19 related cognitive impairment varied from 2.6% to 81%, while the studies after 12 weeks reported rates from 21% to 65%.5 Neuroimaging exams did not found specific structural alteration, excepting two reports of cranial positron emission tomography scan with a finding of frontoparietal hypometabolism in patients with encephalopathy during the acute phase.6,7 Although the high frequency of cognitive impairment after COVID-19 infection is evident, differentiation between mild cognitive impairment and dementia is not achievable.5,8
A meta-analysis of 81 studies, in patients with confirmed COVID-19 found persistent fatigue in approximately a third of the individuals and cognitive impairment in over a fifth of individuals after 12 or more weeks following COVID-19 diagnosis, suggesting a possible association with elevations in pro-inflammatory markers.8 The mechanisms of persistent fatigue and cognitive impairment attributable to COVID-19 could involve the direct viral encephalitis, neuro-inflammation, hypoxia, and cerebrovascular disease, but there are also possible synergistic interactions with other conditions, as systemic sequelae including endothelial dysfunction, hyperinflammation, autoimmunity, latent viral reactivation, multi-organ pathology, and autonomic nervous system dysfunction.9,10
Post-COVID-19 syndrome is defined as a neurological and neuropsychiatric disorder including symptoms such as fatigue, brain fog, memory issues, attention disorder, myalgia, anosmia, dysgeusia and headache, sleep disturbances, anxiety, and depression that persist or develop 12 weeks after the onset of COVID-19.11 All long-term neuropsychiatric sequelae of COVID-19 infection remain unknown, particularly those that may involve alterations in brain development in the offspring of infected pregnant women.12 Similar to neuropsychiatric sequelae after 1918 influenza pandemic, we could expect a greater mental health burden on society in the coming years.10
The purpose of this study is to assess the cognitive disorders associated with moderate and severe forms of COVID-19. To our knowledge, this is the first prospective study in South-East of Romania, on cognitive impairment in hospitalized patients, followed up 1 year after the COVID-19 diagnostic. While most published studies are limited to 12-week evaluation of long COVID syndrome, we have sequentially assessed the cognitive abilities in three steps: in early convalescence considered on hospital discharge, following up after 6 months and 12 months.
Materials and Methods
We conducted a prospective study, carried out between August 2020 and October 2021, in Infectious Diseases Clinic Hospital from Galati, situated in the south-east of Romania.
Patients’ Selection
The criteria for inclusion in the study were the virological confirmation of SARS-CoV2 infection by polymerase chain reaction (PCR) tests of Romanian patients admitted to Infectious Diseases Clinic Hospital from Galati, aged between 18 and 60 years old, with moderate and severe forms of the disease, who gave written consent to research participation. We used the criteria for classification of COVID-19 disease severity infection into mild, moderate, severe, and critical forms, according to WHO definitions for adults and the national protocol for the management of COVID-19 infection that were available at the enrollment date.13–15
The exclusion criteria were pregnant women during COVID-19 or within following 12 months, illiterate or elementary school patients, patients with a history of head trauma, carbon monoxide poisoning, drug abuse, and those with a family history of Alzheimer’s disease or known mild cognitive impairment were excluded. We excluded from the statistical analysis patients who received the COVID-19 vaccine in the follow-up stage or experienced another confirmed episode of COVID-19.
Data Collection
We assessed the demographic characteristics: age (number of years), male or female, urban or rural living environment, educational attainments middle school, high school, associate degree or above and occupation student, employed, retired or unemployed. Depending on age, patients were grouped in two categories, 18–44 years or 45–60 years. We considered behaviors at risk represented by smoking, alcohol, and obesity. The medical history of patients in the study group and the control group was assessed by the Charlson Comorbidity Index.16,17 In addition, we analyzed the clinical, biological, radiological, and therapeutic characteristics of patients with COVID-19 during hospitalization. The data was collected in accordance with the form “Core CRF captures clinical information of individuals hospitalized for COVID-19”, recommended by the World Health Organization (WHO).18
Patients were assessed using the questionnaire method, using the psychometric scales MMSE and MoCA and the time taken to complete these scales. Cognitive assessment screening was performed at three stages: at the baseline (in the day of the hospital discharge) and follow-up at 6 months and 12 months. The Romanian version of MoCA tests was applied by an infectious diseases’ clinician trained and certified to administer, score, and interpret the MoCA. The instrument was used as a rapid screening tool for mild cognitive impairment, assessing several cognitive domains: attention, concentration, executive functions, memory, language, visual-constructive skills, conceptual thinking, computation, and orientation. The total score was adjusted by adding 1 point if a person has 12 years or less of formal education. Total scores greater than or equal to 26 are considered normal, with a maximum value of 30 points.19,20 The time required to administer MoCA is approximately 10 min. MMSE was applied under the supervision of a licensed clinical psychologist. We used a brief version of the “MINI-MENTAL STATE Examination” (MMSE) test consisting of 11 questions to screen for cognitive dysfunctions, testing 6 areas of mental functioning: spatial and temporal orientation, attention and concentration, short-term memory (recall), visual-spatial ability, and the ability to understand and follow instructions. The test requirements are memorizing the names of objects and repeating them later, copying and drawing, writing a grammatically correct sentence, correctly identifying the date, month, season, year, and place of the patient.21,22 The maximum value of the score is 30, considering values lower than 24 as abnormal. The data collected were recorded as numerical variables and grouped into categories (abnormal/normal).
Statistical Analysis
Statistical analysis used IBM SPSS Statistics version 25 software and included the patients with completed the 12 months follow-up. The statistical analysis was aimed at providing an accurate description of the demographics, signs and symptoms of the detailed cognitive performance and evolution from baseline to 6-month and 12-month follow-up. We perform descriptive analysis of numerical data using the mean and standard deviation for the symmetric distribution, and the mean, median, skew and extreme values (maximum and minimum) for asymmetric distribution. The descriptive analysis of the categorical data used the frequency calculation and the ANOVA significance test. Pearson’s chi-squared test was used to analyze the dependence of variables (the interaction between variables). We used the chi-square test or Fisher's exact test to compare the nominal data and the t-test or Mann–Whitney U-test to compare the continuous variables. The accepted significance limit of the tests was p<0.001.
Results
Characteristics of COVID-19 in Hospitalized Patients
The study group consisted of 137 patients of whom 54% were women, 82% lived in urban areas, 64% graduated from high school or a technical/vocational school and 78.8% were employed. Patients under the age of 45 represent 48.9%, while 51.1% are 45 years old or older. The health risk behaviors were smoking (81.8%), alcohol use (13.1%) and obesity with body mass index ≥ 30kg/m2 (21.9%) (Table 1). Charlson Comorbidity Index mean was 0.700 ±1.066.
 |
Table 1 Demographic and Behavioral Characteristics of Patients with COVID-19 |
The mean duration of symptoms from onset to admission was 5.03±1.24 days. The most commonly recorded clinical manifestations were fever (87.59%), cough (86.68%), shortness of breath (33.57%), headache (43.06%), anosmia (45.92%) and dysgeusia (29.19%).
By severity, 71% had moderate forms of the disease and 29% severe forms, but none were critical. Exhaled air oxygen saturation was below 91% in 28.5% of patients, with a minimum value of 76% requiring supplemental oxygen via the nasogastric tube or mask. Other complications during hospitalization for COVID-19 were secondary hepatitis (21.2%), de novo diabetes mellitus (10.2%), and cardiac arrhythmias (1.5%). The rate of healthcare-associated infections with Clostridioides difficile was 4.4%. The most prominent biological changes were increased neutrophil/lymphocyte ratio over 3 (100%), increased C-reactive protein (100%), lactic dehydrogenase (99.3%) and creatine kinase (86.9%).
All patients were discharged cured or with evident improvement of the clinical and laboratory test values.
Outcome of Psychometric Scales Evaluation
The average MoCA global score was 25.07 in baseline and has improved to 26.25 after 6 months and to 26.82 after 12 months. MoCA global scores between 18 and 25 points were found in 19.7% of hospital discharged patients, indicating mild neurocognitive impairment, while 80.03% of patients presented normal scores. The rate of scores corresponding to mild neurocognitive impairment decreased to 5.10% after 6 months and to 1.46% after 12 months. According to the MoCA subscales, attention was the most frequently diminished task in baseline persisting on 92.7% of patients in the 12 month follow-up. Repetition and visuospatial tasks impairment have baseline interested 88.32% and 64.96% of patients, respectively, following slow improvement in the sixth month, but returned to normal to the end of study by 95% of cases. Other impaired skills were language in 28.46% and abstraction in 32.11% of cases that have recovered little after 6 months, but returned to normal after 12 months. Naming and orientation were the least compromised tasks that were completely normal after 12 months of follow-up (Figure 1).
 |
Figure 1 Evolution of the frequencies of MoCA overall scores and by domains scores from baseline to 12 months follow-up. |
The global MMSE global score of hospitalized patients with COVID-19 in the discharge day was 26.86 and has improved to 27.62 in 6 months and to 27.91 in 12 months. The rates of global MMSE scores under 26 points decreased from 12.40% in the COVID-19 hospital discharge to 1.46% after 6 months and 0.74% after 12 months of follow-up (Table 2).
 |
Table 2 Statistical Results of Overall and by Domains Scores on MoCA Test in Patients Experienced COVID-19 Hospitalization |
The evolution of the MMSE assessment confirmed the preservation of naming and repetition tasks, which were observed by the MoCA test. The memory task was impaired only in terms of working memory, but this was the most severe and persistently compromised domain. Orientation skills were impaired in 9.48%, in the baseline, but returned to normal in 6 months, as by MoCA score. Concentration, writing, reading and drawing are other diminished skills that improved after 6 months and 12 months (Table 3, Figure 2).
 |
Table 3 Statistical Results of Overall and by Domains Scores on MMSE Test in Patients Experienced COVID-19 Hospitalization |
 |
Figure 2 Evolution of the frequencies of MMSE overall scores and by domains scores from baseline to 12 months follow-up. |
Correlations of Neurocognitive Impairment with Characteristics of COVID-19 Patients
The age of 45 or more is correlated with mild cognitive impairment in COVID-19 convalescence, both in MoCA (p<0.001; OR=7.70; CI95% 2.80–21.20) and in MMSE test (p<0.001; OR=5.33; CI95% 2.26–14.20). Alteration of the screening cognitive scores in convalescence by gender reveal that men are more severely affected than women, but the statistical difference with p<0.001 is significant for MoCA (OR=5.55; CI95%2.26–14.20), not for MMSE (p=0.007). A moderate correlation between oxygen saturation and MoCA global scores was found (correlation coefficient 0.777). No statistical correlation between neutrophil/lymphocyte ratio during COVID-19 episode and MoCA or MMSE scores was found.
Discussion
Romanian patients previously unknown with cognitive dysfunction, family history or risk factors for mental health diseases have performed 19.70% mild cognitive impairment according to MoCA scores and 12.40% by MMSE scores, when they were discharged, after experience of COVID-19 hospitalization. The rate of abnormal MoCA scores is higher than MMSE, which is concordant with previously reported better sensitivity of MoCA in detecting mild cognitive impairment than MMSE.23 About 95% of both global scores returned to normal after 6 months. A follow-up evaluation after 12 months found persistence of low scores in 2 patients by MoCA test and in 1 patient by MMSE test, both being investigated for dementia-related diseases. Attention, working memory and execution are the main severe and persistent impaired tasks. Visuospatial, repetition, language, abstraction, concentration and drawing functions are also impaired during COVID-19 convalescence but returned to normal after 6 months. The correlation between hypoxia and early cognitive impairment scores in our study is expressed mostly with MoCA rather than MMSE tests.23
Cognitive impairment related to the COVID-19 disease severity and hypoxia was reported in moderate forms, with the frequency of low MoCA scores ranged from 36% to 60%, but as high as 94% in forms with BIPAP respiratory support.24–26
The cognitive domains affected by hypoxia are attention, learning ability, memory, processing speed, and executive function. The severity of neurocognitive deficits correlates with the duration and severity of hypoxia.27 The molecular substrate of neurocognitive disorders associated with acute hypoxia include increased glycolysis, oxidative stress, calcium accumulation, mitochondrial alteration, inflammation and excitotoxicity. Changes occurring after acute hypoxia may recover, whereas chronic hypoxia may be followed by sequelae, or even dementia, probably explained by different molecular mechanisms.28–30
Sequelae of hypoxic encephalopathy can range from attention deficits and discrete memory impairments to dementia and severe mental impairment.31 Given that cognitive disorders correspond to lesions localized in specific cortical areas, our results suggested that the COVID-19 brain injuries are longer interesting to parietal and occipital lobes, expressed by working memory and, respectively, attention, while other cortical impairments are transitory, recovering after at least 6 months, in majority of patients.32
The frequency of cognitive dysfunction in patients with COVID-19, as evidenced by a decrease in the MoCA score, varies according to different studies and selection criteria from 17% to 80%.25–33 A cross-sectional study comparing cognitive impairment in patients with COVID-19 without complications treated as outpatients and patients with COVID-19 hospitalized for hypoxic pneumonia, found that 18% of cases had memory impairment and 11% attention dysfunction, significantly more common in the group hospitalized with hypoxia.34 The domains of cognitive impairment correspond to our results, although we find higher frequencies of low scores of these tasks.
In a prospective 12-month study, the frequency of abnormal MoCA score decreased from 25% at baseline to 13% at 12 months, compared to our study, in which the frequency decreased from 34.3% at baseline to 15.3% at 12 months.35
Recently, the risk of persistent neurocognitive disorders defining “long COVID” was assessed from the perspective of identifying distinct neurophenotypes. The assessment was conducted within the first 12 weeks after the PCR confirmation of the SARS-CoV-2 infection (post-acute recovery stage) and at 6 months (chronic recovery stage) with 205 patients completing a computer outcome assessment. The post-acute recovery stage assessment grouped patients into three clusters: 69% had normal cognitive functions, although mild attention and memory impairment were reported, 16% had memory impairment, slow processing speed, fatigue and 15% had predominantly executive dysfunction.36 The neurophenotype “with impaired memory speed” correlated with anosmia and severe forms of COVID-19, and the “dysexecutive” neurophenotype associated with obesity and isolation. Results after 6 months showed improvement in verbal memory and psychomotor speed in the normal cognition group, improvement in cognitive flexibility in the dysexecutive group but no objective improvement or even worsening in the impaired memory speed group. These data highlight the relationship between post-COVID-19 neurophenotypes and different pathogenic mechanisms, with implications for the prognosis and efficacy of some therapeutic interventions.36
The neurocognitive consequences of COVID-19 are not fully recognized, due to the short perspective since the beginning of the pandemic. However, there are data that support the links between inflammatory process and cognitive impairment or dementia. Identification of SARS-CoV-2 in the human brain, mainly in astrocytes and less common in neurons, involves consecutive changes in the cellular energy metabolism that impact the synthesis of neurotransmitters and their neuronal viability.37 The viral activation of microglia produces neuroinflammation, which contributes to the death of nervous cells and the alteration of the blood–brain barrier, by permeable to cytokines and activated immune cells. Therefore, neuronal damage and the progression of dementia are generated in a vicious circle.38 The hypothetical mechanism of neurodegenerative impact of SARS-CoV-2 is sustained by in vitro studies on brain cells exposed to some pathogens and evidence of producing amyloid proteins and lesions in neuronal cells, distinctive for Alzheimer's disease. Immunohistochemistry and epigenetic studies in neurodegenerative dementia have evidenced that the loss of cortical neurons is related to the increased production of cytokines and microglial and astroglia activation. Neuroinflammation and chronic glial activation are the consequences of abnormal protein aggregation and amyloidogenesis, exacerbating neuronal damage and cytotoxicity.39–41
Gender difference in cognitive dysfunction related to COVID-19 could be explained by the role of sex hormones in modulating immune cells, regarding phagocytic activity, cytokine secretion and production of antibodies.40 Innate and adaptive immune responses to pathogens are more rapid in female than male, with higher T-lymphocytes activation, leading to faster viral clearance and increased susceptibility to autoimmune and inflammatory diseases. Males have a more attenuated viral response, but they are at higher risk to severe COVID-19 diseases, related to increased plasma cytokines of innate immunity. Angiotensin-converting enzyme-2 (ACE-2) receptors, which are essential for COVID-19 pathogen mechanisms, have a higher density in males than females, owing to genetic determinism and modulation of ACE2 expression by sex steroid hormones, estrogen, progesterone, and testosterone.42,43
COVID-19 vaccination and therapeutic interventions for acute viral disease could be speculating about long-term influence on the hypoxic brain death and neurodegeneration.44–46 Further studies dedicated to post-COVID-19 are expected to clarify the type of cognitive disorders, frequency, severity and profile specific to cognitive dysfunction, taking into account the increased variability between individuals, which is given by contextual factors and impacts cognition.29,47
Limits of the Study
The study was conducted during the first pandemic year, when there were not yet available vaccines or specific antiviral medication, the knowledge about the new virus was poor, and there were not clarified data about neurocognitive impairment or long-COVID consequences. Versions of protocols for the management of COVID-19 were often changed during the study, including regulations for isolation time of acute COVID-19. A control group of study was not available. The tests used for the study are dedicated to dementia early detection (MoCA) or dementia monitoring (MMSE) that could be a sequela of COVID-19, but the results are not able to accurately identify the pathological substrate of cognitive impairment.48,49
The role of affective disorders was not examined, although the prevalence of depressive symptoms after COVID-19 increased significantly between mid- and long-term follow-up. Depression can lead to cognitive deficits, such as attention, concentration and working memory, that were frequently notified in our cohort and a further study will analyze the probable interference. MMSE and MoCA tests are used only for the screening of neurocognitive impairments and need supplementary evaluation for validation.
Conclusion
The mild cognitive impairment in convalescent patients hospitalized with COVID-19 was more accurately detected by MoCA than MMSE test. Although most abnormal global scores returned to normal in 6 months, some patients experienced persistent disabilities involving attention, working memory and execution, requiring additional investigations and support interventions. Systematic evaluation of hospitalized COVID-19 patients should be considered for screening of cognitive impairments and follow up on them for possible long-term sequela, by integrated multidisciplinary networks.
Abbreviations
COVID-19, Corona-Viral Disease; Max, Maximum; Min, Minimum; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment (MoCA); mo, month, SD, Standard deviation.
Data Sharing Statement
Data are available on request from the corresponding author due to privacy restrictions.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of “Sf. Cuv. Parascheva” Clinical Hospital for Infectious Diseases No.65/30.07.2020.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Acknowledgments
We acknowledge the clinical psychologist Mrs. Simona-Dana Caramfil for MMSE use and interpretation.
Funding
This research received no external funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Mao L, Jin H, Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi:10.1001/jamaneurol.2020.1127
2. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi:10.1016/S2215-0366(20)30287-X
3. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383(10):989–992. doi:10.1056/NEJMc2019373
4. Vasile MC, Arbune AA, Lupasteanu G, Vlase CM, Popovici GC, Arbune M. Epidemiologic and clinical characteristics of the first wave of the COVID-19 pandemic in hospitalized patients from Galați County. J Clin Med. 2021;10(18):4210. doi:10.3390/jcm10184210
5. Tavares-Júnior JWL, de Souza ACC, Borges JWP, et al. COVID-19 associated cognitive impairment: a systematic review. Cortex. 2022;152:77–97. doi:10.1016/j.cortex.2022.04.006
6. Blazhenets G, Schroeter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive Impairment in a series of chronic COVID-19 patients. J Nucl Med. 2021;62(7):910–915. doi:10.2967/jnumed.121.262128
7. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144(4):1263–1276. doi:10.1093/brain/awab009
8. Crivelli L, Palmer K, Calandri I, et al. Changes in cognitive functioning after COVID-19: a systematic review and meta-analysis. Alzheimers Dement. 2022;18(5):1047–1066. doi:10.1002/alz.12644
9. Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi:10.1016/j.bbi.2021.12.020
10. Losilla-Rodríguez B, Maldonado N, Moreno-Mellado E, López-Díaz Á. COVID-19 natural herd immunity and risk of neuropsychiatric disorders. Rev Psiquiatr Salud Ment. 2020;13(4):228–229. doi:10.1016/j.rpsm.2020.07.002
11. Premraj L, Kannapadi NV, Briggs J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. doi:10.1016/j.jns.2022.120162
12. Ayesa-Arriola R, López-Díaz Á, Ruiz-Veguilla M, Leza JC, Saura LF, Crespo-Facorro B. COVID-19 una oportunidad única para explorar la relación entre la infección prenatal materna, el desarrollo cerebral y los trastornos neuropsiquiátricos en la descendencia [COVID-19 as a unique opportunity to unravel the link between prenatal maternal infection, brain development and neuropsychiatric disorders in offspring]. Rev Psiquiatr Salud Ment. 2021;14(1):1–3. Spanish. doi:10.1016/j.rpsm.2020.12.003
13. Ministerul Sănătăţii din Romania. ORDIN nr. 487 din 23 martie 2020 pentru aprobarea protocolului de tratament al infecţiei cu virusul SARS-Cov-2. Monitorul Oficial nr. 242 din 24 martie; 2020. Available from: http://www.cnscbt.ro/index.php/lex/1591-ordinul-nr-487-2020-pentru-aprobarea-protocolului-de-tratament-covid/file.
14. Ministerul Sanatatii Ordin 1418 privind modificarea anexei la Ordinul ministrului sănătăţii nr. 487/2020 pentru aprobarea protocolului de tratament al infecţiei cu virusul SARS - Cov – 2. Monitorul Oficial nr. 719 din 10 aug; 2020. Available from: https://legislatie.just.ro/Public/DetaliiDocument/229019.
15. World Health Organization. Clinical management of COVID-19: interim guidance. World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/332196.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
17. Charlson Comorbidity Index (CCI). Available from: https://www.mdcalc.com/calc/3917/charlson-comorbidity-index-cci.
18. Global COVID-19 clinical platform case report form (CRF) for post COVID condition (post COVID-19 CRF). Available from: https://www.who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form-(crf)-for-post-covid-conditions-(post-covid-19-crf-).
19. MoCA Version 7.1; 2010. Available from: www.mocatest.org.
20. Dautzenberg G, Lijmer J, Beekman A. Diagnostic accuracy of the Montreal Cognitive Assessment (MoCA) for cognitive screening in old age psychiatry: determining cutoff scores in clinical practice. Avoiding spectrum bias caused by healthy controls. Int J Geriatr Psychiatry. 2020;35(3):261–269. doi:10.1002/gps.5227
21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
22. Folstein MF, Folstein SE, Adaptat în România de Cornelia-Eugenia Munteanu, Dragoș Iliescu, Raluca Livinți. MMSE-2. Mini-Mental State Examination, 2nd Edition Manual de utilizare a testului. O.S. Romania, 2010. Available from: https://monitorulpsihologiei.com/mmse-2-mini-mental-state-examination-second-edition/.
23. Gamberini G, Masuccio FG, Cerrato M, Strazzacappa M, Ferraro D, Solaro C. Previously independent patients with mild-symptomatic COVID-19 are at high risk of developing cognitive impairment but not depression or anxiety. J Affect Disord. 2023;324:645–651. doi:10.1016/j.jad.2022.12.100
24. Pilotto A, Cristillo V, Cotti Piccinelli S, et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol Sci. 2021;42(12):4903–4907. doi:10.1007/s10072-021-05586-4
25. Velichkovsky BB, Razvaliaeva AY, Khlebnikova AA, Manukyan PA, Kasatkin VN. Attention and memory after COVID-19 as measured by neuropsychological tests: systematic review and meta-analysis. Acta Psychol. 2023;233:103838. doi:10.1016/j.actpsy.2023.103838
26. Solaro C, Gamberini G, Masuccio FG. Cognitive impairment in young COVID-19 patients: the tip of the iceberg? Neurol Sci. 2021;42(12):4865–4866. doi:10.1007/s10072-021-05534-2
27. Wang X, Cui L, Ji X. Cognitive impairment caused by hypoxia: from clinical evidences to molecular mechanisms. Metab Brain Dis. 2022;37(1):51–66. doi:10.1007/s11011-021-00796-3
28. Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699–702. doi:10.1002/jmv.25915
29. Vasile CI, Vasile MC, Zlati ML, et al. Post COVID-19 infection psychosis: could SARS-CoV-2 virus infection be a neuropsychiatric condition that triggers psychotic disorders? - A case-based short review. Infect Drug Resist. 2022;15:4697–4705. doi:10.2147/IDR.S373578
30. Li J, Long X, Zhang Q, et al. Emerging evidence for neuropsycho-consequences of COVID-19. Curr Neuropharmacol. 2021;19(1):92–96. doi:10.2174/1570159X18666200507085335
31. Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol. 2021;58(2):564–575. doi:10.1007/s12035-020-02152-5
32. Dondaine T, Ruthmann F, Vuotto F, et al. Long-term cognitive impairments following COVID-19: a possible impact of hypoxia. J Neurol. 2022;269(8):3982–3989. doi:10.1007/s00415-022-11077-z
33. Aiello EN, Fiabane E, Manera MR, et al. Screening for cognitive sequelae of SARS-CoV-2 infection: a comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Neurol Sci. 2022;43(1):81–84. doi:10.1007/s10072-021-05630-3
34. Patel R, Savrides I, Cahalan C, et al. Cognitive impairment and functional change in COVID-19 patients undergoing inpatient rehabilitation. Int J Rehabil Res. 2021;44(3):285–288. doi:10.1097/MRR.0000000000000483
35. Alemanno F, Houdayer E, Parma A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16(2):e0246590. doi:10.1371/journal.pone.0246590
36. Latronico N, Peli E, Calza S, et al. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax. 2022;77(3):300–303. doi:10.1136/thoraxjnl-2021-218064
37. Prabhakaran D, Day GS, Munipalli B, et al. Neurophenotypes of COVID-19: risk factors and recovery outcomes. Res Sq. 2023. doi:10.21203/rs.3.rs-2363210/v2
38. Crunfli F, Carregari VC, Veras FP, et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci U S A. 2022;119(35):e2200960119. doi:10.1073/pnas.2200960119
39. Chai YL, Lee JH, Chong JR, et al. Inflammatory panel cytokines are elevated in the neocortex of late-stage Alzheimer’s disease but not Lewy body dementias. J Neuroinflammation. 2023;20(1):111. doi:10.1186/s12974-023-02789-8
40. Madetko N, Migda B, Alster P, Turski P, Koziorowski D, Friedman A. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol Neurochir Pol. 2022;56(2):148–155. doi:10.5603/PJNNS.a2022.0014
41. Alster P, Madetko N, Friedman A. Neutrophil-to-lymphocyte ratio (NLR) at boundaries of Progressive Supranuclear Palsy Syndrome (PSPS) and Corticobasal Syndrome (CBS). Neurol Neurochir Pol. 2021;55(1):97–101. doi:10.5603/PJNNS.a2020.0097
42. Sciarra F, Campolo F, Franceschini E, Carlomagno F, Venneri MA. Gender-specific impact of sex hormones on the immune system. Int J Mol Sci. 2023;24(7):6302. doi:10.3390/ijms24076302
43. Michelutti M, Furlanis G, Buoite Stella A, et al. Sex-dependent characteristics of neuro-long-COVID: data from a dedicated neurology ambulatory service. J Neurol Sci. 2022;441:120355. doi:10.1016/j.jns.2022.120355
44. Chaurasia B, Chavda V, Lu B, Garg K, Montemurro N. Cognitive deficits and memory impairments after COVID-19 (Covishield) vaccination. Brain Behav Immun Health. 2022;22:100463. doi:10.1016/j.bbih.2022.100463
45. Huang YF, Ho TC, Chang CC, et al. A rare adverse effect of the covid-19 vaccine on autoimmune encephalitis. Vaccines. 2022;10(7). doi:10.3390/vaccines10071114
46. Chavda V, Chaurasia B, Fiorindi A, Umana GE, Lu B, Montemurro N. Ischemic stroke and SARS-CoV-2 infection: the bidirectional pathology and risk morbidities. Neurol Int. 2022;14(2):391–405. doi:10.3390/neurolint14020032
47. Moscu CA, Marina V, Dragomir L, Anghele AD, Anghele M. The impact of burnout syndrome on job satisfaction among emergency department nurses of emergency clinical county hospital “Sfântul Apostol Andrei” of Galati, Romania. Medicina. 2022;58(11). doi:10.3390/medicina58111516
48. Mazza MG, Palladini M, Poletti S, Benedetti F. Post-COVID-19 depressive symptoms: epidemiology, pathophysiology, and pharmacological treatment. CNS Drugs. 2022;36(7):681–702. doi:10.1007/s40263-022-00931-3
49. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi:10.1017/s003329171
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
