Back to Journals » Nature and Science of Sleep » Volume 14
Cognitive Appraisal of Sleep and Brain Activation in Response to Sleep-Related Sounds in Healthy Adults
Authors Hwang Y , Lee KH, Kim N, Lee J, Lee HY , Jeon JE, Lee YJ, Kim SJ
Received 20 January 2022
Accepted for publication 3 August 2022
Published 15 August 2022 Volume 2022:14 Pages 1407—1416
DOI https://doi.org/10.2147/NSS.S359242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Yunjee Hwang,1 Kyung Hwa Lee,2,3 Nambeom Kim,4 Jooyoung Lee,5 Ha Young Lee,2 Jeong Eun Jeon,2 Yu Jin Lee,2 Seog Ju Kim5
1Department of Brain and Cognitive Engineering, Korea University, Seoul, Republic of Korea; 2Department of Psychiatry and Center for Sleep and Chronobiology, Seoul National University, College of Medicine and Hospital, Seoul, Republic of Korea; 3Division of Child and Adolescent Psychiatry, Department of Psychiatry, Seoul National University Hospital, Seoul, Republic of Korea; 4Department of Biomedical Engineering Research Center, Gachon University, Incheon, Republic of Korea; 5Department of Psychiatry, Sungkyunkwan University College of Medicine, Samsung Medical Center, Seoul, Republic of Korea
Correspondence: Yu Jin Lee, Department of Psychiatry and Center for Sleep and Chronobiology, Seoul National University, College of Medicine and Hospital, Seoul, Republic of Korea, Email [email protected] Seog Ju Kim, Department of Psychiatry, Sungkyunkwan University College of Medicine, Samsung Medical Center, Seoul, Republic of Korea, Email [email protected]
Purpose: Sounds play important roles in promoting and disrupting sleep. How our brain processes sleep-related sounds and individual differences in processing sleep-related sounds must be determined to understand the role of sound in sleep. We investigated neural responses to sleep-related sounds and their associations with cognitive appraisals of sleep.
Participants and Methods: Forty-four healthy adults heard sleep-related and neutral sounds during functional magnetic resonance imaging using a 3T scanner. They also completed the Dysfunctional Beliefs and Attitudes about Sleep (DBAS) questionnaire, which was used to assess cognitive appraisals of sleep. We conducted a voxel-wise whole-brain analysis to compare brain activation in response to sleep-related and neutral sounds. We also examined the association between the DBAS score and brain activity in response to sleep-related sounds (vs neutral sounds) using region of interest (ROI) and whole-brain correlation analyses. The ROIs included the anterior cingulate cortex (ACC), anterior insula (AI), and amygdala.
Results: The whole-brain analysis revealed increased activation in the temporal regions and decreased activation in the ACC in response to sleep-related sounds compared to neutral sounds. The ROI and whole-brain correlation analyses showed that higher DBAS scores, indicating a negative appraisal of sleep, were significantly correlated with increased activation of the ACC, right medial prefrontal cortex, and brainstem in response to sleep-related sounds.
Conclusion: These results indicate that the temporal cortex and ACC, which are implicated in affective sound processing, may play important roles in the processing of sleep-related sounds. The positive association between the neural responses to sleep-related sounds and DBAS scores suggest that negative and dysfunctional appraisals of sleep may be an important factor in individual differences in the processing of sleep-related sounds.
Keywords: anterior cingulate cortex, appraisal, functional magnetic resonance imaging, sleep-related sounds
Introduction
Sounds can promote or disrupt sleep.1 Calming and soothing music helps people fall asleep, while short-duration, repetitive sounds (eg, clock-ticking) may prevent people from falling asleep. Sounds that disrupt sleep obviously affect sleep quality.2,3 Examining how the brain processes sleep-related sounds could help us understand how sounds promote or disrupt sleep. In addition, exploring the neural substrates of individual differences in the processing of sleep-related sounds may improve understanding of neurobiological vulnerability to sleep problems.
Neural activity differs among sound stimuli.4–6 Sleep-related sounds may be associated with sleep hygiene. Sleep-related sounds are likely to elicit a neural response in brain regions involved in the affective processing of sound. The auditory cortex plays a key role in affective processing of sound.7–9 The superior temporal cortex responds to sounds that elicit an automatic emotional response.10,11 The neural network engaged in affective processing of sound extends to other brain areas as well as the auditory cortex.7 Affective sounds enhance activity in the medial prefrontal cortex, which plays a role in emotional appraisal and evaluation.12–14 The insula shows activity in response to all types of affective sounds, including human verbalizations,15–17 and the amygdala is responsive to the sounds with negative valence.9,18
Interindividual differences exist in affective and neurobiological sensitivity to sleep-related sounds. Some people appraise the sound of a ticking clock to be sufficiently bothersome to prevent sleep. These differences can be explained by cognitive appraisals of sleep, ie, subjective assessments of sleep-related cognitions.19 Subjects with “dysfunctional” appraisals of sleep exhibit excessive worry and helplessness in relation to it.19,20 They are also more likely to perceive sleep-related sounds as unpleasant and stressful.21 Although dysfunctional sleep-related cognition is associated with neural activity in response to sleep-related sound in insomnia patients,22 no studies have investigated the relationship between sleep-related cognition and brain activity in response to sleep-related sound in those without a sleep disorder.
Affective and neurobiological responses to sleep-related sounds can be changed by subjective reevaluation of sleep-related cognitions. Cognitive appraisal of sleep may be associated with neural activation in several brain areas involved in affective stimuli processing, including the anterior cingulate cortex (ACC; involved in the cognitive regulation of emotions and integration of cognitive-affective processes),23,24 anterior insula (AI; plays a role in detecting emotional salience),25,26 and amygdala (preferentially processes the stimuli with a negative valence).27
This study compared neural responses to sleep-related and neutral sounds. We examined whether individual differences in the neural processing of sleep-related sounds are associated with cognitive appraisals of sleep. Our first hypothesis was that brain activity in response to sleep-related sounds is different from that in response to neutral sounds. Specifically, we hypothesized that sleep-related sounds would induce greater activity in the auditory cortex and affective sound processing areas (eg, insula and amygdala) compared to neutral sounds. Our second hypothesis was that cognitive appraisals of sleep correlate with neural activity in response to sleep-related sounds in brain areas involved in the cognitive regulation of emotions and affective processing, such as the ACC, AI, and amygdala.
Materials and Methods
Participants
We recruited 47 healthy adults (23 females; age, 37.69 ± 12.03 years [range: 24–63 years]) via an advertisement placed on the notice board at Seoul National University Hospital. The exclusion criteria were a history of serious medical or neurological illness, current medical or neurological illness, any Axis I psychiatric disorder according to the Fourth Edition of the Diagnostic and Statistical Manual of Mental Disorders, sleep disorders, shift work, borderline or antisocial personality disorder, pregnancy, and any contraindication for magnetic resonance imaging (MRI).
The Structural and Clinical Interview for DSM disorders was administered to all participants by trained psychologists. Nocturnal polysomnography was conducted to screen out common sleep disorders, such as obstructive sleep apnea or periodic limb movement disorder (sleep apnea was defined as an Apnea-Hypopnea Index > 15, and limb movement during sleep as a Periodic Limb Movement Index > 15). All participants were provided with a description of the study and instructed to voluntarily provide written informed consent before the experiment. All participants received monetary compensation for participating in the experiment. This study was approved by the Institutional Review Board of Seoul National University Hospital and conducted in accordance with the declaration of Helsinki.
Self-Reported Questionnaires of Sleep-Related Variables
All participants completed the self-reported Dysfunctional Beliefs and Attitudes about Sleep (DBAS) questionnaire.19 The DBAS was used to evaluate dysfunctional appraisals of sleep (eg, unrealistic sleep expectations, perception of a lack of control regarding sleep, faulty beliefs, etc.). The DBAS is divided into five sub-scales: consequences of insomnia (1), control and predictability of sleep (2), sleep expectations (3), causal attributions of insomnia (4), and sleep-promoting practices (5).19 We used the Korean version of the Pittsburgh Sleep Quality Index (PSQI) to measure overall sleep quality and assess the severity of sleep problems.28,29
Functional MRI (fMRI) Procedure
The design of experimental procedure is presented in Figure 1. The experiment was based on a block design, and consisted of four sleep-related sound (SS) blocks and four neutral sound (NS) blocks. The blocks were randomized, but were presented in the same order across participants as follows: SS-NS-NS-SS-SS-NS-NS-SS. Each block lasted for 20s, followed by rest periods of 13–19s. The entire task lasted for approximately 5 min.
 |
Figure 1 The design of experimental procedure. |
The SS consisted of two alarm sounds, one ticking clock sound, and one heartbeat sound were from online sources. Sounds commonly heard during bedtime were defined as SS based on discussions with two sleep medicine specialists. Alarm sounds are usually heard in bed before awakening, while heartbeat and ticking clock sounds are commonly perceived to be loud before falling asleep. The SS was validated in a previous study,22 in which > 80% of the participants reported that the SS was associated with sleep. The NS consisted of white noise. The original SS and NS were electronically manipulated using Audacity software (ver. 3.0, https://www.audacityteam.org). The pitch of the NS was between 0 and 22,000 Hz, which was the same as for the SS. All stimuli have the same root mean square power, ie, normalized perceived amplitude intensity (−20 dBfs). Thus, SS and NS also had the same mean pitch (Hz) and amplitude.
All participants were instructed to listen to sound stimuli via MR-compatible headphones. The participants were asked to focus on the sounds while staring at the fixation cross on the screen with their eyes opened. All participants reported their level of sleepiness before the MRI scan, as assessed by the Stanford Sleepiness Scale.30 MRI examination was performed in participants who reported feeling wide awake. After the MRI scan, we asked the participants whether they fell asleep.
fMRI Data Acquisition and Analyses
A 3T whole-body Tim Trio scanner (Siemens, Erlangen, Germany) with a 12-channel birdcage head coil was used to acquire the functional images, with an interleaved T2*-weighted echo-planar imaging gradient echo sequence (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, slice thickness = 4.0 mm, in-plane resolution = 3.4×3.4 mm, 33 slices, field of view = 220 mm, matrix size = 64×64). We acquired 159 functional image volumes for each participant. The structural image was also acquired using a T1-weighted, three-dimensional gradient echo pulse sequence with magnetization-prepared rapid gradient echo (repetition time = 1670 ms, echo time = 1.89 ms, TI = 900 ms, flip angle = 9°, slice thickness = 1.0 mm, in-plane resolution = 1 mm × 1 mm, field of view = 250 mm, matrix size = 256×256).
The fMRI data were preprocessed using SPM12 software (Wellcome Department of Cognitive Neurology, London, UK). The following preprocessing steps were applied to functional and structural images before statistical analyses. First, functional and structural images were visually inspected and reoriented to the anterior commissure. All functional volumes were corrected for slice timing and then realigned to the first image for motion correction. The realigned volumes were co-registered to an anatomical (T1) image. The volumes were spatially normalized to the Montreal Neurological Institute (MNI) template using a transformation matrix derived from T1 structural image segmentation (eg, gray and white matters and cerebrospinal fluid). The voxel sizes were resampled to 2 × 2 × 2 mm3. Finally, the normalized images were smoothed using a Gaussian kernel with a full width at half maximum of 6 mm. We used the Artifact Detection Tool (ART, http://www.nitrc.org/projects/artifact_detect/) to identify outlier volumes. Outliers with significant head motion in each participant were detected at > 2 mm composite motion or a larger global mean intensity (ie, the difference in global mean intensity across functional volumes > 3 SD). Participants were excluded if the outlier volumes exceeded 20% of the total volume; therefore, one participant was excluded from the final analyses.
A general linear model was used to estimate neural activation of conditions of interest (SS and NS blocks). Two regressors pertaining to the presentation of sounds (one for SS and the other for NS) were entered into the model. Head motion parameters and outliers identified from the ART were also entered in the model to control for effects of motion and outliers. The design matrix was convolved temporally with a canonical hemodynamic response function to ensure a better fit. Two contrast images (SS vs implicit baseline [0] and NS vs implicit baseline [0]) for each participant were created to compare neural activation in response to SS or NS compared to implicit baseline.
The contrast images were compared using a whole brain paired t-test to explore neural activation differences between SS and NS. In addition, whole-brain correlation analysis was conducted to investigate the relationships between neural activity in response to SS (vs NS) and sleep-related variables, such as cognitive appraisal of sleep assessed by the DBAS. Sex and age were entered into the regression model to control for sex and age. A cluster-wise correction was performed using the 3dClustSim program, and smoothing was estimated using the 3dFWHMx function of the Analysis of Functional Neuroimages (v20.03.01, https://afni.nimh.nih.gov) program, with the “acf” option selected. Cluster size was determined using Monte Carlo simulations, second nearest-neighbor clustering, and a two-sided threshold. Cluster-wise significance was evaluated based on a p-value of 0.001 and cluster sizes (k) to achieve a corrected significance threshold of p < 0.05. Brain areas were labeled based on peak z values using the automated anatomical labeling atlas (AAL) 3. The MRIcroGL online tool (NITRC: MRIcroGL: Tool/Resource Info) was used to visualize the MRI results. To conduct ROI-based correlation analyses, ROI masks of the ACC, AI and amygdala were created using the human atlas in pickatlas (https://www.nitrc.org/projects/wfu_pickatlas/) from the SPM toolbox based on the AAL. The parameter estimates were extracted from each ROI mask using the marsbar toolbox (https://imaging.mrc-cbu.cam.ac.uk/imaging/CbuImaging) and exported to SPSS software (version 24.0; SPSS Inc., Chicago, IL, USA) for statistical analysis
Pearson’s correlation analysis was performed to investigate the association between the parameter estimates extracted from the ROIs (bilateral ACC, AI, and amygdala) and sleep-related variables (DBAS and PSQI scores). All analyses were two-tailed. A P-value < 0.05 was considered significant.
Results
Demographics and Questionnaire Scores
Three participants were excluded because they failed to adhere to the experimental procedure due to a lack of understanding of the study protocol (n = 1), severe image distortion (n = 1), and excessive head motion (n = 1). Finally, 44 healthy adults (22 females; age, 37.5 ± 12.04 years) were included in the final analysis. No significant differences in age or sex were detected between the three excluded and 44 included participants.
No significant sex differences were found in the DBAS or PSQI scores. Age was positively correlated with the PSQI score (r = 0.42, p < 0.01) but not with the DBAS score. Older participants were more likely to report poor sleep quality. The DBAS score was correlated with the PSQI score (r = 0.41, p < 0.01). Participants with poor sleep quality were more likely to show maladaptive appraisals of sleep.
Comparison of Neural Responses to SS and NS
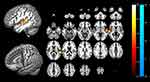
Bilateral temporal regions exhibited greater activation in response to SS than NS (left MNI 64, −24, 6; k = 1554; z = 7.16, right MNI: −52, −38, 14; k = 2163; z = 7.06) (cluster-wise-corrected p < 0.05; Table 1, Figure 2). The left ACC showed less activation following exposure to SS than to NS (MNI: 0, 40, −4; k = 101; z = 3.82) (cluster-wise-corrected p < 0.05; Table 1, Figure 2).
 |
Table 1 Brain Areas Showing Different Activation Between Sleep-Related Sound and Neutral Sound |
Associations Between Brain Activity and the DBAS Scores
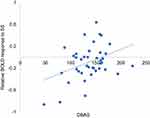
The ROI-based correlation analysis found that the higher DBAS score was correlated with increased ACC activation in response to SS compared with NS (r = 0.37, p = 0.017, Pearson’s correlation, Figure 3). However, no significant associations were detected between the DBAS score and the activity changes in response to SS (vs NS) among the other ROIs, such as the insula or amygdala. The correlation between PSQI and brain activity in response to SS (vs NS) was not associated with any brain area.
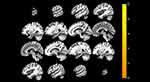
The whole-brain regression analysis indicated that higher DBAS score was significantly associated with an increased neural response in the right medial superior frontal gyrus (MNI 14, 50, 26; k = 154; z = 4.86), the medial raphe nucleus (MNI −4, −26, −28; k = 145; z = 4.58), the left precentral gyrus (MNI −48, 2, 38; k = 105; z = 4.56), the left superior frontal gyrus (MNI −22, 52, 28; k = 205; z = 4.33), and the left middle frontal gyrus (MNI −32, 28, 28; k = 314; z = 4.28) in response to SS compared with NS (cluster-wise-corrected p < 0.05; Table 2, Figure 4).
 |
Table 2 Brain Areas Where Activation to Sleep-Related Sound Was Associated with the Cognitive Appraisal of Sleep |
Additional Analyses
Pearson’s correlation analysis was conducted to investigate the relationships between each sub-scales of the DBAS and ACC activity. DBAS scales are divided into 5 sub-scales: Consequences of insomnia (1), Control and predictability of sleep (2), Sleep requirement expectations (3), Causal attributions of insomnia (4), and Sleep-promoting practices (5). Among 5 sub-scales, ACC activity was positively correlated with “Consequences of insomnia (1)” and “Causal attributions of insomnia (4)” sub-scales (r = 0.38, p < 0.05, r = 0.32, p < 0.05, respectively), but not with the other sub-scales.
Discussion
In this study, stronger activation of the temporal cortex and deactivation of the ACC were observed in response to SS than NS. In addition, cognitive appraisals of sleep were associated with activation of the ACC, prefrontal cortex, and brainstem in response to SS compared to NS. To our knowledge, this is the first study to investigate neural responses to sleep-related sounds and their association with the cognitive appraisals of sleep.
Consistent with our hypothesis, the temporal cortex activity was greater in response to SS compared to NS. The temporal cortex plays a role in interpretations of auditory information.31 Previous studies reported that the temporal cortex is involved in affective sound processing.7 In particular, the superior temporal cortex is more strongly activated in response to sounds with negative than neutral valence.10,18,32 In addition, temporal cortex highly responds to arousing sounds that can induce automatic response from listener.33,34 Thus, sleep-related sounds may also elicit temporal cortical activation; SS are often appraised as negative and arousing sounds before or during sleep. These properties of SS might be prominent compared to NS, which was white noise.
The finding that the ACC was deactivated in response to SS was unexpected. Deactivation, indicated by a negative value in BOLD response, represents decreased neural activity or increased neural inhibition.35 Deactivation of the default mode network (DMN) has been suggested to imply efficient cognitive control, suppressing endogenous activity to facilitate exogenous task-relevant processes.36,37 The ACC and medial prefrontal cortex are key DMN nodes38,39 that play a critical role in the management of self-referential cognition. Therefore, deactivation of the ACC suggests inhibitory control of self-referential processing in response to sleep-related sounds.
Furthermore, individual variations in neurological sensitivity to sleep-related sounds may be detected depending on the cognitive appraisal of sleep, even in healthy people without a sleep disorder. Dysfunctional appraisals of sleep are associated with higher neural activity in the ACC and medial frontal cortex. These findings are consistent with those of a previous study reporting hyperactivity in the prefrontal cortex and DMN in response to sleep-related stimuli,40 and with the finding that the ACC in insomnia patients has a lower gamma-aminobutyric acid level,41 which is usually related to dysfunctional sleep appraisals. Psychiatric disorders, such as bipolar disorder, major depressive disorder, and schizophrenia, commonly accompany insomnia, and are related to functional and structural abnormalities in the ACC.42–44
In contrast to ACC hyperactivity in insomnia patients, ACC deactivation in response to SS suggests that those without insomnia successfully inhibit self-referential processing in response to SS. However, dysfunctional cognitive sleep appraisals were associated with sleep-elicited “reversed activity” in the ACC and medial frontal cortex, along with stronger cortical activity in insomnia patients. With consideration of previous results from insomnia studies, the current results suggest that ACC activity could be a biomarker for the risk of insomnia. Maladaptive appraisals of sleep may modulate activity in the ACC and medial prefrontal cortex in response to SS. Increased DMN activation in response to SS may reflect impaired inhibition of self-referential cognition by SS.
More negative appraisals of sleep were associated with increased activity in the prefrontal cortex and raphe. Given that these are important brain structures in the ascending arousal system,45 dysfunctional appraisals of sleep may be related to hyperarousal of the ascending arousal system from the brainstem to the prefrontal cortex in response to SS. Thus, subjects with maladaptive appraisals of sleep are likely to show greater prefrontal activity in response to SS, as they may be more sensitive to sleep-related stimuli. The raphe nucleus, a key region in the serotonergic pathway, plays an important role in initiating and terminating sleep.46,47 Thus, hyperarousal related to serotonergic activity in response to SS may be associated with a negative appraisal of sleep.
The current study provides novel data showing that dysfunctional cognitive sleep appraisals can alter neural signals in the ACC-mPFC and limbic system in response to SS. These findings suggest that even normal sleepers can exhibit cortical arousal or impaired inhibition in response to sleep-related sounds if they have maladaptive appraisals of sleep. Thus, cognitive appraisals of sleep could be a target for interventions aiming to address sleep problems. Moreover, failure to regulate ACC-mPFC activation to sleep-related stimuli could be a neurobiological risk factor for cortical hyperactivation and insomnia. In particular, the ACC response to sleep-related stimuli could be associated with scores on two DBAS sub-scales, indicating consequences of insomnia and casual attributions of insomnia. However, we could not compare the neurobiological pathway of negative sleep cognition in normal sleepers to that in clinical patients with a sleep disturbance. Thus, follow-up studies are needed to investigate whether healthy subjects with hyperactivity to SS develop sleep disorders. These findings will contribute to the identification of risk factors and neurobiological markers for sleep disorders.
In the current study, there was no correlation between neural response to SS and subjective sleep quality. This seems counterintuitive as neural response to SS would be implicated with sleep quality. The lack of correlation between neural response to SS and subjective sleep quality may be due to the fact that all participants of the current study did not have any clinically significant sleep disorders. Study participants reported lower PSQI scores (7.78±4.29) representing good sleep quality. Based on the best cut-off point of Korea version of the PSQI29 distinguishing poor and good sleepers (8.5), 66% of study participants can be classified into those with good sleep quality. In addition, neural response was measured while listening to sleep-related sounds rather than during sleep. Therefore, neural response to SS would be more likely to be associated with cognitive appraisal of good sleeping people rather than sleep quality of sleep disorder patients.
This study had several limitations. First, we had a relatively small sample size. Second, we did not include patients with sleep disorders, such as chronic insomnia. Therefore, we cannot compare results between normal sleepers and insomnia sufferers. Future studies using larger sample sizes and clinical populations are needed to obtain a more detailed evaluation and clear neurobiological substrates for cognitive appraisals of sleep. Third, our study was cross-sectional; therefore, we could not determine whether brain activation in response to SS was associated with the subsequent development of clinically significant sleep disturbances. Fourth, SS was carefully selected by two sleep medicine specialists and validated as sleep-related sounds in participants with insomnia. Future research for validation of SS was needed in healthy population to confirm whether SS is an appropriate stimulation in healthy population. Fifth, although the white noise used as NS in the current study was matched for acoustic properties, using salient sounds unrelated to sleep or sleep-promoting sounds as NS would help us to specify the brain activation in response to SS, which interrupt sleep. However, given that sleep-unrelated salient sounds or sleep-promoting sounds may be meaningful, these sounds may cause other problems with interpreting results.
In conclusion, the current study revealed increased activation of the temporal cortex and decreased activation of the ACC in response to sleep-related sounds compared to neutral sounds. A dysfunctional appraisal of sleep was correlated with increased activation of the ACC, medial frontal cortex, prefrontal cortex, and brainstem in response to sleep-related sounds. These results suggest that negative and dysfunctional appraisals of sleep are associated with cortical hyperarousal and impaired inhibition in response to sleep-related sounds.
Acknowledgments
This research was supported by the Brain Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (No. 2016M3C7A1904338; No. 2020M3E5D9080561, No.2022R1A2C2008417), and the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HR21C0885).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bion V, Lowe AS, Puthucheary Z, Montgomery H. Reducing sound and light exposure to improve sleep on the adult intensive care unit: an inclusive narrative review. J Intensive Care Soc. 2018;19(2):138–146. doi:10.1177/1751143717740803
2. Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep. 1996;19(9):707–710. doi:10.1093/sleep/19.9.707
3. Xie H, Kang J, Mills GH. Clinical review: the impact of noise on patients’ sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care. 2009;13(2):208. doi:10.1186/cc7154
4. Griffiths TD. The neural processing of complex sounds. Ann N Y Acad Sci. 2001;930:133–142. doi:10.1111/j.1749-6632.2001.tb05729.x
5. Theunissen FE, Elie JE. Neural processing of natural sounds. Nat Rev Neurosci. 2014;15(6):355–366. doi:10.1038/nrn3731
6. Seifritz E, Esposito F, Hennel F, et al. Spatiotemporal pattern of neural processing in the human auditory cortex. Science. 2002;297(5587):1706–1708. doi:10.1126/science.1074355
7. Frühholz S, Trost W, Kotz SA. The sound of emotions-Towards a unifying neural network perspective of affective sound processing. Neurosci Biobehav Rev. 2016;68:96–110. doi:10.1016/j.neubiorev.2016.05.002
8. Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12(6):718–724. doi:10.1038/nn.2331
9. Kumar S, von Kriegstein K, Friston K, Griffiths TD. Features versus feelings: dissociable representations of the acoustic features and valence of aversive sounds. J Neurosci. 2012;32(41):14184–14192. doi:10.1523/JNEUROSCI.1759-12.2012
10. Frühholz S, Grandjean D. Multiple subregions in superior temporal cortex are differentially sensitive to vocal expressions: a quantitative meta-analysis. Neurosci Biobehav Rev. 2013;37(1):24–35. doi:10.1016/j.neubiorev.2012.11.002
11. Szameitat DP, Kreifelts B, Alter K, et al. It is not always tickling: distinct cerebral responses during perception of different laughter types. Neuroimage. 2010;53(4):1264–1271. doi:10.1016/j.neuroimage.2010.06.028
12. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi:10.1038/nrn1884
13. Bach DR, Grandjean D, Sander D, Herdener M, Strik WK, Seifritz E. The effect of appraisal level on processing of emotional prosody in meaningless speech. Neuroimage. 2008;42(2):919–927. doi:10.1016/j.neuroimage.2008.05.034
14. Ethofer T, Kreifelts B, Wiethoff S, et al. Differential influences of emotion, task, and novelty on brain regions underlying the processing of speech melody. J Cogn Neurosci. 2009;21(7):1255–1268. doi:10.1162/jocn.2009.21099
15. Bamiou DE, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev. 2003;42(2):143–154. doi:10.1016/S0165-0173(03)00172-3
16. Sander K, Scheich H. Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci. 2005;17(10):1519–1531. doi:10.1162/089892905774597227
17. Brown S, Martinez MJ, Parsons LM. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport. 2004;15(13):2033–2037. doi:10.1097/00001756-200409150-00008
18. Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002;16(3 Pt 1):746–753. doi:10.1006/nimg.2002.1115
19. Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8(3):463–467. doi:10.1037/0882-7974.8.3.463
20. Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs’ attributions. psychometric properties of the dysfunctional beliefs and attitudes about sleep scale and the sleep disturbance questionnaire. J Psychosom Res. 2000;48(2):141–148. doi:10.1016/S0022-3999(99)00090-2
21. Sandru C, Voinescu BI. The relationship between emotion regulation, dysfunctional beliefs about sleep and sleep quality-an exploratory study. J Evid Based Psychother. 2014;14(2):249.
22. Kim N, Kang SG, Lee YJ, et al. Decreased regional brain activity in response to sleep-related sounds after cognitive behavioral therapy for psychophysiological insomnia. Psychiatry Clin Neurosci. 2019;73(5):254–261. doi:10.1111/pcn.12822
23. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi:10.1016/S1364-6613(00)01483-2
24. Stevens FL, Hurley RA, Taber KH, Hurley RA, Hayman LA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci. 2011;23(2):121–125. doi:10.1176/jnp.23.2.jnp121
25. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi:10.1007/s00429-010-0262-0
26. Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi:10.1038/nrn3857
27. Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi:10.1038/sj.mp.4000812
28. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
29. Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16(3):803–812. doi:10.1007/s11325-011-0579-9
30. MacLean AW, Fekken GC, Saskin P, Knowles JB. Psychometric evaluation of the Stanford sleepiness scale. J Sleep Res. 1992;1(1):35–39. doi:10.1111/j.1365-2869.1992.tb00006.x
31. Binder JR, Frost JA, Hammeke TA, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10(5):512–528. doi:10.1093/cercor/10.5.512
32. Ethofer T, Anders S, Wiethoff S, et al. Effects of prosodic emotional intensity on activation of associative auditory cortex. Neuroreport. 2006;17(3):249–253. doi:10.1097/01.wnr.0000199466.32036.5d
33. Wiethoff S, Wildgruber D, Kreifelts B, et al. Cerebral processing of emotional prosody–influence of acoustic parameters and arousal. Neuroimage. 2008;39(2):885–893. doi:10.1016/j.neuroimage.2007.09.028
34. Concina G, Renna A, Grosso A, Sacchetti B. The auditory cortex and the emotional valence of sounds. Neurosci Biobehav Rev. 2019;98:256–264. doi:10.1016/j.neubiorev.2019.01.018
35. Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG-BOLD-CBF study in humans. Neuroimage. 2014;94:263–274. doi:10.1016/j.neuroimage.2014.02.029
36. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi:10.1016/j.tics.2012.10.008
37. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi:10.1146/annurev-neuro-071013-014030
38. Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi:10.1038/35094500
39. Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi:10.1002/hbm.20113
40. Kim SJ, Lee YJ, Kim N, et al. Exploration of changes in the brain response to sleep-related pictures after cognitive-behavioral therapy for psychophysiological insomnia. Sci Rep. 2017;7(1):12528. doi:10.1038/s41598-017-13065-0
41. Plante DT, Jensen JE, Schoerning L, Winkelman JW. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: a link to major depressive disorder? Neuropsychopharmacology. 2012;37(6):1548–1557. doi:10.1038/npp.2012.4
42. Fountoulakis KN, Giannakopoulos P, Kövari E, Bouras C. Assessing the role of cingulate cortex in bipolar disorder: neuropathological, structural and functional imaging data. Brain Res Rev. 2008;59(1):9–21. doi:10.1016/j.brainresrev.2008.04.005
43. de Kwaasteniet B, Ruhe E, Caan M, et al. Relation between structural and functional connectivity in major depressive disorder. Biol Psychiatry. 2013;74(1):40–47. doi:10.1016/j.biopsych.2012.12.024
44. Dehaene S, Artiges E, Naccache L, et al. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: the role of the anterior cingulate. Proc Natl Acad Sci U S A. 2003;100(23):13722–13727. doi:10.1073/pnas.2235214100
45. Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(18):3817. doi:10.1002/cne.22781
46. Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake–sleep states. Neuroscience. 2001;104(4):1141–1155. doi:10.1016/S0306-4522(01)00103-8
47. Monti JM. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14(5):319–327. doi:10.1016/j.smrv.2009.10.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.