Back to Journals » Drug Design, Development and Therapy » Volume 17
Coenzyme Q10 Stimulate Reproductive Vatality
Authors Nie X , Dong X, Hu Y , Xu F, Hu C , Shu C
Received 25 April 2023
Accepted for publication 15 August 2023
Published 30 August 2023 Volume 2023:17 Pages 2623—2637
DOI https://doi.org/10.2147/DDDT.S386974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Xinyu Nie,1,2 Xinru Dong,1,2 Yuge Hu,1,2 Fangjun Xu,1 Cong Hu,2 Chang Shu1
1Obstetrics and Gynecology Center, First Hospital of Jilin University, Changchun, Jilin, People’s Republic of China; 2Reproductive Medicine Center, Prenatal Diagnosis Center, First Hospital of Jilin University, Changchun, Jilin, People’s Republic of China
Correspondence: Chang Shu, Email [email protected]
Abstract: Female infertility and pregnancy maintenance are associate with various factors, including quantity and quality of oocytes, genital inflammation, endometriosis, and other diseases. Women are even diagnosed as unexplained infertility or unexplained recurrent spontaneous abortion when failed to achieve pregnancy with current treatment, which are urgent clinical issues need to be addressed. Coenzyme Q10 (CoQ10) is a lipid-soluble electron carrier in the mitochondrial electron transport chain. It is not only essential for the mitochondria to produce energy, but also function as an antioxidant to maintain redox homeostasis in the body. Recently, the capacity of CoQ10 to reduce oxidative stress (OS), enhance mitochondrial activity, regulate gene expression and inhibit inflammatory responses, has been discovered as a novel adjuvant in male reproductive performance enhancing in both animal and human studies. Furthermore, CoQ10 is also proved to regulate immune balance, antioxidant, promote glucose and lipid metabolism. These properties will bring highlight for ovarian dysfunction reversing, ovulation ameliorating, oocyte maturation/fertilization promoting, and embryonic development optimizing. In this review, we systematically discuss the pleiotropic effects of CoQ10 in female reproductive disorders to investigate the mechanism and therapeutic potential to provide a reference in subsequent studies.
Keywords: coenzyme Q10, oxidative stress, reactive oxygen species, reproductive, oocyte, endometriosis
Introduction
Coenzyme Q10 (CoQ10) is abundant in biological membranes and mitochondria and is synthesized in all human cells.1 It is the only vitamin-like compound that can be completely synthesized internally and the only lipid antioxidant that can be synthesized autologously.2 CoQ10 has three different redox states: the oxidized form ubiquinone (CoQ), semi-oxidized (or semi-reduced) form semiquinone (CoQH), and reduced form ubiquinol (CoQH2).3 CoQ10 has been shown to reduce oxidative stress (OS) and participate in energy metabolism, gene regulation, anti-apoptotic, and anti-inflammatory reactions.4–8 Any deficiency of regulatory factors, raw materials, and enzymes in the synthesis process may affect energy production and oxidative phosphorylation in the mitochondria and aggravating oxidative stress.9 The lipid-soluble nature of CoQ10 allows it to move freely in the inner mitochondrial membrane, and its different redox states make it possible to participate in redox reactions and adenosine triphosphate (ATP) production processes by transferring protons and electrons.
The process of ATP production coupling during electron transfer in the mitochondrial electron transport chain (ETC) is defined as oxidative phosphorylation.10 Energy production depends on the normal operation of the ETC in mitochondria, and CoQ10 plays a central role in the ETC. The ETC is composed of four protein complexes, together with ATP synthase to produce ATP. Complex I (NADH: ubiquinone oxidoreductase) and Complex II (succinate dehydrogenase complex) transfer the obtained electrons to CoQ, which is reduced to CoQH2 after receiving one electron. Complex III (the cytochrome c reductase complex) receives electrons from CoQH2, which is oxidized to CoQ in a process known as the Q-cycle. Complex IV (cytochrome C oxidase complex) transfers electrons to oxygen, which is then reduced to H2O as the final electron acceptor. During electron transfer, protons are transferred to the mitochondrial membrane gap, forming an electrochemical proton gradient. ATP Synthase uses the energy of proton gradient across the membrane formed in the process of electron transfer to drive the synthesis of ATP from adenosine diphosphate (ADP) and inorganic phosphorus (Pi)10–12 (Figure 1). CoQ10 also exerts its unique function in biological membranes outside the mitochondria. Ferroptosis suppressor protein 1 (FSP1) is an effective ferroptosis resistance factor that is recruited to the plasma membrane and acts as an oxidoreductase to reduce oxidized CoQ10.13 CoQ10 relies on its anti-lipid oxidation effects to protect the integrity of cell membranes and various organelle membranes, thereby maintaining normal cell morphology.
CoQ10 is mainly self-synthesized in the human body, while a small portion comes from exogenous supplements.16 Reactive oxygen species (ROS) are byproducts of aerobic respiration in mitochondria and play an important role in maintaining cell growth, metabolism, and other physiological phenomena.17,18 Most free radicals are ROS, including superoxide anions (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (HO•).19 However, when the amount of ROS exceeds the scavenging capacity of the endogenous antioxidant system, the balance of the redox system is disrupted, ROS accumulates in the body, and OS occurs. Theoretically, anti-oxidant function of CoQ10 may reduce the ROS releasing. During the process of electron transfer from complex I to CoQ10, CoQH is formed transiently after the first electron is transferred. CoQH then acts as an electron delivery body to combine with oxygen to generate superoxide, a process called electron leakage.20 OS can break the structure of the biofilm, resulting in protein, lipid, and DNA peroxidation, which further causes mitochondrial dysfunction and apoptosis.21 Furthermore, at least 10 genes are necessary for CoQ10 synthesis these crucial genes mutation may also affect this loop to result in CoQ10 deficits.14 CoQ10 is synthesized in the mitochondria and then transported to cell membrane.22 Recently, two unidentified UbiB proteins have been found to regulate CoQ10 distribution, however the exact mechanism for the regulation of CoQ10 transport and distribution is remains unclear.23 The dual role of CoQ10 as a key component of the mitochondrial ETC on the one hand, and its antioxidant effect to reduce OS damage on the other hand.
Oocytes are the most abundant mitochondria-containing cells, and mitochondria are the organelles with the largest proportion in oocytes.24 Oocyte maturation, fertilization, and embryonic development require high amounts of ATP produced by mitochondria.25 In the ovaries, OS is a key factor that induces apoptosis of oocytes and granulosa cells (GCs), and the depletion of oocytes leads to a decline in fertility.26,27 High inflammatory responses are equally responsible for mediating the occurrence of apoptosis in GCs.28 The homeostasis at the fetal-maternal interface requires many factors to be maintained together, however, endometritis can disrupt this homeostasis and affect embryo implantation.29,30 Mitochondrial dysfunction leads to an inadequate supply of ATP, which affects oocyte maturation, fertilization and embryo development.25 Animal and clinical studies have shown that CoQ10 reduces ROS levels in oocytes, inhibits apoptosis, and enhances mitochondrial function, thereby improving the ovarian reserve.31,32 The beneficial effects of CoQ10 on oxidative stress have been supported by many studies; however, the underlying mechanisms remain to be explored.33–35
In this review, we summarize the therapeutic effects of CoQ10 on various disorders of the female reproductive system and outline a proposed agenda for future research on CoQ10 with respect to female infertility.
CoQ10 and Ovulation Disorders
CoQ10 Enhances Quantity and Quality of Oocytes
The average childbearing age of women worldwide is gradually increasing due to the influence of social and educational factors. Starting at the age of 35, follicles presenting in the ovary declined rapidly, leading to a decrease in female fertility.36 Although assisted reproductive technology has developed fast, ameliorate quality and quantity of oocytes remains a significant challenge. Ovarian dysfunction are mostly attribute to OS, telomere shortening, inflammatory reaction, mitochondrial dysfunction and apoptosis,37–39 especially OS.40–42
CoQ10 has been widely used in disease treatment because of its numerous biological functions, particularly its powerful antioxidant effects.43–47 In recent years, CoQ10, as an antioxidant, has also been discovered to play important roles in the male reproductive system.48 A case-control study showed that CoQ10 not only improved sperm parameters, but also reduced testicular OS and sperm DNA fragmentation in infertile patients with idiopathic oligospermia.35 Due to the fertility-enhancing effects of antioxidants in men, their effects on the female reproductive system have been explored in animal experiments and clinical trials.
CoQ10 Promotes Oocytes Maturation
The maturation process of oocytes can be categorized into three stages, germinal vesicle (GV), metaphase I (MI), and metaphase II (MII), depending on whether meiosis is initiated and the first polar body is extruded.49 Coenzyme Q6 (COQ6) and polyprenyldiphosphate synthase subunit 2 (PDSS2) are enzymes responsible for CoQ10. It was found that the expression of COQ6 and PDSS2 was significantly decreased in GV stage oocytes obtained from women over 39 years of age compared to those from women under 31 years of age.50 The same phenomenon was observed in 12 months old mice compared with 3 months young mice.51 The study also found that respiratory mitochondrial pools, mitochondrial ROS, and ATP were significantly reduced in oocytes from PDSS2 gene knockout mice, meanwhile meiotic spindle abnormalities were increased, and the ovarian reserve was severely impaired. However, mitochondrial membrane potential, ROS levels, ATP production, and spindles moved in a favorable direction with CoQ10 administration in PDSS2-deficient mice, although the respiratory mitochondrial pool remained unchanged.51 Thus, CoQ10 supplementation may improve oocyte quality by reducing ROS levels and enhancing mitochondrial function. Ben-Meir et al52 also found that GCs counts surrounding the oocytes in aging mice and women decreased, accompanied by COQ6 and PDSS2 expression reduced. It is well documented that GCs provide the growth factors and metabolites that support oocyte growth. CoQ10 supplementation not only decreased GC apoptosis, but also increased GCs cell counts. Oocyte quality could be compromised by decreased CoQ10 and GCs activity. At the same time, CoQ10 treatment may uptake glucose absorption and mitochondrial function was restored.53 Furthermore, in women aged 38–46 years, supplementation with 50 μmol/L CoQ10 significantly increased the oocyte maturation rate (82.6% vs 63.0%; P=0.035) and decreased the percentage of oocyte aneuploidy (36.8% vs 65.5%; P=0.020) and chromosome aneuploidy frequency (4.1% vs 7.0%; P=0.012) in vitro.54 CoQ10 may promote oocyte maturation through gene regulation and energy metabolism in oocytes and GCs, thereby improving oocyte maturation.
CoQ10 Resists Oocytes Aging
The probability of aneuploidy errors in oocytes increases with age and is inextricably linked to mitochondrial dysfunction caused by OS.55,56 Aging affects CoQ10 levels in female oocytes to varying degrees, impairing oocyte quality, and possibly leading to infertility. The number of CoQ10-synthesising enzymes declines with age. CoQ10 enhanced ovarian reserves in animal models of ovarian failure via gene regulation. As documented that, proliferating cell nuclear antigen (PCNA) is required for DNA repair and replication in cells and plays a significant function in the regulation of follicle development.57,58 Follicle-stimulating hormone (FSH) is a marker used to assess the ovarian functional reserve, and its receptor, follicle-stimulating hormone receptor (FSHR), is mainly expressed on the surface of GCs. FSH stimulation of pre-ovulatory follicles promotes the development and proliferation of GCs.59 Both anti-Müllerian hormone (AMH) and FSH are utilized to speculate ovarian function, whereas AMH is the preferred indicator produced by GCs for assessing ovarian functional reserve.60 In a mouse model of ovarian failure induced by cyclophosphamide (CTX), CoQ10 treatment significantly improved the increased level of ROS and the number of atresia follicles, while PCNA and FSHR mRNA expression increased.61 Cisplatin may cause oxidative stress-related ovarian damage; however, increased serum AMH levels with reduced follicular atresia were observed in cisplatin-treated mice supplemented with CoQ10.62 CoQ10 also stimulates the differentiation of ovarian stem cells derived from ovarian epithelial cells. In a mouse model of ovarian failure, it greatly boosted the number of primordial and antral follicles, markedly improved ovarian function and oocyte quality, decreased ROS levels, and increased AMH levels.63 In addition, bone morphogenetic protein 15 (Bmp-15), growth differentiation factor 9 (Gdf-9) and KIT proto-oncogene, receptor tyrosine kinase (c-Kit), the representative regulators of follicle development and oocyte mass expression are also upregulated by CoQ10 treatment.63–65 These results demonstrate that CoQ10 enhances ovarian function in mouse models of ovarian failure by altering hormone levels, follicle counts, and OS.
RNA sequencing analysis of MII oocytes from older women over 40 years of age and younger women under 30 years of age identified 2807 differentially expressed genes identified. Seven hub targets that bind closely to CoQ10, namely Peroxisome (PPARA), Catalase (CAT), Mitogen-activated protein kinase 14 (MAPK14), Sequestosome-1 (SQSTM1), Heme oxygenase 1 (HMOX1), Growth factor receptor-bound protein 2 (GRB2), and Glutathione reductase (GSR) were finally determined by the method of bioelectricity analysis and network pharmacology. They play a vital role in preventing oocyte aging via CoQ10.66 Under in vitro maturation conditions, PPARA was confirmed to prevent porcine oocytes from aging by bezafibrate (PPARA agonist), and CoQ10 and its derivatives have been found to be direct partial agonists of PPARA.67,68 CoQ10 has also been shown to improve the viability of preantral follicles by increasing CAT activity and protecting the ovarian reserve by inhibiting OS.69 At present, there is still a lack of experiments proving how CoQ10 affects ovarian aging through the remaining five key genes; however, they have been shown to support oocytes.27,66,70,71 Thus, CoQ10 may protect the ovarian reserve by regulating the expression of genes associated with oocyte aging.
Follicular fluid is a microenvironment necessary for the growth and development of follicles at all stages. CoQ10 appears to improve the quality of oocytes, especially in women over 35 years old, by improving the oxidative metabolism of follicular fluid.72,73 In addition to reducing OS, CoQ10 restores the function of senescent oocytes by alleviating mitochondrial dysfunction. A clinical study found that the activity of the CoQ10-dependent mitochondrial respiratory chain in oocytes decreased with age, and this decrease in activity was reduced when CoQ10 was supplemented in vitro.74 Although the ability of CoQ10 to prevent telomere attrition and reduce cardiovascular mortality has been demonstrated,75 there is still lack of clinical application standards for ovarian aging reverse using of CoQ10. Research focusing on the use of CoQ10 to reverse ovarian aging is summarized in Table 1.
 |
Table 1 Summary of Studies on the Effect of CoQ10 on Ovarian Aging |
CoQ10 Improves Metabolic Disorders in Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS), a common reproductive endocrine condition, is characterized by hyperandrogenism, absence of or oligoovulation, and polycystic ovarian abnormalities, often accompanied by obesity and insulin resistance, which affect women’s reproductive function, metabolic status, and psychological well-being.76 Currently, the pathogenesis of the disease is not fully understood; however, hyperandrogenism and insulin resistance are considered the main causes of PCOS.77 Additionally, OS is an important component in the pathophysiology of PCOS.78 The interaction between hyperandrogenism, insulin resistance, and oxidative stress leads to a vicious cycle that continuously exacerbates PCOS.79 Although oxidative stress in PCOS pathogenesis and antioxidants effects in PCOS therapeutic have received widespread attention. Benefit of CoQ10 treatment on oocyte quality in PCOS patients is still obscure.
CoQ10 Ameliorates Lipid Metabolism
More than 60% PCOS patients suffer from overweight or obese.80 Overweight or obese females have inferior antioxidant capacity and CoQ10 concentration is lower in the follicular fluid than healthy control.81 High oxidative stress in an obese hyperglycemic environment may disrupt oocyte metabolism and impede mitochondrial function. More perinuclear mitochondrial distribution is usually observed in high-quality oocytes. The percentage of oocytes with perinuclear mitochondria was significantly lower in obese mice fed a high-fat and high-sugar (HF/HS) diet than in healthy controls; CoQ10 supplementation surprisingly prevented this distribution abnormality.82–84 Meanwhile, obese women showed more oocyte spindles and chromosomal abnormalities. The oocytes of obese mice with CoQ10 supplemented had a higher proportion of normal spindles and chromosomes, up to 90.2%, which was significantly higher than that of mice receiving a vehicle-injected (72.2%).84 These results indicate that CoQ10 supplementation can partially prevent and rescue mitochondrial defects in oocytes induced by obesity in mouse models.
The sirtuin 1 (SIRT1) protein is involved in the regulation of mitochondrial function, energy metabolism, insulin secretion and cellular senescence. The expression of SIRT1 was significantly downregulated in the ovarian tissues of PCOS rats, whereas the low expression of SIRT1 in insulin-sensitive tissues may reflect the impaired adjustment of mitochondrial function related to human insulin resistance.85,86 CoQ10 can prevent the reduction in SIRT1 protein levels in oocytes after ovulation, which may provide benefits to PCOS treatment by regulating the expression of SIRT1.31 Clinical trials have observed the effect of CoQ10 on overweight or obese patients with PCOS, but the mitochondrial function of oocytes was not involved. Based on these findings, by controlling mitochondrial metabolism in oocytes, CoQ10 may reduce reproductive dysfunction caused by aberrant lipid metabolism in patients with PCOS.
Thus, increased adiposity may be associated with aseptic inflammation. Therefore, PCOS is closely associated with chronic systemic inflammation, and inflammatory cytokines produced by circulating adipose or intestinal monocytes directly regulate insulin resistance and androgen production.87,88 Taking CoQ10 (200 mg/day) continuously for 8 weeks can reduce inflammatory factors, such as tumor necrosis factor-a (TNF-α), high sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6), and significantly decrease the endothelial dysfunction markers of vascular cell adhesion molecule-1 (VCAM-1) and E-selectin.89 Another study found that CoQ10 administration reduced the gene expression of interleukin-1 (IL-1) and interleukin-8 (IL-8) in the peripheral blood mononuclear cells of patients with PCOS. The study also showed that the intake of CoQ10 down-regulated the gene expression of oxidized low density lipoprotein receptor 1 (LDLR1) and up-regulated the gene expression of peroxisome proliferator-activated receptor γ (PPARγ) in peripheral blood mononuclear cells of PCOS patients compared with placebo group.90 Both LDLR and PPARγ are involved in lipid metabolism.91,92 CoQ10 inhibits cyclic adenosine monophosphate (cAMP) degrading enzyme gene expression through CaMKII-MEK1/2-ERK1/2 signaling pathway, which increases cAMP and activates AMPK signaling pathway, then induce PPARα expression and inhibit adipogenesis.93–95 Vascular endothelial dysfunction is induced by oxidative low-density lipoprotein (Ox-LDL), and CoQH2 protects LDL from peroxidation by peroxyl radicals.15,96 Additionally, it reduced Ox-LDL-mediated ROS production via the AMPK signaling pathway.97 In conclusion, CoQ10 is beneficial to PCOS patients through lipid metabolism, the underlying mechanism includes regulating gene expression, altering intracellular microstructure, and inhibiting the release of inflammatory factors.
CoQ10 Accelerates Glucose Metabolism
As an antioxidant, CoQ10 may reduce androgen levels and affect glucose metabolism in patients with PCOS by reducing OS. Some researchers have found that oxidative stress can promote hyperandrogenism by reducing sex hormones binding globulin.98 CoQ10 supplementation demonstrated favorable effects on serum fasting blood glucose levels (FBG), total testosterone levels, and the homeostasis model of assessment-IR (HOMA-IR), regardless of whether vitamin E was also used.99 A network meta-analysis of the effects of several oral nutritional agents on PCOS confirmed that CoQ10 significantly reduced FBG and HOMA-IR, although its effect was less pronounced than that of inositol.100
Excessively oxidatively active molecules cause lipid peroxidation, which affect absorption of glucose in fat and muscle and decrease the amount of insulin that pancreatic β-cells secrete. High glucose levels promote the formation of ROS.101,102
Currently, the potential mechanism underlying the effect of CoQ10 on glucose metabolism remains unclear. Currently, it is speculated that it may be related to the antioxidant effect of CoQ10, which not only reduces the influence of OS on insulin secretion, but also inhibits glucose-stimulated insulin release from pancreatic islet cel ls through interleukin-1β (IL-1β) blocking.102,103 In addition, increasing inflammatory cytokines cause insulin resistance by inhibiting the signal transduction of insulin, and the inflammation in PCOS patients increases their risk of developing atherosclerosis and infertility. Therefore, CoQ10 is considered an effective treatment for the prevention of PCOS complications.90 Clomiphene is widely used for ovulation promoting in PCOS. In a prospective randomized controlled trial, CoQ10 combined with clomiphene citrate is identified as an effective treatment for clomiphene citrate-resistant PCOS. Compared to clomiphene alone, patients in the treatment group combined with CoQ10 had increased endometrial thickness and improved ovulation and clinical pregnancy rates, which may be due to a combination of multiple effects of CoQ10, associated with the reduction of OS and inhibition of lipid peroxidation in ovaries by CoQ10.104 CoQ10 may be an important adjuvant in the treatment of PCOS by preventing obesity-induced mitochondrial defects, reducing inflammation and OS damage, lowering androgen levels, and reducing insulin resistance. We summarized the trials related to CoQ10 for the treatment of PCOS (Table 2). In general, CoQ10 may delight hope for a successful pregnancy in women with ovulation disorders through various mechanisms (Figure 2).
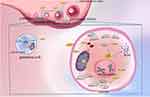 |
Figure 2 Mechanism of Coenzyme Q10 (CoQ10) improving ovulation disorders. CoQ10 has the ability to promote ovarian stem cells differentiation and support function of oocytes and granulosa cells.63 Polyprenyl diphosphate synthase subunit 2 (PDSS2) and Coenzyme Q6 (COQ6) are key genes for the synthesis of CoQ10.50 In oocytes, CoQ10 enhances mitochondrial function, promotes adenosine triphosphate (ATP) production, reduces reactive oxygen species (ROS) production and oxidative stress (OS), and decreases the rate of chromosomal aneuploidy.6,84 In addition, it stimulates the expression of growth differentiation factor 9 (Gdf-9), bone morphogenetic protein 15 (Bmp-15), KIT proto-oncogene, receptor tyrosine kinase (c-Kit) and Catalas (CAT) genes related to oocyte quality and regulates the expression of low-density lipoprotein receptor 1(LDLR1), Peroxisome (PPARA) and sirtuin 1(SIRT1) genes related to metabolism.64–66,85,90 In granulosa cells, CoQ10 promotes follicle-stimulating hormone receptor (FSHR), anti-müllerian hormone (AMH) and proliferating cell nuclear antigen (PCNA) expression and increases cellular uptake of glucose.61,63 Through the above possible mechanisms eventually enhance the quality of oocytes and granulosa cells, increase the number of oocytes, which is beneficial to the functional reserve of the ovary. |
 |
Table 2 Summary of Studies on the Effect of CoQ10 on PCOS |
CoQ10 and Endometriosis
Endometriosis is an estrogen-dependent chronic inflammatory gynecological disease that affects fertility in women of reproductive age. The disease mechanism is complex, and oxidative stress is thought to be a factor in endometriosis-related infertility.105,106 Endometriosis is characterized by the presence of endometrial tissue outside the uterus. CoQ10 has been reported to improve intra-abdominal adhesions in endometriotic implants and has an impact on TNF-α and mast cells in animal studies.107 The electron transfer process of CoQ10 involves ROS generation. Mitochondrial membrane complex I (MMC-I), also known as NADH: ubiquinone oxidoreductase, catalyzes the transfer electrons from NADH to CoQ10. However, this action may involve electron leakage, resulting in the generation of ROS. Patients with endometriosis show a relative increase in MMC-I, and genetic changes in MMC-I may be a hereditary risk factor for endometriosis.108 Theoretically, MMC-I abnormalities affect the normal circulation of CoQ10. CoQ10 may be used to treat endometriosis owing to its anti-inflammatory and antioxidant effects. Because there are few studies in this field, the effectiveness of CoQ10 in the treatment of endometriosis remains unclear.
CoQ10 and Female Genital Inflammation
Female genital inflammation including tubal infections, endometritis, pelvic inflammatory disease, and vaginitis, can increase the risk of infertility.109 OS is a trigger for inflammation, which can lead to vascular endothelial dysfunction, causing a variety of inflammatory reactions, such as cardiovascular inflammation, nephritis, endometritis, etc.110–112 OS can induce apoptosis by promoting mitochondrial damage,112,113 and the level of ROS serves as a diagnostic indicator of endometritis.114 In addition to its antioxidant function, anti-inflammatory and immunomodulatory effects are other dominant characteristics of CoQ10. Inflammatory markers such as TNF-α, IL-8, matrix metalloproteinase 2(MMP-2), and MMP-9 were considerably reduced by CoQ10 supplementation.115–118 CoQ10 and its derivative Mito Q both have been shown to reduce endothelial dysfunction, identified molecular mechanisms including regulating the ratio of H2O2 and O2•− production by nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4),119 and inhibiting endothelial cell ROS and autophagy.120 In addition, due to its inhibition of inflammation, OS, and collagen accumulation, MitoQ prevents surgical adhesions, and its mechanism of action may be related to the NRF2 / HO-1 signaling pathway.121 By preserving endothelial function and reducing inflammatory reactions and oxidative stress, CoQ10 may decrease the fertility damage caused by several inflammatory gynecological disorders.
CoQ10 and Pregnancy Maintenance
Immune crosstalk at maternal-fetal interface is considered to play an especially important role for pregnancy maintenance.122–124 However, the quality of oocytes and antioxidants treatment are found equally crucial to determine the developmental potential of embryos, recently.125–128
CoQ10 Protects Embryo Development
CoQ10 is an important substance that is involved in organisms development. CoQ10 synthesis-related gene knockout mice showed significantly lower mitochondrial CoQ10 levels, severely reduced activity of endogenous CoQ10-dependent complexes, and the mice exhibited severely retarded growth and development compared to controls.129 This indicates that CoQ10 may influence growth and development through its involvement in the mitochondrial respiratory chain function. In addition, CoQ10 concentrations in the follicular fluid are linked to improved embryo quality and pregnancy rates. By measuring CoQ10 levels in the follicular fluid of 60 patients with unexplained infertility who underwent in vitro fertilization, we found that follicular fluid levels of CoQ10 were significantly higher in follicles developing into grade A and B embryos than in grade C and D embryos (0.526±0.64ug/mL vs 0.390±0.55ug/mL; P=0.038). The pregnant group had significantly higher levels of CoQ10 in the follicular fluid than the non-pregnant group (0.603 ± 0.78ug/mL vs 0.379 ± 0.62ug/mL; P=0.044).130 In a prospective randomized controlled study involving 169 consecutive patients with poor ovarian response (POR), 76 patients receiving oral CoQ10 pretreatment showed increased oocyte counts, improved fertilization rates, and increased embryo quality. In the treatment group, the number of females who canceled embryo transfer due to poor embryo development decreased significantly, and more women had available cryopreserved embryos.32 The level of CoQ10 in follicular fluid is correlated with the subsequent embryo development status, whereas artificially supplemented CoQ10 can also increase the number of high-quality embryos.63 CoQ10 application in ovarian failure mice elevates c-Kit expression and upregulates Ets-variant gene 5(ETV5) expression through the MEK/ERK pathway, thereby promoting human preimplantation embryo development.131
Generation of ROS is essential for healthy embryo development; however, excessive ROS levels cause stagnation and death in the developing fetus.132 In assisted reproductive techniques, in vitro culture processes, such as embryo freeze-thaw, disturb the balance between antioxidant and oxidative stress, and in vitro-induced ROS are associated with poorer embryo quality and viability.133 In the presence of 0, 5, 30 or 50μM CoQ10 respectively, when the added concentration was 30μM, blastocyst formation and hatching rates of sheep oocytes were significantly increased compared with other concentrations.134 In bovine oocytes vitrified with 0, 25 or 50μM CoQ10, 50μM was helpful to keep the integrity of oocytes vitrified, improve the survival rate and reduce the negative effects of vitrification.135 Embryo development and CoQ10 supplementation exhibit dose-dependent and species-specific. Maside et al136 found that 10–50μM CoQ10 supplementation did not affect the percentage of porcine MII oocytes, fertilization or embryo development, and even 100μM CoQ10 was not conducive to blastocyst formation.137 These results contradicted the above experimental results. This may be related to OS and seasonal heat stress to which different cultures are subjected.
CoQ10 also has a protective effect on the ovaries of the offspring. Zearalenone (ZEA) inhibits follicle formation in postnatal day 0 mouse ovaries. Epigenetic changes occurred in the ovaries of newborn mice from ZEA-exposed mothers, and oral CoQ10 supplementation in pregnant mice ameliorated DNA damage in the offspring induced by maternal ZEA exposure. The effectiveness of CoQ10 in attenuating ZEA effects suggests that it protects against early follicle production in neonatal mammals by improving mitochondrial function.138 Embryo development is influenced not only by oocyte quality, but also by sperm, with significantly higher blastocyst rates in oocytes fertilized by sperm treated with CoQ10, zinc, and d-aspartate.139 The effect of CoQ10 on embryonic development is not covered from a male perspective because this study was written primarily from the standpoint of women.
CoQ10 Reduces Spontaneous Abortion
Women with recurrent spontaneous abortion (RSA) have decreased antioxidant capacity and lower serum levels of antioxidant enzymes.140,141 The balance of T helper cells (Th) at the maternal-fetal interface is prominent during embryo development. Th1 mainly produces interferon-γ (IFN-γ) and TNF-α,142 whereas ROS significant elevate level of TNF-α in Th1. With CoQ10 treatment, pregnant women with a history of RSA showed a decline in the proportion of Th1 in peripheral blood mononuclear cells and a significant decrease in ROS, IFN-γ and TNF-α levels.143 In conclusion, CoQ10 may maintain immune homeostasis by modulating inflammatory factor levels, altering T cell subpopulation ratios, and reducing OS.
Many connective tissue diseases such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), and antiphospholipid antibody syndrome (AAS) are associated with RSA.34 These disorders emerge due to OS-induced inflammation, apoptosis, and modification of immunological tolerance.115 Anti-phospholipid antibodies and lupus nephritis are significant risk factors for poor maternal pregnancy outcomes.144 The CoQ10 analog idebenone attenuates glomerulonephritis and fibrosis in SLE model mice.145 CoQ10 not only reduces OS in patients with APS and improves mitochondrial function but can also reduce the expression of serum prothrombotic and proinflammatory markers.146 Women with MS have higher rates of miscarriage and intrauterine mortality than normal women.147 CoQ10 supplementation reduces inflammatory markers and OS, and increases antioxidant enzyme activity in patients with MS. Daily CoQ10 supplementation alleviates fatigue and depression in patients.148–150 CoQ10 probably alleviates the symptoms of connective tissue diseases through its anti-inflammatory and antioxidant effects, thus improving pregnancy outcomes in pregnant women with connective tissue diseases.
However, there is insufficient evidence to suggest that antioxidants reduce miscarriage rates in women with low fertility.151 The anabolism of CoQ10 has a high index of effect on RSA.152 This implied that metabolic alterations in CoQ10 may indicate pregnancy failure. Whether CoQ10 reduces the rate of miscarriage by reducing OS remains unclear.
However, clinical use of CoQ10 is limited. First, the oral bioavailability of CoQ10 is low and affected by many factors.44 Secondly, it is not yet approved by the Food and Drug Administration, and its method of application, effective dose, and safety remain controversial.153 There is evidence that reduced CoQ10 levels are associated with an increased lifespan in animal models. Conversely, some experiments have found that exogenous CoQ10 supplementation delays age-related adverse changes and extends lifespan.154 However, the relationship between CoQ10 and aging remains contradictory and requires further investigation. In addition, CoQ10 analogs are gradually being developed that may have better application prospects.155
Conclusions and Prospect
Accumulating data have identified CoQ10 as an endogenous antioxidant, it is essential for mitochondrial respiratory chain activity, ROS levels maintaining and ATP production. Harnessing the power of CoQ10 in reducing cellular OS, enhancing cellular energy metabolism, participating in gene regulation and reducing the inflammatory response, it could have implications for ameliorating the quantity and quality of oocytes in aging ovaries, improving the oxidative metabolism of follicular fluid, boosting the mitochondrial function of oocytes, and increasing pregnancy probability. These advances would enable the development of novel therapy for women with infertility and recurrent pregnancy loss. However, long term safety research based on CoQ10 for offspring, further large-scale clinical investigation is still needed.
Acknowledgment
We appreciate Professor Huanfa Yi (Central Laboratory, First Hospital of Jilin University)’s critical comments and invaluable suggestions on this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Science and Technology Department of Jilin Province (YDZJ202201ZYTS085, 20220204020YY, 20210101315JC), National Natural Science Foundation of China (82201847). Tianhua Health Public Welfare Foundation of Jilin (J2022JKJ026).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7:S41–50. doi:10.1016/j.mito.2007.02.006
2. Pallotti F, Bergamini C, Lamperti C, Fato R. The roles of coenzyme Q in disease: direct and indirect involvement in cellular functions. Int J Mol Sci. 2021;23(1):128. doi:10.3390/ijms23010128
3. Raizner AE. Coenzyme Q10. Methodist Debakey Cardiovasc J. 2019;15(3):185–191. doi:10.14797/mdcj-15-3-185
4. Li X, Zhan J, Hou Y, et al. Coenzyme Q10 regulation of apoptosis and oxidative stress in H2O2 induced BMSC death by modulating the Nrf-2/NQO-1 signaling pathway and its application in a model of spinal cord injury. Oxid Med Cell Longev. 2019;2019:6493081. doi:10.1155/2019/6493081
5. Sadeghiyan Galeshkalami N, Abdollahi M, Najafi R, et al. Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sci. 2019;216:101–110. doi:10.1016/j.lfs.2018.10.055
6. Said RS, Mohamed HA, Kamal MM. Coenzyme Q10 mitigates ionizing radiation-induced testicular damage in rats through inhibition of oxidative stress and mitochondria-mediated apoptotic cell death. Toxicol Appl Pharmacol. 2019;383:114780. doi:10.1016/j.taap.2019.114780
7. Quinzii CM, Luna-Sanchez M, Ziosi M, Hidalgo-Gutierrez A, Kleiner G, Lopez LC. The role of sulfide oxidation impairment in the pathogenesis of primary CoQ deficiency. Front Physiol. 2017;8:525. doi:10.3389/fphys.2017.00525
8. Sabbatinelli J, Orlando P, Galeazzi R, et al. Ubiquinol ameliorates endothelial dysfunction in subjects with mild-to-moderate dyslipidemia: a randomized clinical trial. Nutrients. 2020;12(4):1098. doi:10.3390/nu12041098
9. Ayer A, Fazakerley DJ, Suarna C, et al. Genetic screening reveals phospholipid metabolism as a key regulator of the biosynthesis of the redox-active lipid coenzyme Q. Redox Biol. 2021;46:102127. doi:10.1016/j.redox.2021.102127
10. Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi:10.1016/j.redox.2020.101674
11. AL-Zubaidi U, Liu J, Cinar O, Robker RL, Adhikari D, Carroll J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol Hum Reprod. 2019;25(11):695–705. doi:10.1093/molehr/gaz055
12. Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12(8):961–1008. doi:10.1089/ars.2009.2704
13. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi:10.1038/s41586-019-1705-2
14. Hargreaves I, Heaton RA, Mantle D. Disorders of human coenzyme Q10 metabolism: an overview. Int J Mol Sci. 2020;21(18):6695. doi:10.3390/ijms21186695
15. Wang Y, Hekimi S. Understanding ubiquinone. Trends Cell Biol. 2016;26(5):367–378. doi:10.1080/09513590.2017.1381680
16. Hargreaves IP, Mantle D. Coenzyme Q10 supplementation in fibrosis and aging. Adv Exp Med Biol. 2019;1178:103–112. doi:10.1007/978-3-030-25650-0_6
17. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10(1):49. doi:10.1186/1477-7827-10-49
18. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial Reactive Oxygen Species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950. doi:10.1152/physrev.00026.2013
19. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–462. doi:10.1016/j.cub.2014.03.034
20. Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH: ubiquinone oxidoreductase (complex I). J Biol Chem. 2004;279(38):39414–39420. doi:10.1074/jbc.M406576200
21. Chen Z, Wang C, Yu N, et al. INF2 regulates oxidative stress-induced apoptosis in epidermal HaCaT cells by modulating the HIF1 signaling pathway. Biomed Pharmacother. 2019;111:151–161. doi:10.1016/j.biopha.2018.12.046
22. Subramanian K, Jochem A, Le Vasseur M, et al. Coenzyme Q biosynthetic proteins assemble in a substrate-dependent manner into domains at ER–mitochondria contacts. J Cell Biol. 2019;218(4):1353–1369. doi:10.1083/jcb.201808044
23. Kemmerer ZA, Robinson KP, Schmitz JM, et al. UbiB proteins regulate cellular CoQ distribution in saccharomyces cerevisiae. Nat Commun. 2021;12(1):4769. doi:10.1038/s41467-021-25084-7
24. van der Reest J, Nardini Cecchino G, Haigis MC, Kordowitzki P. Mitochondria: their relevance during oocyte ageing. Ageing Res Rev. 2021;70:101378. doi:10.1016/j.arr.2021.101378
25. Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol. 2009;20(3):354–364. doi:10.1016/j.semcdb.2008.12.005
26. Ding H, Li Z, Li X, et al. FTO alleviates CdCl2-induced apoptosis and oxidative stress via the AKT/Nrf2 pathway in bovine granulosa cells. Int J Mol Sci. 2022;23(9):4948. doi:10.3390/ijms23094948
27. Wang Y, Yang C, Elsheikh NAH, et al. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging. 2019;11(15):5535–5547. doi:10.18632/aging.102136
28. Sammad A, Luo H, Hu L, Zhu H, Wang Y. Transcriptome reveals granulosa cells coping through redox, inflammatory and metabolic mechanisms under acute heat stress. Cells. 2022;11(9):1443. doi:10.3390/cells11091443
29. Garlanda C, Maina V, Martinez de la Torre Y, Nebuloni M, Locati M. Inflammatory reaction and implantation: the new entries PTX3 and D6. Placenta. 2008;29(Suppl B):129–134. doi:10.1016/j.placenta.2008.06.008
30. Wang K, Wang K, Wang J, Yu F, Ye C, Fu Y. Protective effect of Clostridium butyricum on Escherichia coli-induced endometritis in mice via ameliorating endometrial barrier and inhibiting inflammatory response. Microbiol Spectr. 2022;10(6):e0328622. doi:10.1128/spectrum.03286-22
31. Niu Y-J, Zhou W, Nie Z-W, et al. Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging. 2020;12(2):1256–1271. doi:10.18632/aging.102681
32. Xu Y, Nisenblat V, Lu C, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol. 2018;16(1):29. doi:10.1186/s12958-018-0343-0
33. Sangsefidi ZS, Yaghoubi F, Hajiahmadi S, Hosseinzadeh M. The effect of coenzyme Q10 supplementation on oxidative stress: a systematic review and meta-analysis of randomized controlled clinical trials. Food Sci Nutr. 2020;8(4):1766–1776. doi:10.1002/fsn3.1492
34. Gutierrez-Mariscal FM, Arenas-de Larriva AP, Limia-Perez L, Romero-Cabrera JL, Yubero-Serrano EM, López-Miranda J. Coenzyme Q10 supplementation for the reduction of oxidative stress: clinical implications in the treatment of chronic diseases. Int J Mol Sci. 2020;21(21):7870. doi:10.3390/ijms21217870
35. Alahmar AT, Calogero AE, Sengupta P, Dutta S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J Mens Health. 2021;39(2):346–351. doi:10.5534/wjmh.190145
36. Beaujouan E. Latest-late fertility? Decline and resurgence of late parenthood across the low-fertility countries. Popul Dev Rev. 2020;46(2):219–247. doi:10.1111/padr.12334
37. Sasaki H, Hamatani T, Kamijo S, et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front Endocrinol. 2019;10:811. doi:10.3389/fendo.2019.00811
38. Huang Y, Hu C, Ye H, et al. Inflamm-aging: a new mechanism affecting premature ovarian insufficiency. J Immunol Res. 2019;2019:8069898. doi:10.1155/2019/8069898
39. Babayev E, Wang T, Szigeti-Buck K, et al. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and MtDNA quantity. Maturitas. 2016;93:121–130. doi:10.1016/j.maturitas.2016.06.015
40. Oxidative stress induces telomere dysfunction and shortening in human oocytes of advanced age donors Available from: https://pubmed.ncbi.nlm.nih.gov/34440635/.
41. Zhu C, Zhang C, Cui X, Wu J, Cui Z, Shen X. Trichosanthin inhibits cervical cancer by regulating oxidative stress-induced apoptosis. Bioengineered. 2021;12:2779–2790. doi:10.1080/21655979.2021.1930335
42. Wang L, Lu Z, Zhao J, et al. Selective oxidative stress induces dual damage to telomeres and mitochondria in human T cells. Aging Cell. 2021;20:e13513. doi:10.1111/acel.13513
43. Zhao D, Liang Y, Dai S, et al. Dose-response effect of coenzyme Q10 supplementation on blood pressure among patients with cardiometabolic disorders: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2022;13(6):2180–2194. doi:10.1093/advances/nmac100
44. Testai L, Martelli A, Flori L, Cicero AFG, Colletti A. Coenzyme Q10: clinical applications beyond cardiovascular diseases. Nutrients. 2021;13:1697. doi:10.3390/nu13051697
45. Sheykhhasan M, Amini R, Soleimani Asl S, Saidijam M, Hashemi SM, Najafi R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s Disease. Biomed Pharmacother. 2022;152:113224. doi:10.1016/j.biopha.2022.113224
46. Park HW, Park CG, Park M, et al. Intrastriatal administration of coenzyme Q10 enhances neuroprotection in a Parkinson’s Disease rat model. Sci Rep. 2020;10:9572. doi:10.1038/s41598-020-66493-w
47. Shin JY, Choi J-W, Kim D-G, et al. Protective effects of coenzyme Q10 against acute pancreatitis. Int Immunopharmacol. 2020;88:106900. doi:10.1016/j.intimp.2020.106900
48. G L, Lmi J, I F, et al. Coenzyme Q10 and melatonin for the treatment of male infertility: a narrative review. Nutrients. 2022;14. doi:10.3390/nu14214585
49. Arroyo A, Kim B, Yeh J. Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod Sci. 2020;27:1223–1252. doi:10.1007/s43032-019-00137-x
50. Yen H-C, Yeh W-Y, Lee S-H, Feng Y-H, Yang S-L. Characterization of human mitochondrial PDSS and COQ proteins and their roles in maintaining coenzyme Q10 levels and each other’s stability. Biochim Biophys Acta Bioenerg. 2020;1861(7):148192. doi:10.1016/j.bbabio.2020.148192
51. Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi:10.1111/acel.12368
52. Ben-Meir A, Kim K, McQuaid R, et al. Co-enzyme Q10 supplementation rescues cumulus cells dysfunction in a maternal aging model. Antioxidants. 2019;8(3):58. doi:10.3390/antiox8030058
53. Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27(1):27–47. doi:10.1093/humupd/dmaa043
54. Ma L, Cai L, Hu M, et al. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertil Steril. 2020;114(2):331–337. doi:10.1016/j.fertnstert.2020.04.002
55. Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13(7):493–504. doi:10.1038/nrg3245
56. Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Rev Mutat Res. 2020;785:108320. doi:10.1016/j.mrrev.2020.108320
57. Kowalska E, Bartnicki F, Fujisawa R, et al. Inhibition of DNA replication by an Anti-PCNA Aptamer/PCNA complex. Nucleic Acids Res. 2018;46(1):25–41. doi:10.1093/nar/gkx1184
58. Xu B, Hua J, Zhang Y, et al. Proliferating Cell Nuclear Antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS One. 2011;6(1):e16046. doi:10.1371/journal.pone.0016046
59. Ginther OJ. An FSH booster surge for resurgence of the preovulatory follicle in heifers. Domest Anim Endocrinol. 2018;65:90–94. doi:10.1016/j.domaniend.2018.06.002
60. Moolhuijsen LME, Visser JA. Anti-müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–3373. doi:10.1210/clinem/dgaa513
61. Delkhosh A, Delashoub M, Tehrani AA, et al. Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J Biochem Mol Toxicol. 2019;33(11):e22398. doi:10.1002/jbt.22398
62. Özcan P, Fıçıcıoğlu C, Kizilkale O, et al. Can coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016;33(9):1223–1230. doi:10.1007/s10815-016-0751-z
63. Lee HJ, Park MJ, Joo BS, et al. Effects of coenzyme Q10 on ovarian surface epithelium-derived ovarian stem cells and ovarian function in a 4-vinylcyclohexene diepoxide-induced murine model of ovarian failure. Reprod Biol Endocrinol. 2021;19(1):59. doi:10.1186/s12958-021-00736-x
64. Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177. doi:10.1093/humupd/dmm040
65. Kim E, Cai L, Hyun S-H. Effects of stem cell factor/c-kit signaling on in vitro maturation of porcine oocytes and subsequent developmental competence after fertilization. Front Vet Sci. 2021;8:745488. doi:10.3389/fvets.2021.745488
66. Yang L, Wang H, Song S, et al. Systematic understanding of anti-aging effect of coenzyme Q10 on oocyte through a network pharmacology approach. Front Endocrinol. 2022;13:813772. doi:10.3389/fendo.2022.813772
67. Kim J-Y, Zhou D, Cui X-S. Bezafibrate prevents aging in in vitro-matured porcine oocytes. J Anim Sci Technol. 2021;63(4):766–777. doi:10.5187/jast.2021.e64
68. Tiefenbach J, Magomedova L, Liu J, et al. Idebenone and coenzyme Q10 are novel PPARα/γ ligands, with potential for treatment of fatty liver diseases. Dis Model Mech. 2018;11(9):dmm034801. doi:10.1242/dmm.034801
69. Hosseinzadeh E, Zavareh S, Lashkarbolouki T. Antioxidant properties of coenzyme Q10-pretreated mouse pre-antral follicles derived from vitrified ovaries. J Obstet Gynaecol Res. 2017;43(1):140–148. doi:10.1111/jog.13173
70. Yuan X, Tian GG, Pei X, Hu X, Wu J. Spermidine induces cytoprotective autophagy of female germline stem cells in vitro and ameliorates aging caused by oxidative stress through upregulated sequestosome-1/P62 expression. Cell Biosci. 2021;11(1):107. doi:10.1186/s13578-021-00614-4
71. Zhang S, Zhu D, Mei X, et al. Advances in biomaterials and regenerative medicine for primary ovarian insufficiency therapy. Bioact Mater. 2021;6(7):1957–1972. doi:10.1016/j.bioactmat.2020.12.008
72. Giannubilo SR, Orlando P, Silvestri S, et al. CoQ10 supplementation in patients undergoing IVF-ET: the relationship with follicular fluid content and oocyte maturity. Antioxidants. 2018;7(10):141. doi:10.3390/antiox7100141
73. Yang J, Feng T, Li S, Zhang X, Qian Y. Human follicular fluid shows diverse metabolic profiles at different follicle developmental stages. Reprod Biol Endocrinol. 2020;18(1):74. doi:10.1186/s12958-020-00631-x
74. Ben-Meir A, Yahalomi S, Moshe B, Shufaro Y, Reubinoff B, Saada A. Coenzyme Q-dependent mitochondrial respiratory chain activity in granulosa cells is reduced with aging. Fertil Steril. 2015;104(3):724–727. doi:10.1016/j.fertnstert.2015.05.023
75. Opstad TB, Alexander J, Aaseth JO, Larsson A, Seljeflot I, Alehagen U. Selenium and coenzyme Q10 intervention prevents telomere attrition, with association to Reduced Cardiovascular Mortality-Sub-Study of a randomized clinical trial. Nutrients. 2022;14(16):3346. doi:10.3390/nu14163346
76. Dapas M, Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev. 2022;43:927–965. doi:10.1210/endrev/bnac001
77. Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi:10.1016/j.lfs.2019.116940
78. Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):80. doi:10.1186/s12958-018-0391-5
79. Li W, Liu C, Yang Q, Zhou Y, Liu M, Shan H. Oxidative stress and antioxidant imbalance in ovulation disorder in patients with polycystic ovary syndrome. Front Nutr. 2022;9:1018674. doi:10.3389/fnut.2022.1018674
80. Teede HJ, Tay CT, Joham AE. Polycystic ovary syndrome: an intrinsic risk factor for diabetes compounded by obesity. Fertil Steril. 2021;115(6):1449–1450. doi:10.1016/j.fertnstert.2021.03.024
81. Bacchetti T, Morresi C, Vignini A, et al. HDL functionality in follicular fluid in normal-weight and obese women undergoing assisted reproductive treatment. J Assist Reprod Genet. 2019;36(8):1657. doi:10.1007/s10815-019-01523-9
82. Mizgier M, Jarząbek-Bielecka G, Wendland N, et al. Relation between inflammation, oxidative stress, and macronutrient intakes in normal and excessive body weight adolescent girls with clinical features of polycystic ovary syndrome. Nutrients. 2021;13(3):896. doi:10.3390/nu13030896
83. Wang Q, Ratchford AM, Chi MM-Y, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23(10):1603–1612. doi:10.1210/me.2009-0033
84. Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod. 2016;31:2090–2097. doi:10.1093/humrep/dew181
85. Huo P, Li M, Le J, Zhu C, Yao J, Zhang S. Resveratrol improves follicular development of PCOS rats via regulating glycolysis pathway and targeting SIRT1. Syst Biol Reprod Med. 2022;1–13. doi:10.1080/19396368.2022.2125855
86. Rutanen J, Yaluri N, Modi S, et al. SIRT1 MRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59(4):829–835. doi:10.2337/db09-1191
87. Fox CW, Zhang L, Sohni A, et al. Inflammatory stimuli trigger increased androgen production and shifts in gene expression in theca-interstitial cells. Endocrinology. 2019;160(12):2946–2958. doi:10.1210/en.2019-00588
88. Rosenfield RL. Current concepts of polycystic ovary syndrome pathogenesis. Curr Opin Pediatr. 2020;32(5):698–706. doi:10.1097/MOP.0000000000000945
89. Taghizadeh S, Izadi A, Shirazi S, Parizad M, Pourghassem Gargari B. The effect of coenzyme Q10 supplementation on inflammatory and endothelial dysfunction markers in overweight/obese polycystic ovary syndrome patients. Gynecol Endocrinol. 2021;37(1):26–30. doi:10.1080/09513590.2020.1779689
90. Rahmani E, Jamilian M, Samimi M, et al. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(3):217–222. doi:10.1210/clinem/dgaa513
91. Jie J, Ling L, Yi Y, et al. Tributyltin triggers lipogenesis in macrophages via modifying PPARγ pathway. Environ Pollut. 2021;271:116331. doi:10.1111/jog.13173
92. Yu Q, Zheng H, Zhang Y. Inducible degrader of LDLR: a potential novel therapeutic target and emerging treatment for hyperlipidemia. Vascul Pharmacol. 2021;140:106878. doi:10.3390/antiox7100141
93. Lee SK, Lee JO, Kim JH, et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARα induction in 3T3-L1 preadipocytes. Cell Signal. 2012;24(12):2329–2336. doi:10.1210/endrev/bnac001
94. Chen K, Chen X, Xue H, et al. Coenzyme Q10 attenuates high-fat diet-induced non-alcoholic fatty liver disease through activation of the AMPK pathway. Food Funct. 2019;10(2):814–823. doi:10.1007/s10815-019-01523-9
95. Xu Z, Huo J, Ding X, et al. Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition. Sci Rep. 2017;7(1):8253. doi:10.1097/MOP.0000000000000945
96. Raitakari OT, McCredie RJ, Witting P, et al. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radic Biol Med. 2000;28:1100–1105. doi:10.1016/s0891-5849(00)00201-x
97. Tsai K-L, Huang Y-H, Kao C-L, et al. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J Nutr Biochem. 2012;23(5):458–468. doi:10.1016/j.jnutbio.2011.01.011
98. Sun Y, Li S, Liu H, et al. Oxidative stress promotes hyperandrogenism by reducing sex hormone-binding globulin in polycystic ovary syndrome. Fertil Steril. 2021;116(6):1641–1650. doi:10.1016/j.fertnstert.2021.07.1203
99. Izadi A, Ebrahimi S, Shirazi S, et al. Hormonal and metabolic effects of coenzyme Q10 and/or Vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104:319–327. doi:10.1210/jc.2018-01221
100. Zhang J, Xing C, Zhao H, He B. The effectiveness of coenzyme Q10, Vitamin E, Inositols, and Vitamin D in improving the endocrine and metabolic profiles in women with polycystic ovary syndrome: a network meta-analysis. Gynecol Endocrinol. 2021;37(12):1063–1071. doi:10.1080/09513590.2021.1926975
101. de Jesus DS, Bargi-Souza P, Cruzat V, Yechoor V, Carpinelli AR, Peliciari-Garcia RA. BMAL1 modulates ROS generation and insulin secretion in pancreatic β-cells: an effect possibly mediated via NOX2. Mol Cell Endocrinol. 2022;555:111725. doi:10.1016/j.mce.2022.111725
102. Cheng Y-C, Chu L-W, Chen J-Y, et al. Loganin attenuates high glucose-induced schwann cells pyroptosis by inhibiting ROS generation and NLRP3 inflammasome activation. Cells. 2020;9(9):1948. doi:10.3390/cells9091948
103. Schroeder MM, Belloto RJ, Hudson RA, McInerney MF. Effects of antioxidants coenzyme Q10 and lipoic acid on interleukin-1 beta-mediated inhibition of glucose-stimulated insulin release from cultured mouse pancreatic islets. Immunopharmacol Immunotoxicol. 2005;27(1):109–122. doi:10.1081/iph-51755
104. Refaeey AE, Selem A, Badawy A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reprod Biomed Online. 2014;29(1):119–124. doi:10.1016/j.rbmo.2014.03.011
105. Lin X, Dai Y, Tong X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020;30:101431. doi:10.1016/j.redox.2020.101431
106. Hayashi S, Nakamura T, Motooka Y, et al. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020;37:101726. doi:10.1016/j.redox.2020.101726
107. Akarca-Dizakar SÖ, Demirel MA, Coşkun Akçay N, et al. The therapeutic effects of coenzyme Q10 on surgically induced endometriosis in sprague dawley rats. J Obstet Gynaecol. 2022;42(7):3290–3298. doi:10.1080/01443615.2022.2114322
108. Govatati S, Deenadayal M, Shivaji S, Bhanoori M. Mitochondrial NADH: ubiquinone oxidoreductase alterations are associated with endometriosis. Mitochondrion. 2013;13(6):782–790. doi:10.1016/j.mito.2013.05.003
109. Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol. 2021;224(3):251–257. doi:10.1016/j.ajog.2020.10.019
110. Aimo A, Castiglione V, Borrelli C, et al. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27(5):494–510. doi:10.1177/2047487319870344
111. Muñoz M, López-Oliva ME, Rodríguez C, et al. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2020;28:101330. doi:10.1016/j.redox.2019.101330
112. Shahin HI, Radnaa E, Tantengco OAG, et al. Microvesicles and exosomes released by amnion epithelial cells under oxidative stress cause inflammatory changes in uterine cells†. Biol Reprod. 2021;105(2):464–480. doi:10.1093/biolre/ioab088
113. Song P, Liu C, Sun M, et al. Oxidative stress induces bovine endometrial epithelial cell damage through mitochondria-dependent pathways. Animals. 2022;12(18):2444. doi:10.3390/ani12182444
114. Boni R, Cecchini Gualandi S. Relationship between oxidative stress and endometritis: exploiting knowledge gained in mares and cows. Animals. 2022;12(18):2403. doi:10.3390/ani12182403
115. López-Pedrera C, Villalba JM, Patiño-Trives AM, et al. Therapeutic potential and immunomodulatory role of coenzyme Q10 and its analogues in systemic autoimmune diseases. Antioxidants. 2021;10(4):600. doi:10.3390/antiox10040600
116. García-Carpintero S, Domínguez-Bértalo J, Pedrero-Prieto C, et al. Ubiquinol supplementation improves gender-dependent cerebral vasoreactivity and ameliorates chronic inflammation and endothelial dysfunction in patients with mild cognitive impairment. Antioxidants. 2021;10(2):143. doi:10.3390/antiox10020143
117. Dahri M, Tarighat-Esfanjani A, Asghari-Jafarabadi M, Hashemilar M. Oral coenzyme Q10 supplementation in patients with migraine: effects on clinical features and inflammatory markers. Nutr Neurosci. 2019;22(9):607–615. doi:10.1080/1028415X.2017.1421039
118. Alimohammadi M, Rahimi A, Faramarzi F, et al. Effects of coenzyme Q10 supplementation on inflammation, angiogenesis, and oxidative stress in breast cancer patients: a systematic review and meta-analysis of randomized controlled- trials. Inflammopharmacology. 2021;29(3):579–593. doi:10.1007/s10787-021-00817-8
119. Yuan S, Hahn SA, Miller MP, et al. Cooperation between CYB5R3 and NOX4 via coenzyme Q mitigates endothelial inflammation. Redox Biol. 2021;47:102166. doi:10.1016/j.redox.2021.102166
120. Chen S, Wang Y, Zhang H, et al. The antioxidant MitoQ protects against CSE-induced endothelial barrier injury and inflammation by inhibiting ROS and autophagy in human umbilical vein endothelial cells. Int J Biol Sci. 2019;15(7):1440–1451. doi:10.7150/ijbs.30193
121. Hu Q, Lu X, Li G, et al. Mitoquinone treatment for the prevention of surgical adhesions via regulation of the NRF2/HO-1 signaling pathway in mice. Surgery. 2022;171(2):428–436. doi:10.1016/j.surg.2021.08.053
122. Shu C, Yu X, Cheng S, Jing J, Hu C, Pang B. Pristimerin suppresses trophoblast cell epithelial–mesenchymal transition via miR-542-5p/EGFR axis. Drug Des Devel Ther. 2020;14:4659–4670. doi:10.2147/DDDT.S274595
123. Hu C, Zhen Y, Ma Z, et al. Polyamines from myeloid-derived suppressor cells promote Th17 polarization and disease progression. Mol Ther. 2023;31(2):569–584. doi:10.1016/j.ymthe.2022.10.013
124. Pang B, Hu C, Li H, et al. Myeloidderived suppressor cells: escorts at the maternal–fetal interface. Front Immunol. 2023;14:1080391. doi:10.3389/fimmu.2023.1080391
125. Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015;103(2):317–322. doi:10.1016/j.fertnstert.2014.12.115
126. Truong T, Gardner DK. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum Reprod. 2017;32(12):2404–2413. doi:10.1093/humrep/dex330
127. Truong TT, Gardner DK. Antioxidants increase blastocyst cryosurvival and viability post-vitrification. Hum Reprod. 2020;35(1):12–23. doi:10.1093/humrep/dez243
128. Truong TT, Soh YM, Gardner DK. Antioxidants improve mouse preimplantation embryo development and viability. Hum Reprod. 2016;31(7):1445–1454. doi:10.1093/humrep/dew098
129. Wang Y, Oxer D, Hekimi S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun. 2015;6(1):6393. doi:10.1038/ncomms7393
130. Akarsu S, Gode F, Isik AZ, Dikmen ZG, Tekindal MA. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J Assist Reprod Genet. 2017;34(5):599–605. doi:10.1007/s10815-017-0882-x
131. Tan J, Zou Y, Huang Z-H, et al. C-kit signaling promotes human pre-implantation 3PN embryonic development and blastocyst formation. Reprod Biol Endocrinol. 2019;17(1):75. doi:10.1186/s12958-019-0521-8
132. Deluao JC, Winstanley Y, Robker RL, Pacella-Ince L, Gonzalez MB, McPherson NO. Oxidative stress and reproductive function: reactive oxygen species in the mammalian pre-implantation embryo. Reproduction. 2022;164(6):F95–F108. doi:10.1530/REP-22-0121
133. Leite RF, Annes K, Ispada J, et al. Oxidative stress alters the profile of transcription factors related to early development on in vitro produced embryos. Oxid Med Cell Longev. 2017;2017:1502489. doi:10.1155/2017/1502489
134. Heydarnejad A, Ostadhosseini S, Varnosfaderani SR, Jafarpour F, Moghimi A, Nasr-Esfahani MH. Supplementation of maturation medium with CoQ10 enhances developmental competence of ovine oocytes through improvement of mitochondrial function. Mol Reprod Dev. 2019;86:812–824. doi:10.1002/mrd.23159
135. Ruiz-Conca M, Vendrell M, Sabés-Alsina M, Mogas T, Lopez-Bejar M. Coenzyme Q10 supplementation during in vitro maturation of bovine oocytes (bos taurus) helps to preserve oocyte integrity after vitrification. Reprod Domest Anim. 2017;52(Suppl 4):52–54. doi:10.1111/rda.13056
136. Maside C, Martinez CA, Cambra JM, et al. Supplementation with exogenous coenzyme Q10 to media for in vitro maturation and embryo culture fails to promote the developmental competence of porcine embryos. Reprod Domest Anim. 2019;54 Suppl 4:72–77. doi:10.1111/rda.13486
137. Gendelman M, Roth Z. Incorporation of coenzyme Q10 into bovine oocytes improves mitochondrial features and alleviates the effects of summer thermal stress on developmental competence. Biol Reprod. 2012;87:118. doi:10.1095/biolreprod.112.101881
138. Feng Y-Q, Wang -J-J, Li M-H, et al. Impaired primordial follicle assembly in offspring ovaries from zearalenone-exposed mothers involves reduced mitochondrial activity and altered epigenetics in oocytes. Cell Mol Life Sci. 2022;79:258. doi:10.1007/s00018-022-04288-0
139. Gualtieri R, Barbato V, Fiorentino I, et al. Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology. 2014;82(4):592–598. doi:10.1016/j.theriogenology.2014.05.028
140. Omeljaniuk WJ, Socha K, Borawska MH, et al. Antioxidant status in women who have had a miscarriage. Adv Med Sci. 2015;60(2):329–334. doi:10.1016/j.advms.2015.06.003
141. El-Far M, El-Sayed IH, El-Motwally AE, Hashem IA, Bakry N. Serum levels of TNF-alpha and antioxidant enzymes and placental TNF-alpha expression in unexplained recurrent spontaneous miscarriage. J Physiol Biochem. 2009;65:175–181. doi:10.1007/BF03179068
142. Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol. 2020;11:2025. doi:10.3389/fimmu.2020.02025
143. Talukdar A, Sharma KA, Rai R, Deka D, Rao DN. Effect of coenzyme Q10 on Th1/Th2 paradigm in females with idiopathic recurrent pregnancy loss. Am J Reprod Immunol. 2015;74:169–180. doi:10.1111/aji.12376
144. Smyth A, Oliveira GHM, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5(11):2060–2068. doi:10.2215/CJN.00240110
145. Blanco LP, Pedersen HL, Wang X, et al. Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis Rheumatol. 2020;72:454–464. doi:10.1002/art.41128
146. Perez-Sanchez C, Ruiz-Limon P, Aguirre MA, et al. Mitochondrial dysfunction in antiphospholipid syndrome: implications in the pathogenesis of the disease and effects of coenzyme Q(10) treatment. Blood. 2012;119(24):5859–5870. doi:10.1182/blood-2011-12-400986
147. Vanya M, Nyari T, Bencsik K, Bartfai G. Pregnancy and perinatal outcomes among women with multiple sclerosis: a Retrospective Case-Controlled Study in South Hungary. J Matern Fetal Neonatal Med. 2014;27(6):577–581. doi:10.3109/14767058.2013.825596
148. Sanoobar M, Dehghan P, Khalili M, Azimi A, Seifar F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: a double blind randomized clinical trial. Nutr Neurosci. 2016;19(3):138–143. doi:10.1179/1476830515Y.0000000002
149. Sanoobar M, Eghtesadi S, Azimi A, et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutr Neurosci. 2015;18(4):169–176. doi:10.1179/1476830513Y.0000000106
150. Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Jazayeri S, Reza Gohari M. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int J Neurosci. 2013;123(11):776–782. doi:10.3109/00207454.2013.801844
151. Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2020;8:CD007807. doi:10.1002/14651858.CD007807.pub4
152. Zhang Y, Zhang T, Wu L, Li TC, Wang CC, Chung JPW. Metabolomic markers of biological fluid in women with reproductive failure: a systematic review of current literatures. Biol Reprod. 2022;106(6):1049–1058. doi:10.1093/biolre/ioac038
153. Arenas-Jal M, Suñé-Negre JM, García-Montoya E. Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf. 2020;19:574–594. doi:10.1111/1541-4337.12539
154. Díaz-Casado ME, Quiles JL, Barriocanal-Casado E, et al. The paradox of coenzyme Q10 in aging. Nutrients. 2019;11(9):2221. doi:10.3390/nu11092221
155. Gueven N, Ravishankar P, Eri R, Rybalka E. Idebenone: when an antioxidant is not an antioxidant. Redox Biol. 2021;38:101812. doi:10.1016/j.redox.2020.101812
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

