Back to Journals » Infection and Drug Resistance » Volume 17
Coexisting of Primary Central Nervous System Lymphoma and Talaromyces marneffei Brain Abscess in an AIDS Patient, A Case Report and Review of the Literature
Authors Liu X , Xing H, Lin J, Sun J, Wang Y, Liu Y , Cao W, Liu Z, Li T
Received 27 July 2023
Accepted for publication 6 February 2024
Published 22 February 2024 Volume 2024:17 Pages 709—718
DOI https://doi.org/10.2147/IDR.S432697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xinchao Liu,1,* Hao Xing,2,3,* Jing Lin,1,3,* Jian Sun,4 Yu Wang,3 Yaxu Liu,5 Wei Cao,1,6 Zhengyin Liu,1 Taisheng Li1,6,7
1Department of Infectious Disease, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, People’s Republic of China; 3Graduate School, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 4Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, People’s Republic of China; 5Department of International Medical Services, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, People’s Republic of China; 6State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People’s Republic of China; 7Tsinghua-Peking Center for Life Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Taisheng Li, Email [email protected]
Background: Talaromyces marneffei is prevalent in South Asia. Latent Talaromyces marneffei infection of travellers make the diagnosis difficult. There are similarities in clinical manifestations between Talaromyces marneffei infection and lymphoma. Brain abscess is a rare form of Talaromyces marneffei infection.
Case Presentation: We reported a very rare case of a 19-year-old man with HIV infection who suffered from a brain mass and lymphadenopathy. His blood culture, bone marrow culture and sputum culture all grew Talaromyces marneffei. One month after treatment with voriconazole, the symptoms improved except brain mass. Surgical incision of the brain mass showed a compact mass, and pathological analysis showed the coexisting Talaromyces marneffei abscess and lymphoma. The patient is currently in a stable condition after receiving antifungal therapy and chemotherapy.
Conclusion: Based on a case report of a traveller who suffered from a brain mass of Talaromyces marneffei abscess and lymphoma after a visit to an endemic area, this review summarized the cases where there was confusion between lymphoma and the brain abscess of Talaromyces marneffei. Talaromyces marneffei infection can be found globally due to the increasing number of international travels. Talaromyces marneffei infection and lymphoma had similar characteristics which is easy to misdiagnose in clinic. Infection may also be accompanied by tumors, especially in patients infected with HIV. The manifestations and imaging of brain abscess of Talaromyces marneffei were not characteristic in different patients.
Keywords: Talaromyces marneffei, lymphoma, misdiagnose, HIV, travel
Background
Talaromyces marneffei is a dimorphic and opportunistic fungus that can cause multiple organ involvement in humans.1 The geographical distribution of the diseases is South China and Southeast Asia.2 Both residents and travellers are susceptible to Talaromyces marneffei. Furthermore, immunocompromised hosts, such as patients with acquired immunodeficiency syndrome (AIDS), are more prone to develop disseminated Talaromyces marneffei infection.2
The clinical features of disseminated Talaromyces marneffei infection may include fever, lymphadenopathy, hepatomegaly, splenomegaly, respiratory and gastrointestinal abnormalities, and skin lesions.3 The symptoms of Talaromyces marneffei infection are sometimes insidious and atypical, and latent Talaromyces marneffei infections do exist, both of which increase the difficulty of diagnosis. The mortality rate of Talaromyces marneffei infection can be as high as 81% if diagnosis and treatment are delayed.4 Talaromyces marneffei infection can present with multiple organ involvement; however, intracranial infection with Talaromyces marneffei has rarely been reported, accounting for 1.9% of all Talaromyces marneffei infections.5
The pathophysiology underlying the concurrence of lymphomas and opportunistic infections in HIV patients is thought to be multifactorial. On one hand, the impaired cell-mediated immunity predisposes to the proliferation of oncogenic viruses, such as Epstein-Barr virus (EBV), which is implicated in the pathogenesis of certain types of non-Hodgkin’s lymphoma (NHL). On the other hand, the same defect in immunity provides a fertile ground for opportunistic pathogens to thrive. What`s more, the treatments for lymphoma may exacerbate the risk of opportunistic infections by further suppressing immunity. Antiretroviral therapy plays a pivotal role in restoring immune function, which can mitigate the risk of both lymphoma and opportunistic infections.
Lymphoma can present with fever, enlargement of the lymph nodes and multisystem damage. Hence, clinicians have difficulty distinguishing lymphoma and Talaromyces marneffei infection due to their similar clinical characteristics. The presence of both diseases makes the illness more complex and difficult to diagnose and treat. Here, we report a rare case of a patient with AIDS who developed central nervous system lymphoma and disseminated Talaromyces marneffei infection. In addition, we systematically review the cases of the misdiagnosis of lymphoma and Talaromyces marneffei infection and the cases of brain mass of Talaromyces marneffei to improve clinical knowledge.
Case Presentation
A previously healthy 19-year-old male was admitted to our hospital with the complaint of fever and shaking of hands for a period of approximately 1 month. He was diagnosed with human immunodeficiency virus (HIV) after admission. He had moved to Beijing (north China) 10 years earlier from Yunnan (South China) and had last visited Yunnan 1 year before presentation.
Laboratory examination revealed a white blood cell count of 0.81× 109/L, a haemoglobin content of 71 g/L, and a platelet count of 35× 109/L. CD4+ T-cell counts are 2/µL. Blood culture, bone marrow culture and sputum culture all grew Talaromyces marneffei.Coronal T2-weighted MRI showed a high-signal-density mass in the left parietal lobe (Figure 1A). Chest CT examinations showed a hollow plaque in the lower left lung (Figure 1B). PET/CT scan revealed abnormal uptake in the lesions of the lung (SUVmax = 2.0), bones (SUVmax = 5.1), lymph node (SUVmax = 5.7) and brain (SUVmax = 5.2).
 |
Figure 1 (A) Contrast enhanced Magnetic resonance imaging showed the left parietal lobe mass with ring enhancement; (B) computed tomography scan shows hollow shadows in left lung field. |
The patient was started on anti-Talaromyces marneffei therapy with intravenous voriconazole (200 mg) twice a day for 1 month (Neither liposomal amphotericin B nor itraconazole were available in the hospital). One month after treatment, the size of the pulmonary hollow plaques had decreased on CT chest examinations. However, he still presented with recurrent neutropenia, and coronal T2-weighted MRI showed that the brain mass in the left parietal lobe had further enlarged.
Surgical incision of the brain mass showed a compact mass (Figure 2). Subsequently, microscopic morphology of Giemsa stain (Figure 3A) lectophenol cotton blue stain (Figure 3B) and culture in SDA (Figure 3C) of the brain mass showed the presence of Talaromyces marneffei. Furthermore, metagenomic next-generation sequencing (mNGS) of the brain mass was conducted, and Talaromyces marneffei (sequence number: 9) and EBV (sequence number: 6054) were identified. Besides the Talaromyces marneffei infection, histopathologic examination of the brain mass revealed B-cell non-Hodgkin lymphoma!Thus, the patient continued antifungal therapy with voriconazole (200 mg bid po). After the pathology results revealed lymphoma, the patient started chemotherapy with a high-dose methotrexate (5.4 g once ivgtt, 3.5g/m2) regimen. The neurological function of the patient remained stable after surgery. Furthermore, his fever, neutropenia and lymphadenopathy improved after receiving antifungal therapy and chemotherapy.
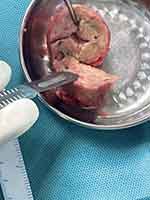 |
Figure 2 Excised brain mass during surgery. |
Discussion and Conclusion
We present a case report focusing on the misdiagnosis of Talaromyces marneffei abscess and lymphoma. This case report describes a young male patient with HIV infection presenting with fever and shaking of hands. Blood and sputum cultures revealed infection with Talaromyces marneffei. Following antifungal treatment, the pulmonary lesions improved, but the cerebral lesions worsened. Subsequent surgical resection of the brain lesion confirmed the presence of central nervous system lymphoma with concomitant Talaromyces marneffei infection, which was histopathologically verified. Ultimately, the addition of chemotherapy led to improvement in the cerebral symptoms and lesion.We performed a review of the medical literature relating to Talaromyces marneffei infection and lymphoma. We searched all articles listed in PubMed, Embase and Web of Science (until February 2023, reported in the last 15 years) using the combination of terms “marneffei” and “lymphoma”. A filter for English and Chinese language was then applied. A flow chart for the search strategy is provided in Figure S1. We included 4 published case reports.6–9 In 2 cases, the coexistence of Talaromyces marneffei and lymphoma was confirmed, while in the other 2 cases, Talaromyces marneffei infection was initially misdiagnosed as lymphoma. No case outside China was found during the literature search. Two patients were diagnosed with disseminated Talaromyces marneffei infection. Three patients received voriconazole and two patients received amphotericin B. The characteristics of the included cases reported are shown in Table 1. We performed a review of the medical literature relating to brain abscesses of Talaromyces marneffei. The article search used the PubMed, Embase and Web of Science databases (until February 2023, reported in the last 15 years) databases. The search terms were “marneffei”. and “brain”, or “central nervous system”, or “intracranial”. A filter for English and Chinese language was then applied. Finally, a flow chart for the search strategy is provided in Figure S2. We found 5 published case reports describing brain abscesses with Talaromyces marneffei in this context.10–14 No case outside China was found during the literature search. Two of the five patients were diagnosed with HIV infection. Three patients were treated with amphotericin B and itraconazole. Two patients received voriconazole. Table 2 summarizes the demographic and epidemiological characteristics of these 5 patients.
 |
Table 1 Clinical Features of the Reported Misdiagnosis Cases of Talaromyces Marneffei infection and Lymphoma |
 |
Table 2 Clinical Features of the Cases of Brain Mass of Talaromyces Marneffei Infection |
To our knowledge, this is the first report describing a brain mass combined with Talaromyces marneffei infection and lymphoma. As a travel-associated infection, Talaromyces marneffei infection is increasingly being recognized in nonendemic areas such as Japan, Australia, Belgium, France, Germany, the Netherlands, Oman, Sweden, Switzerland, Canada and the United States, among patients who have traveled to or lived in endemic regions.15–17 Talaromyces marneffei can affect not only AIDS patients but also transplant recipients,18–20 immunosuppressed patients, and those with underlying medical conditions, such as lymphoma. Contact with bamboo rats and hunting have been proposed as potential risk factors for Talaromyces marneffei infection, but there is no direct evidence.21 It is important to note that Talaromyces marneffei infection can present as a latent infection, and the disease can reactivate at any time in an immunocompromised host.22 Therefore, the clinician must take a detailed epidemiological history to identify any potential exposure to the fungus.
Disseminated Talaromyces marneffei is a potentially lethal condition that primarily affects the peripheral blood, lymph nodes, liver and spleen, bone marrow, lung, digestive tract. According to a study by Qiu et al, 11 out of 14 cases of Talaromyces marneffei infection showed lymphadenopathy, and 10 out of 14 cases presented hepatosplenomegaly.23 Due to the varying clinical patterns of Talaromyces marneffei infection, the associated symptoms are not typical and can often lead to misdiagnosis.24,25 In our case and the previously published cases, an increase in 18F-FDG uptake was detected in the affected lymph nodes, particularly when traditional imaging and culturing methods are not providing sufficient diagnostic information. In cases where Talaromyces marneffei lymphadenopathy presents with nonspecific symptoms, and both laboratory testing and imaging, as well as lymph node biopsy, fail to provide any etiological results, a missed epidemiological history may lead to a misdiagnosis of lymphoma. In febrile travelers with an unknown cause, Talaromyces marneffei infection should also be considered as a possible etiology, especially in HIV or immunocompromised patients.
The diffuse involvement of Talaromyces marneffei in the central nervous system (CNS) is a rare occurrence, and in HIV patients infected with Talaromyces marneffei in the CNS, the mortality is 81%.26,27 A study in Guangxi province, South China, involving HIV/AIDS patients, found that 10 out of 159 patients had meningitis due to Talaromyces marneffei infection, and 9 out of 10 patients had intracranial lesions.28 Another study reported that 4/10 patients had intracranial infection after discovering Talaromyces marneffei in the cerebrospinal fluid.29 In recent years, there have also been sporadic reports of Talaromyces marneffei causing intracranial occupation. The common clinical manifestations of CNS fungal infections include chronic meningitis, meningoencephalitis, abscesses, and fungal ventriculitis.30 In our case and the previously published cases included in the systematic review, patients showed neuroimaging findings in single or multiple sites, and 3 out of 6 cases demonstrated abnormal ring enhancement in contrast-enhanced brain MRI. The primary symptoms exhibited by these patients were fever and changes in mental status. Therefore, in patients presenting with symptoms of fever and neuropathy, who have either lived in or traveled to endemic regions, Talaromyces marneffei infection should be considered as a differential diagnosis.
In a study conducted in Guangdong Province (South China), approximately 9.36% of HIV-infected patients were found to develop disseminated Talaromyces marneffei infection.31 Out of these patients, 4/12 died during treatment, while 4/12 relapsed after treatment.23 In other studies, the mortality rate for disseminated Talaromyces marneffei infection was found to be 20%. This indicates that disseminated Talaromyces marneffei has a high mortality and is prone to recurrence. Therefore, early identification and treatment of this infection are of crucial importance. The effective antifungal drugs for treating disseminated Talaromyces marneffei infection are amphotericin B and voriconazole.32 In addition, oral itraconazole is recommended for patients with CD4<100 and an epidemiological history to prevent Talaromyces marneffei infection. Because amphotericin B and itraconazole were not readily available at our hospital, the voriconazole regimen was adopted.
In our case, the mNGS analysis of the resected brain tissue revealed a significant increase in EBV, while the EBV DNA in the cerebrospinal fluid tested negative. Previous studies have established a strong link between EBV and the development of lymphoma.33 The incidence of EBV infection is higher among patients with HIV-related diffuse large B-cell lymphoma as compared to the general population.34,35 In this case, it is speculated that the intracranial lymphoma is primary central nervous system lymphoma (PCNSL), while the systemic lymphadenopathy could be due to Talaromyces marneffei infection, rather than lymphoma. PCNSL is a common type of lymphoma in in individuals infected with HIV,36 and approximately 80% of HIV-associated PCNSLs are linked to EBV infection.37 The current recommended therapy for HIV-associated EBV PCNSL is the high-dose methotrexate regimen, and thus, this therapy was employed in our case.38,39 In addition, The mNGS technique played an important role in confirming the link of EBV and PCNSL, and mNGS technique in differential diagnosis are essential. In our case, the tissue mNGS of Talaromyces Marneffei could be affected by blood-borne infection, however culture of the brain tissue also grew Talaromyces Marneffei, which confirmed Talaromyces Marneffei brain abscess.
In clinical practice, dualism is often observed in AIDS, such as coinfection of different pathogens or combined infection with tumors. Previous reports have shown AIDS patients with Talaromyces marneffei combined with tuberculosis, or nontuberculous mycobacteria. In one study conducted in China, tumours complicated with Talaromyces marneffei infection accounted for 16/212 cases in HIV-infected patients.28 Due to the dualism frequently observed in AIDS, the diagnosis of etiology can be challenging. Histopathology is crucial for identifying Talaromyces marneffei when traditional culture has not yielded any results. However, it is noteworthy that histopathological examination has only been conducted in 32.34% of confirmed Talaromyces marneffei cases.24 Recent studies show that the use of mNGS has contributed to an increase in the diagnosis of Talaromyces marneffei cases.40–42 However, some lymphomas can be challenging to detect accurately, and there may have been potential for misdiagnoses and missed suspicious cases, particularly among patients infected with HIV.
PCNSL is a rare form of non-Hodgkin lymphoma confined to the brain, spinal cord, leptomeninges, or eyes, without evidence of systemic disease. In HIV-negative patients, PCNSL usually presents as a solitary lesion, predominantly affecting the immunocompetent elderly population, although cases have been reported across a wide age range.43 The pathogenesis of PCNSL in immunocompetent individuals is not fully understood, but it is thought to involve genetic mutations and aberrant responses to viral infections, such as EBV, albeit less commonly than in immunocompromised individuals.44 Talaromyces marneffei is a well-recognized opportunistic infection in HIV-positive individuals, and is also an important emerging pathogen in HIV-negative patients who are immunocompromised due to other causes, such as autoimmune diseases, cancer, or immunosuppressive therapy.45 The incidence of Talaromyces marneffei infection among HIV-negative individuals has been increasing, likely due to greater clinical awareness and improved diagnostic capabilities.
In conclusion, we have presented an exceptionally rare case of PCNSL complicated by a Talaromyces marneffei brain abscess in an HIV-infected patient. If someone has a history of travelling to endemic areas, Talaromyces marneffei infection must be considered as a differential diagnosis in cases of fever, lymphadenopathy, and multisite lesions. Physicians should consider endemic infections, including Talaromyces marneffei, when examining patients with lymphadenopathy who have a medical history of travelling to endemic regions. However, physicians should also be alert to the possibility of lymphoma, especially if the effects of anti-infection therapy are ineffective in AIDS patients with lymphadenopathy. Indeed, at times, both conditions can exist simultaneously in the same patient.
Ethics Approval and Consent to Participate
We obtained the patient’s written informed consent to publish this case report. This study was approved by Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College (Protocol No. JS-3029B)
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Xinchao Liu: Conceptualization, Methodology, Visualization, Investigation, Writing – original draft, Resources. Hao Xin: Writing – original draft. Jing Lin: Writing – review & editing, Supervision. Jian Sun: Supervision. Yu Wang: Supervision. Wei Cao: Supervision. Zhengyin Liu: Supervision. Taisheng Li: Writing – review & editing, Validation, Supervision.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by grants from National High Level Hospital Clinical Research Funding (grant number: 2022-PUMCH-A-044).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344(8915):110–113. doi:10.1016/S0140-6736(94)91287-4
2. Narayanasamy S, Vu Quoc D, Nguyen Tat T, et al. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Global Health. 2021;9(11):E1618–E1622. doi:10.1016/S2214-109X(21)00350-8
3. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17(11):E334–E343. doi:10.1016/S1473-3099(17)30303-1
4. Le T, Chi NH, Cuc NTK, et al. AIDS-associated penicillium marneffei infection of the central nervous system. Clinl Infect Dis. 2010;51(12):1458–1462. doi:10.1086/657400
5. Zhou Y, Liu Y, Wen Y. Gastrointestinal manifestations of Talaromyces marneffei infection in an HIV-infected patient rapidly verified by metagenomic next-generation sequencing: a case report. BMC Infect Dis. 2021;21(1):1. doi:10.1186/s12879-021-06063-1
6. Yang Z, Zeng W, Qiu Y, Liu G, Zhang J. Nodular sclerosing Hodgkin lymphoma combined with disseminated talaromyces marneffei infection: a case report. Infect Drug Resist. 2021;14:5671–5678. doi:10.2147/IDR.S340192
7. Wanzhen L, Weihua P, Xuefei HU, Aidi SUN, Yang YU, Jianchun LI. AIDS combinated with penicilliosis marneffei and malignant lymphoma: the first case report. Chin J Nosocomiol. 2008;18(7):1038–1040.
8. Chen D, Chang C, Chen M, et al. Unusual disseminated Talaromyces marneffei infection mimicking lymphoma in a non-immunosuppressed patient in East China: a case report and review of the literature. BMC Infect Dis. 2020;20(1):1. doi:10.1186/s12879-020-05526-1
9. Cheng Y, Li WM, Huang JJ, Wang SX, Shao D. 18F-FDG PET/CT image analysis of penicilliosis marneffei in an immunocompetent child patient. Clin Nucl Med. 2022;47(4):e358–e359. doi:10.1097/RLU.0000000000004075
10. Zhu X, Liu X, Cao Q, et al. Multiple intracranial space-occupying lesions caused by Talaromyces marneffei infection in an AIDS patient returning from Cambodia. Travel Med Infectious Dis. 2022;3:50.
11. Lei M, Yu U, Zhang N, Deng J. An HIV-negative infant with systemic Talaromyces marneffei infection. Inter J Infect Dis. 2018;77:3–4. doi:10.1016/j.ijid.2018.06.003
12. Wang P, Chen Y, Xu H, et al. Acute disseminated talaromyces marneffei in an immunocompetent patient. Mycopathologia. 2017;182(7–8):751–754. doi:10.1007/s11046-017-0127-7
13. Zhu Y-M, Ai J-W, Xu B, et al. Rapid and precise diagnosis of disseminated T. marneffei infection assisted by high-throughput sequencing of multifarious specimens in a HIV-negative patient: a case report. BMC Infect Dis. 2018;18(1):18. doi:10.1186/s12879-017-2906-7
14. Xie S, Zhang L, Huang D, et al. Case Report: A case of intracranial space-occupying lesion caused by infection of AIDS-associated Talaromyces marnefei. Curr Med Imaging. 2022;3:4.
15. Yoshimura Y, Sakamoto Y, Lee K, Amano Y, Tachikawa N. Penicillium marneffei infection with beta-d-glucan elevation: a case report and literature review. Internal Medicine. 2016;55(17):2503–2506. doi:10.2169/internalmedicine.55.6173
16. Hatakeyama S, Yamashita T, Sakai T, Kamei K. Case report: disseminated talaromyces (Penicillium) marneffei and mycobacterium tuberculosis coinfection in a Japanese patient with acquired immunodeficiency syndrome. Am. J. Trop. Med. Hyg. 2017;97(1):38–41. doi:10.4269/ajtmh.16-1004
17. Waters M, Beliavsky A, Gough K. Talaromyces marneffei fungemia after travel to China in a Canadian patient with AIDS. Can. Med. Assoc. J. 2020;192(4):E92–E95. doi:10.1503/cmaj.191136
18. Gupta P, Kaur H, Kenwar DB, Gupta P, Agnihotri S, Rudramurthy SM. First case of subcutaneous infection by Talaromyces marneffei in a renal transplant recipient from India and review of literature. Journal De Mycologie Medicale. 2022;32:1.
19. Hart J, Dyer JR, Clark BM, McLellan DG, Perera S, Ferrari P. Travel-related disseminated Penicillium marneffei infection in a renal transplant patient. Transplant Infect Dis. 2012;14(4):434–439. doi:10.1111/j.1399-3062.2011.00700.x
20. Stathakis A, Lim KP, Boan P, Lavender M, Wrobel J, Musk M. Heath CH: penicillium marneffei infection in a lung transplant recipient. Transplant Infect Dis. 2015;17(3):429–434. doi:10.1111/tid.12377
21. Wang S, Chen Y, Wang D, et al. The feasibility of metagenomic next-generation sequencing to identify pathogens causing tuberculous meningitis in cerebrospinal fluid. Front Microbiol. 2019;10:10. doi:10.3389/fmicb.2019.00010
22. Chastain DB, Henao-Martinez AF, Franco-Paredes C. Opportunistic invasive mycoses in AIDS: cryptococcosis, histoplasmosis, coccidiodomycosis, and talaromycosis. Curr Infect Dis Rep. 2017;19(10):10. doi:10.1007/s11908-017-0592-7
23. Qiu Y, Zhang J, Liu G, et al. Retrospective analysis of 14 cases of disseminated Penicillium marneffei infection with osteolytic lesions. BMC Infect Dis. 2015;15:15. doi:10.1186/s12879-014-0739-1
24. He L, Mei X, Lu S, et al. Talaromyces marneffei infection in non-HIV-infected patients in mainland China. Mycoses. 2021;64(10):1170–1176. doi:10.1111/myc.13295
25. Sethuraman N, Thirunarayan MA, Gopalakrishnan R, Rudramurthy S, Ramasubramanian V, Parameswaran A. Talaromyces marneffeiOutside endemic areas in India: an emerging infection with atypical clinical presentations and review of published Reports from India. Mycopathologia. 2020;185(5):893–904. doi:10.1007/s11046-019-00420-0
26. Gao Y, Qu M, Song C, Yin L, Zhang M. Cerebral vasculitis caused by Talaromyces marneffei and Aspergillus Niger in a HIV-positive patient: a case report and literature review. J Neurovirol. 2022;28(2):274–280. doi:10.1007/s13365-021-01032-5
27. Wang D-M, Ma H-L, Tan M-Q, Wu Y-M, Wang S-N. Next-generation sequencing confirmed the diagnosis of isolated central nervous system infection caused by Talaromyces marneffei in an immunocompetent patient. Chinese Med J. 2020;133(3):374–376. doi:10.1097/CM9.0000000000000593
28. Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect. 2019;25(2):233–241. doi:10.1016/j.cmi.2018.04.018
29. Li -Y-Y, Dong R-J, Shrestha S, et al. AIDS-associated Talaromyces marneffei central nervous system infection in patients of southwestern China. AIDS Res Ther. 2020;17(1):1. doi:10.1186/s12981-020-00281-4
30. Gavito-Higuera J, Mullins CB, Ramos-Duran L, Olivas Chacon CI, Hakim N, Palacios E. Fungal infections of the central nervous system: a pictorial review. J Clin Imaging Sci. 2016;6:24. doi:10.4103/2156-7514.184244
31. Wang YF, Xu HF, Han ZG, et al. Serological surveillance for Penicillium marneffei infection in HIV-infected patients during 2004–2011 in Guangzhou, China. Clin Microbiol Infect. 2015;21(5):484–489. doi:10.1016/j.cmi.2014.12.014
32. Ouyang Y, Cai S, Liang H, Cao C. Administration of voriconazole in disseminated talaromyces (Penicillium) marneffei infection: a retrospective study. Mycopathologia. 2017;182(5–6):569–575. doi:10.1007/s11046-016-0107-3
33. Rosemarie Q, Sugden B. Epstein-Barr virus: how its lytic phase contributes to oncogenesis. Microorganisms. 2020;8:11. doi:10.3390/microorganisms8111824
34. Arvey A, Ojesina AI, Pedamallu CS, et al. The tumor virus landscape of AIDS-related lymphomas. Blood. 2015;125(20):E14–E22. doi:10.1182/blood-2014-11-599951
35. Chao C, Silverberg MJ, Martinez-Maza O, et al. Epstein-Barr virus infection and expression of b-cell oncogenic markers in HIV-related diffuse large B-cell lymphoma. Clin Cancer Res. 2012;18(17):4702–4712. doi:10.1158/1078-0432.CCR-11-3169
36. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. doi:10.1002/ijc.23487
37. Kaulen LD, Galluzzo D, Hui P, et al. Prognostic markers for immunodeficiency-associated primary central nervous system lymphoma. J Neuro-oncol. 2019;144(1):107–115. doi:10.1007/s11060-019-03208-w
38. Han CH, Batchelor TT. Diagnosis and Management Of Primary Central Nervous System Lymphoma. Cancer. 2017;123(22):4314–4324. doi:10.1002/cncr.30965
39. Miralles P, Tomas Navarro J, Berenguer J, et al. Recommendations of GeSIDA/PETHEMA on the diagnosis and treatment of lymphomas in patients infected by the human immunodeficiency virus. Med Clin. 2018;151(1):39.
40. Liu L, Sun B, Ying W, et al. Rapid diagnosis of Talaromyces marneffei infection by metagenomic next-generation sequencing technology in a Chinese cohort of inborn errors of immunity. Front Cell Infect Microbiol. 2022;2:12.
41. Peng L, Shi Y-B, Zheng L, Hu L-Q, Weng X-B. Clinical features of patients with talaromycosis marneffei and microbiological characteristics of the causative strains. J Clin Lab Analysis. 2022;36(11):11. doi:10.1002/jcla.24737
42. Xing S, Zhang H, Qiu Y, Pan M, Zeng W, Zhang J. Clinical characteristics of transplant recipients infected with talaromyces marneffei: 2 case reports and a literature review. Infect Drug Resist. 2022;15:2879–2890. doi:10.2147/IDR.S363362
43. O’Neill BP, Decker PA. Primary central nervous system lymphoma: an update. J clin oncol. 2016;34(14):1588–1595.
44. Küppers R, Engert A, Hansmann M-L. Hodgkin lymphoma. J Clin Investig. 2012;122(10):3439–3447. doi:10.1172/JCI61245
45. Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1–2):57–67. doi:10.1007/s11046-012-9577-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

