Back to Journals » Infection and Drug Resistance » Volume 16
Co-Infection Talaromyces marneffei and Pneumocystis jirovecii in a Patient with Systemic Lupus Erythematosus
Authors Chen R , Li X, Zheng D, Cao C, Su J
Received 3 April 2023
Accepted for publication 21 June 2023
Published 27 July 2023 Volume 2023:16 Pages 4913—4918
DOI https://doi.org/10.2147/IDR.S414763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Rifeng Chen, Xiuying Li, Dongyan Zheng, Cunwei Cao, Jiaguang Su
Department of Dermatology, The First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, People’s Republic of China
Correspondence: Jiaguang Su, Department of Dermatology, The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road, Qingxiu District, Nanning, Guangxi Province, 530021, People’s Republic of China, Tel +86 13878106965, Email [email protected]
Abstract: Talaromyces marneffei (TM) and Pneumocystis jirovecii (PJ) infection are opportunistic infections that typically affect individuals with compromised immune systems, such as those with HIV or immunodeficiency. However, these infections are rarely seen in patients with systemic lupus erythematosus (SLE). We present a case study of a 52-year-old woman diagnosed with SLE who developed a co-infection of TM and PJ after receiving glucocorticoids, mycophenolate mofetil (MMF), and belimumab therapy. The patient’s pneumonia improved following treatment with voriconazole, clarithromycin, and compound sulfamethoxazole. This case highlights the potential risk of serious opportunistic infections in SLE patients receiving a combination of glucocorticoids, MMF, and belimumab. Close monitoring of lymphocyte count, immunoglobulin levels, and chest computed tomography scans can aid in the early detection of infections. To the best of our knowledge, this is the first reported case of TM and PJ co-infection in an SLE patient.
Keywords: Talaromyces marneffei, Pneumocystis jirovecii, systemic lupus erythematosus, co-infection, belimumab
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that affects multiple organ systems. Treatment options for SLE include non-specific therapies such as glucocorticoids, immunomodulators, and immunosuppressants, as well as targeted biological agents.1 Due to inherent immune system defects and the immunosuppressive nature of these therapies, SLE patients are susceptible to bacterial, fungal, parasitic, and viral infections.2 Infections not only exacerbate SLE activity but also contribute to increased mortality in these patients.3 While infections are common in SLE, cases of Talaromyces marneffei (TM) or Pneumocystis jirovecii (PJ) infection, particularly TM infection, are rarely reported.4–6 Here, we present a case of co-infection involving PJ and TM in a female patient with SLE.
Case Presentation
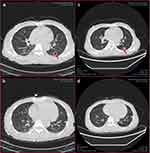
In July 2022, a 52-year-old woman was diagnosed with SLE, lupus nephritis, and polyserositis. She presented with symptoms of facial edema, anorexia, nausea, abdominal distention, cough, and white phlegm. The severity of her condition was measured using the SLE Disease Activity Index (SLEDAI), which scored 20. Initially, the patient received treatment with glucocorticoids (methylprednisolone, prednisone), immunosuppressants (cyclosporine, mycophenolate mofetil), and hydroxychloroquine sulfate. After confirming no contraindications such as infection or allergies, the patient began receiving belimumab (480mg) on July 22, 2022. Four rounds of therapy were completed by September 29, 2022. During this period, the patient’s symptoms gradually improved, and her SLEDAI score decreased. As a result, the dosage of glucocorticoids and mycophenolate mofetil was gradually reduced. However, in October, the patient developed an infection with the herpes zoster virus. Although she did not experience a noticeable cough or expectoration, she had occasional chest tightness and mild shortness of breath. Before the fifth belimumab therapy, on November 1, 2022, a chest computed tomography scan revealed patchy, nodular, and flocculent ground-glass density shadows in all lung lobes, as well as nodules and cavities in the dorsal segment of the left lower lobe. These findings indicated pulmonary infiltration associated with SLE and pulmonary infectious lesions (Figure 1a and b). HIV and anti-IFN-γ autoantibody test results were negative. White blood count and C-reactive protein were normal. Serum beta-D-glucan, serum galactomannan, cryptococcal capsular antigen, tuberculosis antibody, candida IgG antibody and candida antigen test were negative. CD4+T lymphocyte count was 332/ul (reference range, 180–324/ul), CD8+T lymphocyte count was 646/ul (reference range, 1185–1901/ul), absolute NK cell count was 16/ul (reference range, 200–587/ul), IgG 5.44 g/L (reference range, 8–18g/L), IgA 1.16g/L (reference range, 0.9–4.5g/L), IgM 0.64g/L (reference range, 0.84–1.32g/L). The next generation sequencing (NGS) of bronchoalveolar lavage fluid (BALF) showed that Pneumocystis jirovecii (sequence number 178940) and Talaromyces marneffei (sequence number 7480). TM was cultured from the patients’ BALF (Figure 2a and b). Meanwhile, BALF was positive for PJ by polymerase chain reaction (PCR). Finally, we discontinued the fifth belimumab therapy. Voriconazole (0.6g/d on day 1 and 0.4g/d from day 2) was used to treat TM infection. She had a history of glucose-6-phosphate dehydrogenase deficiency. Therefore, PJ pneumonia was treated with clarithromycin 1.8g/d for the first week. Our test for glucose-6-phosphate dehydrogenase was normal. Clarithromycin was changed to sulfamethoxazole. The treatment of SLE, maintenance of prednisone 5mg/d, MMF 0.75g/d, hydroxychloroquine sulfate 0.2g/d. After about one month of therapy, the reexamination of chest computed tomography showed that the pneumonia was improved (Figure 1c and d). The patient is still under follow-up.
Discussion
In patients with SLE, there are multiple factors that contribute to their susceptibility to infections. Firstly, inherent immune system dysfunction in these patients, exacerbated by disease activity, increases the risk of infection. Secondly, genetic factors that contribute to SLE can also lead to certain primary immunodeficiencies. Lastly, the therapies used to treat SLE, such as glucocorticoids and immunosuppressants, further compromise the immune system, making patients more vulnerable to infections. A review of literature has demonstrated that the most common infections in SLE patients align with those seen in the general population, with bacterial infections being the most prevalent, followed by viral infections. Although opportunistic infections are less common than typical bacterial and viral infections, they carry a high mortality rate.7 PJ or TM infection represents a rare and severe opportunistic infection observed in SLE patients.
In our patient’s case, the HIV and anti-IFN-γ autoantibody tests were negative. However, it is important to note that there could be other immune deficiencies that have not been identified. Additionally, the patient’s SLE disease activity was effectively controlled following therapy. We believe that the treatments for SLE likely played a significant role in predisposing this patient to the infection. Immunosuppressive therapies used for autoimmune diseases, including glucocorticoids, cyclophosphamide, methotrexate, azathioprine, and biologic agents (especially TNF-A inhibitors and rituximab), have been associated with the development of Pneumocystis jirovecii pneumonia (PCP).8 The use of additional immunosuppressants (such as cyclophosphamide, methotrexate, or azathioprine) within the first two weeks of glucocorticoid therapy has been identified as an independent risk factor for PCP.9 In a review of SLE patients infected with TM, it was found that 9 patients received prednisone prior to infection, and 3 of them were also treated with additional immunosuppressants like MMF, azathioprine, or hydroxychloroquine.4 Considering our patient’s treatment history, they were maintained on a dose of prednisone >30mg/d and MMF 1.5g/d for nearly 2 months, in addition to belimumab therapy. This combination may significantly increase the risk of developing co-infection with PJ and TM. MMF has been found to have dual activity, depleting guanosine monophosphate in B and T cells, resulting in a semi-specific inhibition of T and B lymphocyte proliferation and antibody production.10 However, recent studies have associated the development of PJ pneumonia in SLE patients with the use of MMF and high-dose glucocorticoids.6,11 Glucocorticoids and other immunosuppressants reduce total and CD4+ lymphocyte counts, and the immunosuppression caused by low CD4+ lymphocyte counts, along with changes in pulmonary surfactant, have been proposed as potential mechanisms by which glucocorticoids and other drugs contribute to the development of PCP.12,13 T Therefore, PCP can occur in SLE patients undergoing treatment with glucocorticoids and MMF.
Belimumab is a biological agent that specifically targets B lymphocytes. It binds to BLyS and prevents its interaction with B cell surface receptors, leading to the inhibition of B cell proliferation, differentiation, and antibody production. By promoting the apoptosis of autoreactive B cells and reducing the production of autoantibodies, belimumab effectively treats SLE.14 Over the past decade, studies have provided compelling evidence on the tolerability and efficacy of belimumab in reducing SLE activity, enabling corticosteroid tapering, and limiting disease-induced damage accumulation.15–17 There have been limited reports of severe opportunistic infections associated with belimumab treatment for SLE. Two cases of opportunistic infections, including cytomegalovirus pneumonia and coccidioidomycosis, occurred in patients during the fourth year of belimumab therapy.18 Additionally, there have been reports of progressive multifocal leukoencephalopathy (PML), a progressive and fatal infection caused by the JC virus, in SLE patients after receiving belimumab therapy.19,20 Numerous studies have identified joint pain, upper respiratory tract infection, headache, rash, diarrhea, fatigue, and nausea as the most common adverse events associated with belimumab.18,21 However, there have been no reported cases of TM or PJ infections in SLE patients treated with belimumab. It is important to note that a decrease in lymphocyte count is a significant risk factor for major infections in SLE patients.22 B lymphocytes play a role in cytokine secretion and have direct interactions with T cells. The depletion of B cells can impair T cell immunity and increase the susceptibility to opportunistic infections.23 From July to November 2022, our patient’s laboratory tests consistently showed a gradual decrease in immune-related indicators such as T lymphocytes, B lymphocytes, IgG, and IgM, falling below the normal range. However, the doses of glucocorticoids and MMF were gradually reduced during this period. Belimumab therapy, known to affect B lymphocyte function and reduce immunoglobulin levels, might have contributed to the impairment of host cell production of crucial virulence factors against TM/PJ infection. While we cannot exclude the possibility that belimumab therapy played a role in the development of TM and PJ infection in our SLE patient, further studies are necessary to investigate whether the combination of conventional drugs and belimumab carries a higher risk of serious opportunistic infections compared to treatment with conventional drugs alone. Additionally, it is important to assess how these factors relate to the severity of TM or PJ infections. We recommend considering discontinuation of belimumab therapy once infections develop and closely monitoring patients in such cases.
Non-HIV-infected patients with TM or PJ infection typically experience a more acute onset, rapid disease progression, increased severity, and higher mortality compared to HIV-infected patients.24,25 Currently, there are no established clinical guidelines for preventing opportunistic infections in SLE patients undergoing treatment with glucocorticoids, cytotoxic agents, or biologic agents. Long-term antifungal therapy and regular follow-up are necessary for SLE patients with TM/PJ infection who are on long-term immunosuppression. Important factors for discontinuing antifungal therapy include clinical presentation, imaging findings, and laboratory tests. Studies have demonstrated that SLE patients with PCP infection tend to have lower lymphocyte counts and CD4+ lymphocyte counts compared to those without PCP infection.26 Some research suggests that a CD4+ lymphocyte count below 200/ul should be considered as a preventive threshold for PJ infection.27,28 Additionally, low IgG levels are indicative of severe infections in SLE patients.29 When SLE patients are affected by opportunistic fungal infections, it is crucial to comprehensively assess the activity of lupus and carefully evaluate the use of glucocorticoids, immunosuppressants, especially biologic agents. We believe that following the administration of new biologic agents, the dosage of traditional immunosuppressants can be tapered more rapidly or even discontinued to minimize the risk of serious infections. Simultaneously, close monitoring of the patient’s immune status and infection indicators is essential.
Conclusion
The combination therapy of glucocorticoids, immunosuppressants, and belimumab can effectively reduce disease activity in SLE patients. However, it is important to be aware that this treatment approach may increase the risk of serious opportunistic infections, particularly in cases where lymphocyte count and IgG levels are significantly decreased. Therefore, diligent monitoring of lymphocyte count, immunoglobulin (Ig) levels, and chest computed tomography is crucial in the early detection of infections. By closely monitoring these indicators, healthcare providers can intervene promptly and manage potential infections at an early stage.
Ethical Statement and Informed Consent
The study was approved by the Ethics Committee at the First Affiliated Hospital of Guangxi Medical University. The patient provided written consent for the publication of this report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; approved final version of the manuscript; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
None of the authors has any conflict of interest.
References
1. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update omicron the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80(1):14–25. doi:10.1136/annrheumdis-2020-218272
2. Jung JY, Suh CH. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med. 2017;32(3):429–438. doi:10.3904/kjim.2016.234
3. Fei Y, Shi X, Gan F, et al. Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin Rheumatol. 2014;33(1):57–63. doi:10.1007/s10067-013-2383-3
4. Wei J, Qiu Y, Zeng W, Pan M, Zhang J. Talaromyces marneffei infection in systemic lupus erythematosus patients: report of two cases and review of the literature. Infect Drug Resist. 2020;13:3811–3816. doi:10.2147/IDR.S265479
5. Chong YB, Tan LP, Robinson S, et al. Penicilliosis in lupus patients presenting with unresolved fever: a report of 2 cases and literature review. Trop Biomed. 2012;29(2):270–276.
6. Wang WH, Lai CC, Huang YF, et al. Pneumocystis jirovecii pneumonia in systemic lupus erythematosus: a nationwide cohort study in Taiwan. Arthritis Care Res. 2022;74(9):1444–1450. doi:10.1002/acr.24584
7. Barber MRW, Clarke AE. Systemic lupus erythematosus and risk of infection. Expert Rev Clin Immunol. 2020;16(5):527–538. doi:10.1080/1744666X.2020.1763793
8. Wolfe RM, Peacock JE Jr. Pneumocystis pneumonia and the rheumatologist: which patients are at risk and how can PCP be prevented? Curr Rheumatol Rep. 2017;19(6):35. doi:10.1007/s11926-017-0664-6
9. Ogawa J, Harigai M, Nagasaka K, Nakamura T, Miyasaka N. Prediction of and prophylaxis against Pneumocystis pneumonia in patients with connective tissue diseases undergoing medium- or high-dose corticosteroid therapy. Mod Rheumatol. 2005;15(2):91–96. doi:10.1007/pl00021707
10. Oz HS, Hughes WT. Novel anti-Pneumocystis carinii effects of the immunosuppressant mycophenolate mofetil in contrast to provocative effects of tacrolimus, sirolimus, and dexamethasone. J Infect Dis. 1997;175(4):901–904. doi:10.1086/513988
11. Yeo KJ, Chen HH, Chen YM, et al. Hydroxychloroquine may reduce risk of Pneumocystis pneumonia in lupus patients: a Nationwide, population-based case-control study. BMC Infect Dis. 2020;20(1):112. doi:10.1186/s12879-020-4826-1
12. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–2498. doi:10.1056/NEJMra032588
13. Schmidt JJ, Lueck C, Ziesing S, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. 2018;22(1):307. doi:10.1186/s13054-018-2221-8
14. Dennis GJ. Belimumab: a BLyS-specific inhibitor for the treatment of systemic lupus erythematosus. Clin Pharmacol Ther. 2012;91(1):143–149. doi:10.1038/clpt.2011.290
15. Singh JA, Shah NP, Mudano AS. Belimumab for systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;2(2):CD010668. doi:10.1002/14651858.CD010668.pub2
16. Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–1128. doi:10.1056/NEJMoa2001180
17. Levy RA, Gonzalez-Rivera T, Khamashta M, et al. 10 Years of belimumab experience: what have we learnt? Lupus. 2021;30(11):1705–1721. doi:10.1177/09612033211028653
18. Merrill JT, Ginzler EM, Wallace DJ, et al. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64(10):3364–3373. doi:10.1002/art.34564
19. Fredericks CA, Kvam KA, Bear J, Crabtree GS, Josephson SA. A case of progressive multifocal leukoencephalopathy in a lupus patient treated with belimumab. Lupus. 2014;23(7):711–713. doi:10.1177/0961203314524292
20. Raisch DW, Rafi JA, Chen C, Bennett CL. Detection of cases of progressive multifocal leukoencephalopathy associated with new biologicals and targeted cancer therapies from the FDA’s adverse event reporting system. Expert Opin Drug Saf. 2016;15(8):1003–1011. doi:10.1080/14740338.2016.1198775
21. Furie R, Stohl W, Ginzler EM, et al. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a Phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10(5):R109. doi:10.1186/ar2506
22. Ng WL, Chu CM, Wu AK, Cheng VC, Yuen KY. Lymphopenia at presentation is associated with increased risk of infections in patients with systemic lupus erythematosus. QJM. 2006;99(1):37–47. doi:10.1093/qjmed/hci155
23. Luu VP, Vazquez MI, Zlotnik A. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity. 2014;47(1):1–12. doi:10.3109/08916934.2013.856006
24. Sone K, Muramatsu H, Nakao M, et al. Pneumocystis pneumonia secondary to idiopathic CD4+ T-lymphocytopenia: a comparison of AIDS and Non-AIDS patients. Intern Med. 2018;57(3):383–386. doi:10.2169/internalmedicine.8746-16
25. He L, Mei X, Lu S, et al. Talaromyces marneffei infection in non-HIV-infected patients in mainland China. Mycoses. 2021;64(10):1170–1176. doi:10.1111/myc.13295
26. Lertnawapan R, Totemchokchyakarn K, Nantiruj K, Janwityanujit S. Risk factors of Pneumocystis jeroveci pneumonia in patients with systemic lupus erythematosus. Rheumatol Int. 2009;29(5):491–496. doi:10.1007/s00296-008-0721-6
27. Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis. 2017;57:108–115. doi:10.1016/j.ijid.2017.02.010
28. Braga BP, Prieto-Gonzalez S, Hernandez-Rodriguez J. Pneumocystis jirovecii pneumonia prophylaxis in immunocompromised patients with systemic autoimmune diseases. Med Clin (Barc). 2019;152(12):502–507. doi:10.1016/j.medcli.2019.01.010
29. Md Yusof MY, Vital EM, McElvenny DM, et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 2019;71(11):1812–1823. doi:10.1002/art.40937
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.