Back to Journals » Drug Design, Development and Therapy » Volume 17
Co-Housing and Fecal Microbiota Transplantation: Technical Support for TCM Herbal Treatment of Extra-Intestinal Diseases Based on Gut Microbial Ecosystem Remodeling
Authors Sun X, Zhou X, He W, Sun W, Xu Z
Received 6 October 2023
Accepted for publication 13 December 2023
Published 24 December 2023 Volume 2023:17 Pages 3803—3831
DOI https://doi.org/10.2147/DDDT.S443462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Video abstract of “Technical support for TCM herbal treatment of extra-intestinal diseases” [443462].
Views: 65
Xian Sun,1 Xi Zhou,1 Weiming He,2 Wei Sun,2 Zheng Xu1
1School of Chinese Medicine & School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210023, People’s Republic of China; 2Department of Nephrology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, 210029, People’s Republic of China
Correspondence: Zheng Xu, School of Chinese Medicine & School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210023, People’s Republic of China, Tel +86-15951978958, Email [email protected] Wei Sun, Department of Nephrology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, 210029, People’s Republic of China, Tel +86-13505199810, Email [email protected]
Abstract: Dysregulation of the gut microbial ecosystem (GME) (eg, alterations in the gut microbiota, gut-derived metabolites, and gut barrier) may contribute to the onset and progression of extra-intestinal diseases. Previous studies have found that Traditional Chinese Medicine herbs (TCMs) play an important role in manipulating the GME, but a prominent obstacle in current TCM research is the causal relationship between GME and disease amelioration. Encouragingly, co-housing and fecal microbiota transplantation (FMT) provide evidence-based support for TCMs to treat extra-intestinal diseases by targeting GME. In this review, we documented the principles, operational procedures, applications and limitations of the key technologies (ie, co-housing and FMT); furthermore, we provided evidence that TCM works through the GME, especially the gut microbiota (eg, SCFA- and BSH-producing bacteria), the gut-derived metabolites (eg, IS, pCS, and SCFAs), and intestinal barrier to alleviate extra-intestinal diseases. This will be beneficial in constructing microecological pathways for TCM treatment of extra-intestinal diseases in the future.
Keywords: gut microbial ecosystem, TCM herb, extra-intestinal disease, co-housing, fecal microbiota transplantation
Introduction
Dysregulation of the gut microbial ecosystem (GME) (eg, alterations in the gut microbiota, gut-derived metabolites, and intestinal barrier) may contribute to the onset and progression of extra-intestinal diseases.1–3 A healthy gut microbiota produces dynamic changes according to the body’s biorhythms to maintain host homeostasis. In contrast, dysbiosis of the gut microbiota (eg, alterations in composition, abundance, and interactions) may lead to the onset and progression of extra-intestinal diseases.4–6 For example, alterations in microbiota composition can transform normal commensal bacteria into pathogenic agents that can adversely affect target organs. Similarly, interactions between commensal bacteria and the immune system can lead to maturation of the human immune system and relative stabilization of the gut microbiota structure. Conversely, an imbalance in this interaction can lead to the pathogenesis of many extra-intestinal diseases. In addition, gut-derived metabolites are intermediate or final products of bacterial fermentation. They are one of the important molecules in the gut-organ crosstalk. Depending on the level of specific metabolites produced in the host, they can have beneficial or detrimental effects on the organs and are associated with a wide range of extra-intestinal diseases.7–9 In addition, the intestinal barrier is the first line of defense between the external environment and the gut. It plays an important role in nutrient absorption and also serves as a natural barrier to prevent and inhibit microbial translocation. A compromised intestinal barrier contributes to the disruption of intestinal integrity and immune homeostasis, ultimately affecting extra-intestinal diseases.10–12
Given the impact of GME on susceptibility to extra-intestinal diseases, its utilization for therapeutic purposes may be promising. Previous studies have found that Traditional Chinese Medicine herbs (TCMs) have a close interaction with the GME. In short, herbal treatments affect the gut microbiota, gut-derived metabolites, or intestinal barrier.13–15 In turn, GME plays an important role in converting carbohydrates, proteins, lipids, and non-nutritive small molecule compounds from herbs into chemical metabolites that may be beneficial or harmful to the host.16 In addition, there is growing evidence that herbal interventions are highly effective in alleviating extra-intestinal disease, accompanied by improved GME.
However, a prominent obstacle to current TCM research is the causal relationship between GME and disease improvement. Encouragingly, GME remodeling based on co-housing or fecal microbiota transplantation (FMT) has provided technical support and data evidence for the above obstacles (Figure 1). Specifically, microbial transfer properties are core elements of co-housing and FMT. Recent studies have shown that rodents with different gut microbiota can achieve microbial transfer in a shared environment.17,18 In short, co-housing techniques exploit the coprophagia behavior of rodents and use their shared living environment as a medium for the ultimate purpose of microbial transfer. In addition, FMT involves the transplantation of gut microbiota obtained from the feces of healthy donors into the gastrointestinal tract of a patient (or an animal model of disease), with the ultimate goal of restoring the recipient’s normal microbiota.19 As reported in a large number of studies, co-housing- or FMT-based remodeling of the GME effectively replaces the indigenous bacterial community and contributes to the mitigation of extra-intestinal diseases.20 These studies exploited the characteristics of microbial transfer to achieve microbiota remodeling, which contributed to the elucidation of GME as one of the important mechanisms of disease remission. Notably, a growing number of studies have used co-housing and FMT as technical means to verify the mediating role of GME in TCM treatment. In most of TCM study protocols, mice that had been treated with herbs or herbal components for a period of time were selected as co-housed companions or donors for FMT.
 |
Figure 1 Mediation of gut microbial ecosystem: the manipulation power behind the improvement of extra-intestinal diseases by Traditional Chinese Medicine herbs. |
In this review, we document the principles, operational procedures, applications, and limitations of the key technologies (ie, co-housing and FMT); furthermore, we provide evidence that herbal treatments alleviate extra-intestinal diseases through the GME, especially the gut microbiota (eg, SCFA- and BSH-producing bacteria), gut-derived metabolites, and intestinal barriers for the alleviation of extra-intestinal diseases. This will be beneficial in constructing microecological pathways for TCM treatment of extra-intestinal diseases in the future.
Key Technologies for GME Remodeling: Co-Housing and FMT
Co-Housing
Principle of Co-Housing
Recent studies have shown that the gut microbiota of rodents can be transferred in a shared environment (eg, co-housing). For example, Surana et al found that rodents with different gut microbiota were able to produce hybrid microbiota (intermediate phenotype) after co-housing.17,18 The research results indicated that co-housing could achieve bidirectional transfer of microbial communities. Specifically, this strategy exploits the coprophagia behavior of rodents and uses their shared living environments as a medium for the ultimate purpose of microbial transfer (Figure 2). Therefore, research on co-housing has been largely focused on animals, and human research is still lacking.
 |
Figure 2 Microbiota-transfer strategy: Co-housing. |
Operation Process of Co-Housing
Environmental conditions: All experimental murine were housed in specific-pathogen-free barrier conditions. Specifically, murine were housed in conventional plastic (or individually ventilated) cages and were provided with adequate standard food and water AD libitum. The indoor environmental conditions in which the murine were housed included temperature (22°C±2°C), humidity (50%±10%), and 12-hour light-dark cycle.
Allocation ratio per cage: Typically, murine (from two experimental groups) were co-housed in a cage at a 1:1 ratio. The raising density of murine can be adjusted according to the specific experimental requirements, but the number of murine per cage should be controlled below five.
Time setting: The duration of co-housing was set based on the specific experimental purpose and the maximum avoidance of adverse factors that could interfere with the experimental results. ①Continuous short-term co-housing: It was mainly suitable for experiments with strict requirements on time and gut microbiota. In order to achieve microbial transfer and maximize the control of changes caused by time factors (eg, gut microbiota, intestinal tissue), continuous short-term co-housing was often the preferred strategy in the study of intestinal diseases21,22 and extra-intestinal diseases (eg, nonalcoholic fatty liver disease (NAFLD), asthma, nephrocalcinosis).23–25 Ji et al achieved gut microbiota remodeling in DSS-induced colitis mice based on a continuous short-term co-housing strategy, demonstrating that daphnetin ameliorated experimental colitis by modulating microbiota composition and Treg/Th17 balance.26 ② Intermittent co-housing: Co-housing for more than six weeks resulted in some degree of chronic stress and behavioral disorder.27 According to previous studies, chronic stress reduced body weight and disrupted gut microbiota (eg, Helicobacter, Peptostreptococcaceae, Enterococcus faecalis, and Streptococcus) associated with inflammation.28 Therefore, several studies have applied intermittent co-housing strategy to reshape the gut microbiota to avoid unexpected chronic stress or behavioral changes caused by continuous co-housing. In a study on whether gut microbiota remodeling could reverse aging-related inflammation and systemic bile acid dysfunction, an intermittent co-housing approach was used. Intermittent co-housing consisted of two rounds lasting 10 weeks in their study. Specifically, the first and second rounds of co-housing lasted two and four weeks, respectively, with two weeks of separate-housing at the end of each round.29 ③Continuous long-term co-housing: It was typically used to analyze the effects of environmental and microbial factors on neurological and mental behavior. Abdel-Aziz et al applied a six month continuous long-term co-housing strategy to demonstrated the contribution of microbial and environmental factors to cognitive and affective behavior in C57BL/6 sub-strains (C57BL/6J and C57BL/6N mice).30
Precautions: ①Animal ethics: The experimental operating procedures need to be approved by the institutional animal care and use committee. ②Animal monitoring: The co-housed animals need to be monitored for any signs of fighting (ie, wounds, tail lesions, or any injuries) for timely improvements (eg, maintaining stable environmental temperature and humidity, reducing raising density, providing sufficient padding, and wound treatment), ultimately ensuring the safety of co-housing and the accuracy of experimental results.
The Application of Co-Housing in Mechanism Research
Study on the microbial mechanism of drug intervention: drug interventions have shown considerable effects in alleviating diseases, accompanied by improved GME. Wu et al reported that Linggui Zhugan formula improved glucose levels and increased the abundance of SCFA-producing bacteria in high-fat diet (HFD)-induced diabetic mice.31 Ji et al confirmed that rhubarb enema effectively reduced the level of gut-derived metabolite (TMAO) and improved renal function in 5/6 nephrectomized chronic kidney disease (CKD) rats.32 Liu et al indicated that vitamin E α- and γ- Tocopherol alleviated DSS-induced colitis and protected the intestinal barrier function in mice.33 However, the causal relationship between reshaped GME and disease remission has not been fully elucidated, and relevant research efforts are actively underway. Co-housing with microbial transfer properties is one of the key technologies driving mechanistic studies. Grant et al found that circulating inflammatory factor (IL-1β) was significantly reduced when chemotherapy-treated female mice were co-housed with healthy female mice.34 Luccia et al suggested that co-housing with responsive mice improved the immune response to cholera toxin in hypo-responsive mice.35 These results suggested that remission of the disease might depend on the mediation of the gut microbiota. Therefore, the mining and regulation of targeted microbiota may establish part of the microecological pathway for disease treatment. Wang et al found that Tetrastigma hemsleyanum leaves (THLW) significantly decreased the severity of ulcerative colitis (UC), which was characterized by improvements in body weight and colon length, and reductions in disease activity index and histological score. Further co-housing experiments confirmed that the protective effect of THLW was mediated by regulating gut microbiota, especially by increasing the abundance of Oscillospiraceae, Prevotellaceae and Corynebacterium.22 Hong et al demonstrated that the remodeled gut microbiota during co-housing contributed to the therapeutic effects of Astragalus polysaccharides on NAFLD. Furthermore, by bacterial screening, a potent acetic acid-producing bacterium, Desulfovibrio vulgaris, was identified as a potential biomarker for the remission of NAFLD.23 In summary, co-housing provides technical means and experimental evidence for assessing the contribution of gut microbiota to the effect of drug on disease treatment.
Mining of novel biomarkers and therapeutic targets for disease: studies have reported that depletion of related genes or biomarkers can affect host susceptibility to disease. Agudelo et al suggested that Gpr35−/− mice were susceptible to HFD-induced obesity.36 Xie et al showed that Dock2−/− mice were more susceptible to colitis induced by Citrobacter rodentium infection than wild type mice.37 However, the underlying mechanism remains unclear. Previous studies have shown that deletion of specific genes or biomarkers can be accompanied by modulation of the GME. For example, Toll-like receptor 4 (TLR4) has been identified as a potential therapeutic target for acute pancreatitis (AP).38 Mei et al showed that the loss of intestinal epithelial TLR4 aggravated pancreatic and intestinal injury, and also reduced the relative abundance of Lactobacillus.39 In another research of AP, Li et al found that knockout of NLRP3 could reshape the gut microbiota and protect mice from intestinal damage induced by AP. Specifically, wild-type AP mice exhibited significant changes in the gut microbiota characterized by pathogenic bacterium (Escherichia-Shigella) overgrowth compared to NLRP3−/− AP mice, suggesting that NLRP3 deficiency counteracted AP-induced microbial dysregulation.40 These results suggested that host susceptibility to disease might be in gut microbiota-dependent manner. Interactions among gut microbiota, dysregulated immune responses, and genetic factors are involved in the pathogenesis of inflammatory bowel disease (IBD). Leber et al found that loss of Nlrx1 in epithelial cells increased susceptibility to DSS colitis. Gut microbiota analysis showed an increase in Veillonella and Clostridiales in Nlrx1−/−mice. Transfer of Nlrx1−/−associated gut microbiota by co-housing worsened the disease in WT mice, confirming the contribution of the gut microbiota to the Nlrx1−/−phenotype.41 In summary, co-housing provides a technical means for the mechanistic study of therapeutic targets and biomarkers, as well as an experimental basis for precise targeted-intervention.
Impact of microbial and environmental factors on sub-strains: genetic variation resulted in large differences in phenotypic traits among the sub-strains. However, recent studies have shown that inconsistency in phenotypic behavior may be due to certain phenotypes not only derived from host genetics but also attributable to alterations in microbial profiles and environmental factors. Abdel-Aziz et al applied a six month continuous long-term co-housing strategy to demonstrated the contribution of microbial and environmental factors to cognitive and affective behavior in C57BL/6 sub-strains (C57BL/6J and C57BL/6N mice). The results of this study suggest that environmental conditions have a direct effect on gut microbiota, which in turn affects the brain immune cell profile to regulate cognitive and affective behaviors.30
Limitations and Countermeasures of Co-Housing
Induce chronic stress and behavioral disorders: co-housing for more than six weeks resulted in chronic stress and behavioral disorders. According to previous studies, chronic stress lead to adverse outcomes characterized by reduced body weight and disrupted gut microbiota associated with inflammation. Therefore, for experimental purposes, some studies have adopted intermittent co-housing as their preferred strategy to avoid unexpected chronic stress or behaviors caused by continuous co-housing.29
Impact of potential confounders: co-housing involves multiple potential confounding factors, such as food source and processing, ventilation, density per cage, and barrier environment, which may contribute to the differences in co-housing outcomes.42 Therefore, the implementation of co-housing should follow standard operating procedures, and further research on the confounders mentioned above is needed.
Relatively low transfer efficiency: Grant et al partially alleviated inflammatory levels in chemotherapy-treated female mice by co-housing them with healthy female mice. At the same time, the donor (healthy female mice) developed altered gut microbiota profile and chemotherapy-related side effects (a significant decrease in plasma IL-1β level).34 These results suggested that due to bidirectional nature of microbial transfer in co-housing, the gut microbiota in donor murine is altered to varying degrees, which may ultimately lead to poor remodeling of the gut microbiota in recipient murine. Moreover, Zhang et al compared three gut microbiota transfer methods (embryo transfer, cross-fostering, and co-housing) to evaluate transfer efficiency and the differences in disease remission. It was found that the efficiency of microbial transfer in co-housing was significantly lower than that in embryo transfer and cross-fostering. In addition, the therapeutic effect of co-housing on DSS-induced chronic colitis mice was significantly decreased compared with embryo transfer and cross-fostering.43 Therefore, Based on the research purpose and disease, the best matching strategy among the three co-housing strategies (Continuous short-term, Intermittent, Continuous long-term) was selected by researchers to achieve the best microbial transfer efficiency. Furthermore, in order to obtain the satisfactory effect of microbiota transfer, the strategy of co-housing combined with FMT has been employed in some studies.44
FMT
Background and Application of FMT
In recent years, FMT has become a medical technology that has attracted much attention. FMT is the transplantation of gut microbiota obtained from the feces of healthy donor into the gastrointestinal tract of patient (or diseased animal model), with the goal of restoring the normal microorganisms of the recipient. From the perspective of the development of FMT, it can be divided into four stage according to the methods of FMT, namely original, standardized, washed and precision FMT.
The earliest records of FMT date back to the 4th century in China, where human fecal material, known as “Huang Tang”, was used to treat patients with severe diarrhea, setting a precedent for FMT using directly untreated feces. In addition, 23 prescriptions with human feces as components were recorded by Li shizhen in medical book of the Ming Dynasty in the 16th century.45 In the 17th century, the concept of “transfaunation”, the transfer of gastrointestinal contents from healthy to diseased animals, was proposed by Acquapendente, based on the Bedouin experience of treating bacillary dysentery by consuming camel faeces.46 Moreover, Eiseman et al successfully applied FMT to the treatment of disease in 1958. Three patients with pseudomembranous enterocolitis unresponsive to vancomycin and metronidazole were improved by enema with human fecal supernatant.47
Subsequently, clinical cases regarding the use of FMT in the treatment of Clostridium difficile infection (CDI) began to emerge in large numbers. By 2013, the first controlled clinical study of FMT for recurrent CDI-associated diarrhea showed that FMT was significantly more effective than vancomycin.48 In addition, The Food and Drug Administration (FDA) officially declared human feces as drugs in 2013 and recommended strict supervision of samples in clinical trials due to the risk of accidental transmission of pathogens and development of antibiotic resistance.49 In the same year, FMT was included in clinical practice guidelines for the treatment of recurrent CDI.50 These experiments and events contribute to the development of FMT to a new stage.
Manual FMT has been used to treat diseases for thousands of years. However, there are still safety risks in this method, which challenges the psychological endurance and acceptance of doctors, patients and donors. Since 2014, the method (Washed microbiota transplantation, WMT) has been developed and applied in the FMT Center of China. It is based on automatic microfiltration equipment and has multiple washing processes, which can remove undigested food residues, fungi, eggs of parasitic worms and some pro-inflammatory metabolites, ensuring the quality and safety of the microbiota preparation.51 A previous study showed that washed microbiota preparation significantly reduced the incidence of adverse events from 21.7% to 8.7% in patients with Crohn’s disease.52 Notably, the washed microbiota preparations did not affect therapeutic efficacy compared with manual microbiota preparations in the aforementioned study.
The gut microbiota of different individuals is highly heterogeneous, and even healthy individuals have different intestinal types. Therefore, it is of great significance to achieve precision FMT. For example, based on six bacterial indicators (richness, distance, beneficial taxa, harmful taxa, beneficial pathway and harmful pathway), an analytic hierarchy process (AHP)-based donor-recipient matching model was established to select suitable donors for UC patients. The model showed good accuracy (>70%) for the effectiveness of FMT in two previous clinical trials.53 At present, FMT is used to treat many diseases related to gut microbiota imbalance, or as a key technology to reshape GME in animal experiments. However, precision FMT is still in its infancy.
Operation Process of FMT
Donor selection and recipient preparation: strict screening of FMT donors is recommended to reduce and prevent adverse events. The guidelines recommend the use of donor questionnaires to meet exclusion and inclusion criteria.54–56 In addition, screened donors should undergo an additional interview on the day of donation to assess recent potentially harmful behaviors to minimize the risk of FMT.54 It is particularly important that standardized donor screening protocols should be followed to reduce the risk of donor-to-recipient transmission of infection, and suitable donors should undergo blood and stool tests within 4 weeks prior to donation.54–56 Recipients of FMT need to receive support and education prior to treatment.57 They should not be treated with any form of antibiotics 12–48 hours before fecal infusion.54 Preparation for FMT is similar to that for other endoscopic procedures, including standard bowel preparation.
For FMT experiments in rodents, donor selection is based on experimental purposes. Some studies used FMT to achieve microbiota remodeling, verifying that the GME plays an important role in disease treatment. For example, Hu et al transplanted fecal contents from healthy male mice into experimental autoimmune myocarditis mice, in which myocardial damage was improved and inflammatory cell infiltration was reduced.58 Similarly, Ma et al performed gut microbiota remodeling on old mice (> 24 months) by FMT from young mice (8 weeks), and found that FMT significantly improved natural aging-related systemic disorders (eg, glucose sensitivity, hepatosplenomegaly, inflammation, and liver injury).59 In addition, previous studies used FMT as a technical means to verify the mediating role of GME in drug treatment. Li et al transplanted fecal preparation from MCAO mice treated with TCM for two weeks into TCM-untreated MCAO mice. They found that the significant improvement in neurological function of the recipients was attributed to the mediation of SCFA-producing bacteria.60 Moreover, FMT provides experimental basis for novel treatment strategies. Zhang et al demonstrated that FMT from NLPR3−/−mice significantly ameliorated the depressive-like behavior induced by chronic unpredictable stress in recipient mice, suggesting that NLRP3 may be a potential therapeutic target.61 The five most common animal models (eg, germ-free (GF), antibiotic-treated, conventional, laxative-treated, vertical microbiota transmission) can be used as recipient for FMT studies, with each model having advantages and limitations.62 Researchers need to determine the most appropriate approach based on research requirements. For instance, GF model appears as a reliable recipient for FMT studies, which is excellent for testing specific mechanisms of interventions on host. Furthermore, the greatest advantage of utilizing this model to receive exogenous gut microbiota is the lack of competition for gut colonization with resident microbes. Sharon et al transplanted gut microbiota from human donors with autism spectrum disorder (ASD) or typically-developing (TD) controls into GF mice and revealed that colonization with ASD microbiota is sufficient to induce hallmark autistic behaviors.63 However, the antibiotic-treated model is widely used alternative to GF model for a variety of reasons: simpler in experimental design, high cost effectiveness, no need for specialized housing equipment, and circumventing the limitations of GF model. For example, to address the causative relationship between gut dysbiosis and pre-eclampsia (PE), Chen et al used FMT in an antibiotic-treated mouse model.64
Fecal collection and and processing: most of the microorganism of the colon is strictly anaerobic bacteria. Feces can be stored in a sample container with an anaerobic sachet to maintain an oxygen-free environment. Containers containing fecal samples should be traceability labeled, including a unique code for the donor and the date of collection and processing. The collected samples should preferably be delivered to fecal microbiota bank within six hours, where it will be received, weighed, assessed, recorded and processed by qualified staffs.65 As for the collection of rodent feces. Fresh samples can be obtained by gently pressing the rodent’s abdomen to induce spontaneous defecation. However, defecation by pressure is actually based on a stress response and may be in a tense state. A gentler approach is to place the donor rodent in a clean cage and wait for defecation, which usually occurs within 10 to 60 minutes.62 Alternatively, samples can be taken surgically from the cecum or colon without sacrificing the animal.66 The amount of feces required for microbiota preparation depends on the experimental design of the study. Researchers then use individual sterile tools for collection and place fecal pellets into sterile tubes prior to processing in an anaerobic chamber.62 For short-term storage, to maximize microbial preservation, all human or rodent fecal samples should be kept at 4°C or lower after collection and during transport (but should not be frozen, as freeze-thaw cycles can compromise sample quality).67 There are some variations in FMT preparation across institutions and labs. However, the overall process is similar and consists of mixing feces with a bacteriostatic solution, removing particulate matter, and delivering the feces to the recipient. On the day of FMT, fresh feces can be diluted in sterilized phosphate buffered saline (PBS) to obtain an estimated fecal suspension. Feces can be diluted approximately 3–6 times with PBS.68 Notably, the proportion of feces diluted in the preparation of FMT can be adjusted appropriately to meet specific requirements. In addition, frozen aliquot fecal suspension can be thawed in a 37°C water bath for 10–15 minutes with the addition of L-cysteine amino acid (a reducing agent that preserves anaerobic bacteria). It is recommended that FMT be performed within six hours of thawing. Fecal suspensions should be filtered with filters or automated microbial filtration instruments to remove larger particulate matter. Furthermore, centrifugation can be performed to separate undissolved solids.69 Centrifugation speeds vary depending on the FMT preparation method.
Delivery routes: FMT can be delivered via the upper or lower gastrointestinal tract, and common routes include nasogastric/nasojejunal tube, endoscopy (sigmoidoscopy, colonoscopy), oral capsules, retention enema.70 Generally, FMT can be performed via the upper GI route in patients with an inflamed colon; However, the discomfort of intubation, the risk of aspiration, and the inability to collect and evaluate mucosal tissue samples are weaknesses. FMT can also be delivered via the lower GI route. For example, FMT via colonoscopy is superior in recolonizing the entire colon with beneficial bacteria, and preoperative bowel preparation contributes to visualization of the entire colon by reducing the number of residual microorganisms and spores; however, it is a relatively risky and invasive procedure. FMT by retention enema is more cost-effective and less invasive than colonoscopy, but it has the disadvantage that the donor’s fecal bacterial preparation cannot be delivered to the entire colon and is limited to the distal colon.70,71 Conventional gastric delivery capsules and colon-targeted capsules are the most commonly used dosage forms with the advantages of low invasiveness and high patient acceptance, but high cost and capsule burden are disadvantages.70,71 Notably, delivery routes have differences in effectiveness for the treatment of related conditions (eg, recurrent CDI,72 IBD,73 diarrhea,74 and IBS75–78). Therefore, Associated factors (eg, FMT effectiveness, recurrence rate, comfort of administration, invasiveness, patient compliance, cost-effectiveness, admission rates, risk of aspiration and infection) are the main factors influencing the choice of delivery route by physicians.70 Oral-gastric gavage is a commonly used and preferred route of delivery in FMT experiments in rodents, mainly due to the simplicity of the technique, controllable amount of drug administered, etc.79,80 It is not recommended to perform any anesthesia prior to oral-gastric gavage, which can increase the risk of aspiration pneumonia in rodents.81 The route is effective in remodeling the gut microbiota of rodent recipients, but obstacles associated with it include inadvertent drug entry into the trachea, bronchopneumonia, esophageal injury, gastric rupture, and weight loss.82,83
Follow-up and monitoring: FMT is considered a generally safe therapeutic strategy; nevertheless, recipients undergoing FMT need to be informed of its potential risks prior to treatment. Previous clinical trials and systematic reviews have shown some adverse effects after FMT, such as abdominal discomfort, diarrhea, constipation, low-grade fever, and complications that may be associated with endoscopy and anesthesia. Of note, no differences in the proportion of adverse events were observed between FMT delivery routes.84 The European consensus suggests a follow-up period of at least 8 weeks after FMT in patients with CDI.54 Regardless, the details of the content and period of follow-up after recipient FMT have not yet been standardized so far. Rodents may also suffer similar post-FMT adverse effects as humans.85 Moreover, recipients receiving exogenous microbial colonization may cause an increased inflammatory response. Regardless of the route of delivery, it is recommended that enhanced monitoring of the recipient rodent for at least 24 to 48 hours after FMT is required.
Limitations and Countermeasures of FMT
Optimizing donor screening: there are no clear and universal donor screening criteria for FMT. Work is ongoing to study the effect of donor characteristics on recipients in order to develop simple and feasible donor screening criteria. For example, previous studies have shown that factors such as the donor’s diet and fecal butyrate content had no significant effect on FMT for CDI. Moreover, Donor BMI and age might not affect FMT efficacy.86 In addition, a recent clinical study demonstrated through a randomized controlled trial that the metabolic profile of the FMT donor can be “transferred” to the recipient.87 Therefore, donor screening criteria need to be refined and standardized to further optimize FMT.
Post-FMT adverse reactions: FMT is safe overall, including for use in high-risk groups such as immunocompromised individuals and patients with inflammatory bowel disease. Abdominal discomfort, flatulence, short-term low-grade fever, change in bowel habits, abdominal ringing, nausea and vomiting are common GI adverse events, most of which are mild and self-limiting. Serious adverse events are rarer and are mostly caused by coexisting conditions or FMT manipulation. Regardless, potential side effects of FMT, such as chronic diseases or transmission of pathogens due to altered gut microbiota, need to be more studied and reported.88
Infection risk of the FMT process: operators (rodents) should be appropriately protected to avoid and deal with the potential infectious risks associated with FMT.89 The utilization of human feces in animal studies also requires safety considerations. In human-to-human FMT, donors are interviewed and screened to rule out blood- and fecal-related infectious risks. The same screening principles apply to human-to-animal transplants to protect researchers and animal caregivers. Appropriate safety measures should be taken when working with FMT, including decontamination of the work area, use of personal protective equipment, and placement of rodents in protected systems such as individually ventilated cages.
Evidence That TCM Modifies Extra-Intestinal Diseases Through GME Remodeling
Gut Dysbiosis in Extra-Intestinal Diseases
Symbiosis is recognized as a complex and long-term biological interaction between two symbiotic “organisms”, such as the gut microbiota and the human body. A healthy gut microbiota produces dynamic changes in response to the body’s biological rhythms to maintain host homeostasis. Conversely, dysbiosis of the gut microbiota (eg, alterations in composition, abundance, and interactions) may lead to the onset and progression of extra-intestinal diseases. For example, alterations in the composition of the microbiota can transform normally symbiotic microorganisms into pathogenic agents that adversely affect target organs. Then again, the interaction between commensal bacteria and the immune system leads to the maturation of the human immune system and the development of a relatively stable structure of the gut microbiota. Thus, an imbalance in this interaction contributes to the pathogenesis of many extra-intestinal diseases. Encouragingly, large-scale clinical studies on the microbiota (eg, composition, relative abundance, symbiosis, etc.) of extra-intestinal diseases have gradually increased in recent years. Breakthroughs have also been made in exploring the underlying pathogenesis of extra-intestinal diseases through animal models based on microecological pathways. There is growing evidence of gut microbiota dysbiosis in extra-intestinal diseases. Studies have reported changes in the gut microbial composition of patients with major depressive disorder (MDD) compared to healthy individuals, particularly in terms of microbial diversity and abundance of specific bacterial community (enrichment of pro-inflammatory bacteria and reduction of anti-inflammatory bacteria).90 Vogt et al analyzed fecal samples from 25 Alzheimer’s disease (AD) patients and found decreased diversity and differences in the composition of the gut microbiota (ie, decreased Firmicutes and Bifidobacterium, increased Bacteroidetes).91 Shilo et al found significant differences in the gut microbiota of adult patients with type 1 diabetes (T1D) compared to controls, characterized by an enrichment of Prevotella copri and Eubacterium siraeum, however, a decrease in Firmicutes bacterium and Faecalibacterium prausnitzii.92 In addition, dysregulation of beneficial and harmful bacteria is favorable microecological evidence of extra-intestinal disease progression. As reported in a previous study of AD patients, the massive amplification of harmful amyloid-producing and endotoxin-producing bacteria (Escherichia/Shigella) was accompanied by a shrinkage of beneficial bacteria (Eubacterium rectale, Bacteroides fragilis) with anti-inflammatory effects.93 Similarly, Zuo et al indicated that the gut microbiota of COVID-19 patients was characterized by an enrichment of opportunistic pathogenic bacteria and a decrease in beneficial bacteria (butyrate-producing bacteria).94 Of note, previous studies also focused on the correlation between gut microbiota and clinical indicators in patients with extra-intestinal diseases. For instance, in adult patients with T1D, some bacterial taxa were significantly associated with glycemic indices, with Prevotellaceae species (SGB592 and SGB1340) negatively correlated with HBA1c levels, and Enterobacteriaceae bacteria (SGB2483) positively correlated with mean blood glucose values.92 In addition, analysis of the gut microbiota in atherosclerotic cardiovascular disease (ACVD) showed that the abundance of Streptococcus spp. was positively associated with blood pressure, as was the abundance of Enterobacteriaceae spp. with myocardial markers.95
Gut-derived metabolites are intermediate or end products of bacterial fermentation. They are one of the important molecules in the gut-organ crosstalk. Depending on the level of specific metabolites produced in the host, they exert beneficial or deleterious effects on organs and have been linked to several extra-intestinal diseases. For example, gut-derived metabolites can directly or indirectly modulate the central nervous system (CNS) through immune, neuronal, and metabolite-mediated pathways in the microbiota-gut-brain axis. In the gut lumen, dietary products can be metabolized by the microbiota into neuroactive compounds, including neurotransmitters (eg, serotonin, dopamine), amino acids (eg, tryptophan, tryptamine), and other gut-derived metabolites (eg, SCFAs, trimethylamine (TMA)). Metabolites can trigger physiological responses directly by crossing the blood-brain barrier and affecting the CNS, or indirectly via neurotransmitters through the enterochromaffin or immune pathways.96 Conversely, gut imbalances (eg, increased intestinal permeability, changes in gut microbiota), which in turn lead to altered levels of various metabolites. These metabolites can enter the circulation and ultimately be transported to the CNS, contributing to disturbed neuronal activity and neurotransmitter expression.97 On the other hand, some metabolites have been recognized as promising markers for the treatment of extra-intestinal diseases. TMAO is one of the representative gut-derived metabolites, and its main source are l-carnitine, choline and betaine. Specifically, these precursors are metabolized by the gut microbiota in the colon and cecum to produce TMA, which is taken up and reaches the liver via the portal circulation, where it is rapidly oxidized by the flavin-containing monooxygenase to produce TMAO. Of note, TMAO has been extensively studied in cardiovascular diseases.98,99 For example, plasma levels of TMAO were positively correlated with blood pressure in hypertensive patients. Animal study found that TMAO elevated systolic blood pressure and caused vasoconstriction in Ang II–induced hypertensive mice, which was mediated by the PERK/ROS/CaMKII/PLCβ3/Ca2+axis. These findings provide new insights into the pathogenesis of hypertension and identify TMAO as a potential therapeutic target and biomarker.100
The intestinal barrier is the first line of defense between the external environment and the gut. It plays an important role in nutrient absorption, as well as being a natural barrier that prevents and inhibits microbial translocation, ultimately contributing to intestinal integrity and immune homeostasis. Specifically:1) The intestinal barrier is a multilayered structure: it consists of the mucus, epithelial and lamina propria layers, in addition to another key component-the gut microbiota. 2) Mucus and epithelial layers: the mucus layer consists of water and mucins secreted by goblet cells, which has antimicrobial properties and keeps bacteria away from the mucosa. In addition, the intestinal epithelium is a single layer of columnar epithelium that separates the intestinal lumen from the lamina propria. 3) Mechanism: Intestinal immune homeostasis is maintained through the coordinated interplay of intestinal epithelial cells (IECs) and gut microbiota. In a healthy state, a small number of bacteria cross the intestinal lumen; IECs are able to differentiate between commensal bacteria and pathogens and regulate the expression of pattern-recognition receptors (PRRs) to regulate inflammatory responses. However, in the presence of immune system dysfunction and a compromised intestinal barrier (or dysbiosis of the gut microbiota), this balance is disturbed and more bacteria and their products translocate into mesenteric lymph nodes and systemic circulation. Microbial translocation exacerbates mucosal and systemic inflammation, further increasing intestinal permeability and contributing to a vicious cycle. This mechanistic model of gut-driven inflammation is a recognized trigger for the onset and progression of extra-intestinal diseases.101 Other studies provide favorable evidence to support the idea that the integrity and permeability of the intestinal barrier can serve as a disease marker and therapeutic target. As an example, previous studies have found that CNS disorders (cognitive impairment, Parkinson’s disease (PD), AD, multiple sclerosis, autism, etc.) are accompanied by alterations in the intestinal barrier. Clinical and animal evidence suggested that a compromised intestinal barrier lead to translocation of gut microbiota, gut-derived metabolites, or CNS-associated pathogenic proteins into the systemic circulation, either activating an inflammatory response or transporting to the brain, which in turn triggered CNS pathology. On the contrary, improvement of the intestinal barrier through probiotics/prebiotics and microbial metabolism is beneficial for the prevention and treatment of CNS-related diseases.12
Co-Housing- or FMT-Based Remodeling of GME in Extra-Intestinal Diseases
Given the impact of the GME on susceptibility to extra-intestinal diseases, manipulating the microbiota for therapeutic purposes may be promising. The following potential approaches may be preferred for microbial manipulation: (1) direct regulation through the addition or elimination of specific bacterial strains or communities, such as the administration of certain bacteria (antibiotics, etc.); (2) indirect regulation through diet, mediation, and the environment; and (3) replacement of the indigenous microbiota through co-housing or FMT from suitable donors.20
Robust data have confirmed that co-housing- or FMT-based remodeling of the GME effectively replaces the indigenous bacterial community and contributes to the mitigation of extra-intestinal diseases (Table 1). Some studies utilized FMT to achieve microbiota remodeling, validating the important role of the GME in the treatment of extra-intestinal diseases. For example, Wang et al performed gut microbiota remodeling on the MCAO mice by FMT from healthy female mice, and found that a female-like biological community reduced the level of systemic pro-inflammatory cytokines after ischemic stroke. Furthermore, poor stroke outcomes were positively modulated by supplementation of the female gut microbiota.102 Similarly, Chen et al transplanted fecal materials from healthy college students into cognitively impaired patients and found that cognitive function could be improved by altering the structure of the gut microbiota and serum metabolomics.103 In addition, previous studies used FMT as a technical means to verify the mediating role of GME in drug treatment. Zou et al transplanted fecal preparation from Qiong Yu Gao (QYG)-treated AKI mice into TCM-untreated mice. They found that the renal function and fibrosis could be improved by the mediation of gut microbiota (SCFA-producing bacteria) and its metabolites (acetic acid, butyric acid).104 Moreover, a potent acetic acid-producing bacterium, Desulfovibrio vulgaris, was identified as a potential biomarker for NAFLD remission based on co-housing experiment combined with bacterial screening by Hong et al.23
Co-Housing and FMT: A Button for Constructing Microecological Pathways to Treat Extra-Intestinal Diseases via TCM Herbs
Co-Housing and FMT in the Treatment of Extra-Intestinal Diseases by TCM Herbs
TCM herbs (TCMs) interact closely with the gut microbiota, including influencing their composition, abundance and interactions.126 In turn, the gut microbiota plays an important role in converting carbohydrates, proteins, lipids, and non-nutritive small molecule compounds derived from TCMs into chemical metabolites that may be beneficial or harmful to the host. These results suggest that manipulation of gut microbiota may contribute to enhancing the efficacy of TCMs in alleviating disease.
 |
Table 1 Evidence for Gut Microbial Ecosystem Remodeling to Alleviate Extra-Intestinal Diseases |
However, most GME-based research efforts on the mechanisms of TCMs share common shortcomings. The most prominent obstacle is that the causal relationship between GME remodeling and disease amelioration. Encouragingly, the co-housing or FMT-based GME remodeling provides technical support and data evidence for the aforementioned hurdles (Table 2). Therefore, in the research protocols, murine treated for a certain period of time with TCMs or herbal components were mostly selected as co-housed companions or donors for FMT. Standardizing the technique is challenging, as it varies according to research topics, but it is feasible to avoid common pitfalls. The co-housing process is referenced in Operation Process of Co-Housing and illustrated with the following example:44 Sun et al demonstrated that part of the therapeutic mechanism of Yishen Qingli Heluo Granule (YQHG) for CKD was mediated through GME by applying continuous short-term co-housing. ① Environmental conditions: Parameters were set according to established criteria. ②Allocation ratio per cage: Five groups (n=6 in each group) were included in this co-housing experiment: sham group, model group, CoHo-model group, CoHo-YQHG group and YQHG group. In details, the 5/6 nephrectomized rats treated with YQHG for eight weeks were randomly divided into YQHG group and CoHo-YQHG group. At the same time, 5/6 nephrectomized rats treated with sterile water for eight weeks were randomly divided into model group and CoHo-model group. The raising density of rats in each cage was as follows: For the co-housing groups: a total of six cages with two rats per cage (CoHo-model group×1, CoHo-YQHG group×1) (Figure 2). For non-co-housing groups: Sham group (a total of two cages with three rats per cage). The raising density of rats in the model group and YQHG group was the same as that in the sham group. ③Time setting: Three consecutive weeks. ④Precautions: The experimental operating procedures were approved by the institutional animal care and use committee of Nanjing Agricultural University (permission number PZW20200013). During the experiment, signs of fighting between co-housed animals were monitored and emergencies were promptly addressed. In addition, refer to Operation Process of FMT and Figure 3 for the process of FMT.
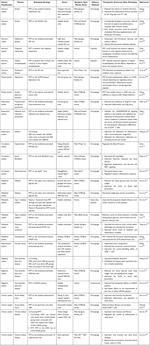 |
Table 2 Evidence That TCM Modifies Extra-Intestinal Diseases Through GME Remodeling |
 |
Figure 3 Microbiota-transfer strategy: FMT. |
Improved Gut Microbiota in the Treatment of Extra-Intestinal Diseases by TCMs
Co-housing and FMT provide a technical and experimental basis for exploring the microecological mechanisms behind TCMs. Recent studies have shown a variety of herbs influence microbial abundance and diversity, which in turn are closely related to the efficacy of TCMs. TCMs for stroke relief as an example: almost all the abundance of bacterial phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Tenericutes) were significantly regulated by herbal administration.128 Further detailed analysis showed that TCMs modulated specific groups of beneficial bacteria (ie, SCFA-producing bacteria).127 Similarly, In a study of asthma improvement with TCMs, Dong et al noted that Guben Fangxiao Decoction (GBFXD) significantly increased the abundance of SCFA-producing bacteria, such as Firmicutes, Lachnospiraceae, and Bifidobacteriaceae.24 Although the digestive activity of the host occurs mainly in the small intestine, the production site of SCFAs is mainly concentrated in the colon, especially the ascending colon. SCFA-producing bacteria located in the colon are capable of efficiently degrading non-digestible carbohydrates to produce SCFAs. Therefore, dysregulation of this group of bacteria can directly affect the production of SCFAs. Most SCFAs are rapidly absorbed into the intestinal epithelium via specific transporters of by diffusion, providing an important source of energy for colonic tissues, which contribute to intestinal health and intestinal barrier homeostasis.24 Improvements in some key bacterial taxa (eg, butyric acid- and BSH-producing bacteria) have also been reported in some recent studies. For example, Liu et al found that Linggui Zhugan Decoction (LZD) could regulated the abundance of butyrate-producing bacteria (eg, Thermotogae, Alistipes putredinis) in obese mice induced by HFD.129 The abundance of BSH-producing bacteria (eg, Clostridium_XlVa, Clostridium_IV) was also improved by Penthorum chinense Pursh. Extract (PCPE) in NAFLD mice.122 BSH-producing bacteria are involved in bile acid metabolism. Therefore, the improved bacterial taxa contribute to the regulation of the balance of primary and secondary bile acids, and ultimately alleviate the related metabolic diseases.122 Furthermore, dysregulation of beneficial and harmful bacteria is favorable microecological evidence of extra-intestinal disease progression. The study reported that treatment with Gegen Qinlian Decoction (GQD) reversed the expansion of harmful bacteria (Escherichia coli) and the contraction of beneficial bacteria (Akkermansia muciniphila, Desulfovibrio C21 c20, Lactobacillus salivarius) in mice with influenza virus-infected pneumonia.111
Regulated Gut-Derived Metabolites in the Treatment of Extra-Intestinal Diseases by TCMs
Gut-derived metabolites are one of the important molecules in the gut-organ crosstalk, among which the SCFAs has been widely studied and reported. The production of SCFAs is concentrated in the colon, especially the ascending colon. SCFA-producing bacteria efficiently degrade indigestible carbohydrates to produce SCFAs, most of which are rapidly absorbed into the intestinal epithelium by specific transporters or by diffusion, and are a source of energy for the colonic tissues. Among them, acetate is an important co-factor for bacterial growth. Propionate and butyrate are key metabolites that provide a major source of energy for colonic cells. Most of the absorbed SCFAs are used as an energy source and a small portion is consumed by the liver. Eventually, the remaining SCFAs may pass through the circulatory system to target organs and tissues where they perform certain functions.130 Mechanistic studies continue to demonstrate the importance of SCFAs in extra-intestinal diseases. For instance, an animal study showed that oral administration of propionic acid to hypertensive mice attenuated its cardiovascular damage by modulating immunity and ameliorating systemic inflammation. These findings suggest that propionic acid, a member of the SCFA, has an important role in cardiovascular health. Increasing propionic acid, either orally or by targeting the gut microbiota, may be a new strategy for preventing hypertensive cardiovascular damage.131 Encouragingly, TCM research targeting SCFAs for the treatment of extra-intestinal diseases is currently flourishing. Dong et al demonstrated that GBFXD could improve asthma in remission stage (ARS) via the microbiota-acetate-Treg cells (Tregs) axis (Figure 4A). Briefly, fecal SCFAs (especially acetate) concentrations were increased in ARS mice treated with GBFXD. Acetic acid is a common mediator of the gut-lung axis, and it promotes the production of Tregs. The role of Tregs involves maintaining peripheral tolerance and suppressing inflammation. In the presence of Tregs dysfunction, the inability to suppress excessive immune responses may contribute to the development and progression of asthma. Interestingly, Tregs were significantly increased by GBFXD treatment. Further experiments showed that the restoration of Treg cell ratio was mediated by acetate. The above results confirmed that modulation of the microbiota-acetate-Tregs axis may be a promising strategy for the treatment of ARS.24 Furthermore, SCFAs are also recognized as signaling molecules that can affect renal physiology or ameliorate kidney injury by acting as natural histone deacetylase (HDAC) inhibitors. For example, acetic acid ameliorated sepsis-induced acute kidney injury (AKI) by decreasing HDAC activity. Treatment with butyric acid inhibited diabetes-induced kidney injury by suppressing the expression of HDAC. Fibrosis, inflammation and apoptosis were found to be the pathogenesis of cisplatin-induced AKI. By acting as HDAC inhibitors, SCFAs have been shown to possess pharmacological activities, including anti-fibrotic, anti-inflammatory, and anti-apoptotic effects in kidney diseases. Notably, Zou et al found an increase in the abundance of SCFA-producing bacteria (Akkermansia, Faecalibaculum, and Bifidobacterium) in cisplatin-treated AKI mice by QYG treatment. Further experiments showed that QYG reduced HDAC expression and activity in renal tissues, which might be attributed to the increased concentrations of acetic and butyric acids, thus reducing nephrotoxicity.104 Moreover, SCFA plays a role in extra-intestinal diseases by modulating the intestinal barrier, which will be described in Impaired Intestinal Barrier in the Treatment of Extra-Intestinal Diseases by TCMs.
Dysbiosis of the gut microbiota may lead to a shift in metabolic pattern from saccharolytic to protein fermentation, which promotes an increase in uremic toxins such as indoxyl sulfate (IS) and p-Cresyl sulfate (pCS).132 Specifically, dietary tryptophan is broken down to indole by intestinal Escherichia coli in the presence of tryptophanase. After absorption from the intestine into the portal circulation, indole is converted to hydroxyindole and IS by two hepatic cytochrome oxidases (CYP 2E1 and SULT1A1), respectively. As for pCS, dietary tyrosine and phenylalanine are broken down by intestinal anaerobes to 4-hydroxyphenylacetic acid, and then decarboxylated to pcresol, which is converted to pCS in the liver by SULT1A1.133 These two substances are mainly excreted through renal tubular secretion under normal renal function; however, in renal insufficiency, they cannot be excreted effectively, which leads to large accumulations.134 It has been documented that the levels of IS and pCS correlate with the severity of AKI patients.135 The major toxic effects of IS and pCS on renal cells include induction of oxidative stress,136 increased inflammatory response,137 enhanced profibrotic expression,138 and down-regulation of the expression of nephro-protective proteins (eg, Klotho proteins).139 However, Zou et al demonstrated that QYG down-regulated the levels of IS and pCS in cisplatin-induced AKI mice.104
Impaired Intestinal Barrier in the Treatment of Extra-Intestinal Diseases by TCMs
IECs play an important role in nutrient absorption and also act as a natural barrier to prevent and inhibit microbial translocation. These columnar epithelial cells are adjacent to each other through tight junctions and form the “seal” of the intestinal barrier.140 Abnormal intestinal epithelial cell differentiation and reduced expression of tight junctions can lead to a compromised intestinal barrier, thereby increasing the risk of extra-intestinal disease. Tight junction (TJ) protein is an important parameter reflecting intestinal epithelial cell barrier. Intestinal barrier dysfunction is associated with a reduction in the expression levels of TJ protein, including Occludin, Claudin-1 and ZO-1. Previous studies have found that the onset and progression of extra-intestinal diseases are strongly associated with impaired intestinal barrier. For example, expression of TJ proteins (including occludin and claudin-1) is significantly reduced in the intestinal epithelium of arthritis patients. Further, in mice with collagen-induced arthritis, it was found that intestinal inflammation and T cell accumulation in the gut occurred prior to the onset of arthritis. However, targeting the intestinal barrier (eg, reduced intestinal permeability) may serve to alleviate arthritis.141 Similarly, a number of herbal studies have provided evidence-based support for targeting the intestinal barrier to alleviate extra-intestinal diseases. Salidroside significantly enhanced the integrity of the intestinal barrier by reducing the degree of intestinal pathological damage (reduced epithelial cell destruction, inflammatory cell infiltration, and crypt loss) in obese mice induced by HFD.116 GQD significantly increased the expression of TJ proteins (eg, Claudin-1, ZO-1, Occludin), preventing inflammatory factors from entering the bloodstream to trigger an immune-inflammatory response in influenza virus-infected pneumonia mice.111 Zhilong Huoxue Tongyu capsule (ZHTC) significantly improved the integrity of the intestinal barrier by ameliorating colonic tissue damage (increased villus length and crypt ratio) and up-regulating the expression level of the TJ protein Occludin-1 in stroke rats.128 Notably, SCFAs are important fuels for IECs. They influence intestinal motility, enhance intestinal barrier function, and host metabolism by regulating IEC function through different mechanisms, such as regulating their proliferation, differentiation, and function of subpopulations such as enteroendocrine cells. Recent findings suggest that SCFAs also have important intestinal and immunomodulatory functions.130 Several studies reported that reduced concentration of SCFAs led to renal dysfunction. Instead, supplementation with SCFAs, especially butyrate, which improved the intestinal barrier and controlled microbial translocation for ultimate nephroprotection.142 Encouragingly, Sun et al demonstrated that YQHG ameliorated renal dysfunction in 5/6 nephrectomized rats by improved SCFAs concentrations and intestinal barrier integrity (Figure 4B).44
Conclusion
In this review, we explored TCM treatment of extra-intestinal diseases based on GME remodeling. We summarized from the following three aspects: (1) Gut dysbiosis in extra-intestinal diseases: ①dysbiosis of the gut microbiota (eg, alterations in composition, abundance, and interactions) may lead to the onset and progression of extra-intestinal diseases. ②gut-derived metabolites are one of the important molecules in the gut-organ crosstalk. Depending on the level of specific metabolites produced in the host, they exert beneficial or deleterious effects on organs and have been linked to several extra-intestinal diseases. ③Intestinal barrier plays an important role in nutrient absorption, as well as being a natural barrier that prevents and inhibits microbial translocation, ultimately contributing to intestinal integrity and immune homeostasis in extra-intestinal diseases. (2) Co-housing- or FMT-based remodeling of GME in extra-intestinal diseases: most GME-based research efforts on the mechanisms of TCMs share common shortcomings. The most prominent obstacle is that the causal relationship between GME remodeling and disease amelioration. Encouragingly, the co-housing or FMT-based GME remodeling provides technical support and data evidence for the aforementioned hurdles. Therefore, in the research protocols, murine treated for a certain period of time with TCMs or herbal components were mostly selected as co-housed companions or donors for FMT. Standardizing the technique is challenging, as it varies according to research topics, but it is feasible to avoid common pitfalls. (3) Therapeutic effect mediated by the GME: Co-housing- or FMT-based techniques have confirmed that the protective effects of TCMs are partly attributable to the mediation of GME, in particular the gut microbiota (eg, SCFA- and BSH-producing bacteria), gut-derived metabolites, and intestinal barrier.
At present, Co-housing and FMT is used to treat many diseases related to gut microbiota imbalance, or as a key technology to reshape GME in animal experiments. However, precision Co-housing and FMT are still in its infancy. Fortunately, advances in the metagenomic studies and the novel computational tools, combined with breakthroughs in the mechanistic studies based on animal experiments, are accelerating our understanding of the causal relationship between the gut microbiota and different diseases, while uncovering the potential therapeutic effects of key bacterium or bacterial taxa. Co-housing and FMT provide technical and experimental basis for exploring the microecological mechanism behind disease amelioration. Most importantly, these efforts will ultimately contribute to the mechanistic elucidation of TCM treatments and the establishment of effective microecological pathways.
Funding
This research was funded by National Natural Science Foundation of the People’s Republic of China (grant number 82174295).
Disclosure
All authors declare no conflicts of interest.
References
1. Cao CJ, Zhu H, Yao Y, Zeng R. Gut dysbiosis and kidney diseases. Front Med. 2022;9:829349. doi:10.3389/fmed.2022.829349
2. Fang J, Yu CH, Li XJ, et al. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front Cell Infect Microbiol. 2022;12:997018. doi:10.3389/fcimb.2022.997018
3. Pan QR, Guo FB, Huang YY, et al. Gut microbiota dysbiosis in systemic lupus erythematosus: novel insights into mechanisms and promising therapeutic strategies. Front Immunol. 2021;12:799788. doi:10.3389/fimmu.2021.799788
4. Chen M, Xie CR, Shi YZ, Tang TC, Zheng H. Gut microbiota and major depressive disorder: a bidirectional Mendelian randomization. J Affect Disord. 2022;316:187–193. doi:10.1016/j.jad.2022.08.012
5. Ma PJ, Wang MM, Wang Y. Gut microbiota: a new insight into lung diseases. Biomed Pharmacother. 2022;155:113810. doi:10.1016/j.biopha.2022.113810
6. Yang ZH, Wang QC, Liu YX, et al. Gut microbiota and hypertension: association, mechanisms and treatment. Clin Exp Hypertens. 2023;45(1):2195135. doi:10.1080/10641963.2023.2195135
7. Hu TT, Wu QQ, Yao Q, Jiang KB, Yu JB, Tang QZ. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev. 2022;81:101706. doi:10.1016/j.arr.2022.101706
8. Praveenraj SS, Sonali S, Anand N, et al. The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol Neurobiol. 2022;59(11):6684–6700. doi:10.1007/s12035-022-02990-5
9. Hung KC, Yao WC, Liu YL, et al. The potential influence of uremic toxins on the homeostasis of bones and muscles in chronic kidney disease. Biomedicines. 2023;11(7):2076. doi:10.3390/biomedicines11072076
10. Lewis CV, Taylor WR. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;319(6):H1227–H1233. doi:10.1152/ajpheart.00612.2020
11. Liu L, Yin MY, Gao JW, et al. Intestinal barrier function in the pathogenesis of nonalcoholic fatty liver disease. J Clin Transl Hepatol. 2023;11(2):452–458. doi:10.14218/JCTH.2022.00089
12. Pellegrini C, Fornai M, D’Antongiovanni V, Antonioli L, Bernardini N, Derkinderen P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol. 2023;8(1):66–80. doi:10.1016/S2468-1253(22)00241-2
13. Zhang BX, Liu K, Yang HY, Jin ZS, Ding QY, Zhao LH. Gut microbiota: the potential key target of TCM’s therapeutic effect of treating different diseases using the same method-UC and T2DM as examples. Front Cell Infect Microbiol. 2022;12:855075. doi:10.3389/fcimb.2022.855075
14. Chen ZW, Wu SB, Zeng Y, et al. FuZhengHuaYuJiangZhuTongLuoFang prescription modulates gut microbiota and gut-derived metabolites in UUO rats. Front Cell Infect Microbiol. 2022;12:837205. doi:10.3389/fcimb.2022.837205
15. Che QY, Luo TT, Shi JH, He YH, Xu DL. Mechanisms by which traditional Chinese medicines influence the intestinal flora and intestinal barrier. Front Cell Infect Microbiol. 2022;12:863779. doi:10.3389/fcimb.2022.863779
16. Lin TL, Lu CC, Lai WF, et al. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell. 2021;12(5):394–410. doi:10.1007/s13238-020-00784-w
17. Surana NK, Kasper DL. Moving beyond microbiome-wide associations to causal microbe identification. Nature. 2017;552(7684):244–247. doi:10.1038/nature25019
18. Ralston JC, Mitchelson KAJ, Lynch GM, et al. Microbiome transfer partly overrides lack of IL-1RI signaling to alter hepatic but not adipose tissue phenotype and lipid handling following a high-fat diet challenge. Mol Nutr Food Res. 2021;65(1):e2000202. doi:10.1002/mnfr.202000202
19. Hamamah S, Gheorghita R, Lobiuc A, Sirbu IO, Covasa M. Fecal microbiota transplantation in non-communicable diseases: recent advances and protocols. Front Med. 2022;9:1060581. doi:10.3389/fmed.2022.1060581
20. Miyauchi E, Shimokawa C, Steimle A, Desai MS, Ohno H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. 2023;23(1):9–23. doi:10.1038/s41577-022-00727-y
21. Wu M, Li P, An YY, et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. 2019;150:104489. doi:10.1016/j.phrs.2019.104489
22. Wang J, Cao W, Tao J, et al. Gut microbiota and transcriptome profiling revealed the protective effect of aqueous extract of Tetrastigma hemsleyanum leaves on ulcerative colitis in mice. Curr Res Food Sci. 2022;6:100426. doi:10.1016/j.crfs.2022.100426
23. Hong Y, Sheng LL, Zhong J, et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes. 2021;13(1):1–20. doi:10.1080/19490976.2021.1930874
24. Dong YM, Yan H, Zhao X, et al. Gu-Ben-Fang-Xiao Decoction Ameliorated murine asthma in remission stage by modulating microbiota-acetate-tregs axis. Front Pharmacol. 2020;11:549. doi:10.3389/fphar.2020.00549
25. Ma QY, Grigorescu M, Schreiber A, et al. Genetic background but not intestinal microbiota after co-housing determines hyperoxaluria-related nephrocalcinosis in common inbred mouse strains. Front Immunol. 2021;12:673423. doi:10.3389/fimmu.2021.673423
26. Ji JJ, Ge XY, Chen YG, et al. Daphnetin ameliorates experimental colitis by modulating microbiota composition and Treg/Th17 balance. FASEB J. 2019;33(8):9308–9322. doi:10.1096/fj.201802659RR
27. Yang H, Jung S, Seo J, et al. Altered behavior and neural activity in conspecific cagemates co-housed with mouse models of brain disorders. Physiol Behav. 2016;163:167–176. doi:10.1016/j.physbeh.2016.05.031
28. Gao XH, Cao QH, Cheng Y, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115(13):E2960–E2969. doi:10.1073/pnas.1720696115
29. Ma JL, Hong Y, Zheng NN, et al. Gut microbiota remodeling reverses aging-associated inflammation and dysregulation of systemic bile acid homeostasis in mice sex-specifically. Gut Microbes. 2020;11(5):1450–1474. doi:10.1080/19490976.2020.1763770
30. Aziz NA, Berkiks I, Mosala P, Brombacher TM, Brombacher F. Environmental and microbial factors influence affective and cognitive behavior in C57BL/6 sub-strains. Front Immunol. 2023;14:1139913. doi:10.3389/fimmu.2023.1139913
31. Wu R, Zhao D, An R, et al. Linggui Zhugan formula improves glucose and lipid levels and alters gut microbiota in high-fat diet-induced diabetic mice. Front Physiol. 2019;10:918. doi:10.3389/fphys.2019.00918
32. Ji CL, Li Y, Mo YN, et al. Rhubarb enema decreases circulating trimethylamine N-oxide level and improves renal fibrosis accompanied with gut microbiota change in chronic kidney disease rats. Front Pharmacol. 2021;12:780924. doi:10.3389/fphar.2021.780924
33. Liu KY, Nakatsu CH, Jones-Hall Y, Kozik A, Jiang Q. Vitamin E alpha-and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic Biol Med. 2021;163:180–189. doi:10.1016/j.freeradbiomed.2020.12.017
34. Grant CV, Loman BR, Bailey MT, Pyter LM. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav Immun. 2021;95:401–412. doi:10.1016/j.bbi.2021.04.014
35. Luccia BD, Ahern PP, Griffin NW, et al. Combined prebiotic and microbial intervention improves oral cholera vaccination responses in a mouse model of childhood undernutrition. Cell Host Microbe. 2020;27(6):899–908. doi:10.1016/j.chom.2020.04.008
36. Agudelo LZ, Ferreira DMS, Cervenka I, et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27(2):378–392. doi:10.1016/j.cmet.2018.01.004
37. Xie Y, Chen J, Wu B, He TS, Xie L, Liu ZP. Dock2 affects the host susceptibility to Citrobacter rodentium infection through regulating gut microbiota. Gut Pathog. 2021;13(1):52. doi:10.1186/s13099-021-00449-x
38. Vaz J, Akbarshahi H, Andersson R. Controversial role of toll-like receptors in acute pancreatitis. World J Gastroenterol. 2013;19(5):616–630. doi:10.3748/wjg.v19.i5.616
39. Mei Q, Fu Y, Huang ZH, et al. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut Microbes. 2022;14(1):2112882. doi:10.1080/19490976.2022.2112882
40. Li XY, He C, Li NS, et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes. 2020;11(6):1774–1789. doi:10.1080/19490976.2020.1770042
41. Leber A, Hontecillas R, Tubau-Juni N, Zoccoli-Rodriguez V, Abedi V, Bassaganya-Riera J. NLRX1 modulates immunometabolic mechanisms controlling the host-gut microbiota interactions during inflammatory bowel disease. Front Immunol. 2018;9:363. doi:10.3389/fimmu.2018.00363
42. Chen JC, Zhang SL, Feng XM, et al. Conventional co-housing modulates murine gut microbiota and hematopoietic gene expression. Int J Mol Sci. 2020;21(17):6143. doi:10.3390/ijms21176143
43. Zhang CY, Shi YS, Burch M, Olthoff B, Ericsson AC, Franklin C. Transfer efficiency and impact on disease phenotype of differing methods of gut microbiota transfer. Sci Rep. 2022;12(1):19621. doi:10.1038/s41598-022-24014-x
44. Sun X, Chen J, Huang YT. Yishen Qingli Heluo Granule ameliorates renal dysfunction in 5/6 nephrectomized rats by targeting gut microbiota and intestinal barrier integrity. Front Pharmacol. 2022;13:858881. doi:10.3389/fphar.2022.858881
45. Zhang FM, Luo WS, Shi Y, Fan ZN, Ji GZ. Should we standardize the 1700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755. doi:10.1038/ajg.2012.251
46. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087. doi:10.1038/ajg.2012.60
47. Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859. PMID: 13592638.
48. Nood EV, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi:10.1056/NEJMoa1205037
49. Sachs RE, Edelstein CA. Ensuring the safe and effective FDA regulation of fecal microbiota transplantation. J Law Biosci. 2015;2(2):396–415. doi:10.1093/jlb/lsv032
50. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48. doi:10.1093/cid/cix1085
51. Zhang T, Lu GC, Zhao Z, et al. Washed microbiota transplantation vs. Manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11(4):251–266. doi:10.1007/s13238-019-00684-8
52. Wang HG, Cui BT, Li QQ, et al. The safety of fecal microbiota transplantation for crohn’s disease: findings from a long-term study. Adv Ther. 2018;35(11):1935–1944. doi:10.1007/s12325-018-0800-3
53. Zhang BZ, Yang LX, Ning HB, et al. A matching strategy to guide donor selection for ulcerative colitis in fecal microbiota transplantation: meta-analysis and analytic hierarchy process. Microbiol Spectr. 2023;11(1):e0215921. doi:10.1128/spectrum.02159-21
54. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580. doi:10.1136/gutjnl-2016-313017
55. Paramsothy S, Borody TJ, Lin E, et al. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis. 2015;21(7):1600–1606. doi:10.1097/MIB.0000000000000405
56. Vindigni SM, Surawicz CM. Fecal microbiota transplantation. Gastroenterol Clin North Am. 2017;46(1):171–185. doi:10.1016/j.gtc.2016.09.012
57. Blackburn LM, Bales A, Caldwell M, Cordell L, Hamilton S, Kreider H. Fecal microbiota transplantation in patients with cancer undergoing treatment. Clin J Oncol Nurs. 2015;19(1):111–114. doi:10.1188/15.CJON.111-114
58. Hu XF, Zhang WY, Wen Q, et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. 2019;139:412–421. doi:10.1016/j.phrs.2018.11.042
59. Ma JL, Liu ZK, Gao XX, et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol Res. 2023;189:106687. doi:10.1016/j.phrs.2023.106687
60. Zhang F, Zhai MT, Wu Q, Jia XY, Wang Y, Wang N. Protective Effect of Tong-Qiao-Huo-Xue Decoction on inflammatory injury caused by intestinal microbial disorders in stroke rats. Biol Pharm Bull. 2020;43(5):788–800. doi:10.1248/bpb.b19-00847
61. Zhang Y, Huang RR, Cheng MJ, et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019;7(1):116. doi:10.1186/s40168-019-0733-3
62. Gheorghe CE, Ritz NL, Martin JA, Wardill HR, Cryan JF, Clarke G. Investigating causality with fecal microbiota transplantation in rodents: applications, recommendations and pitfalls. Gut Microbes. 2021;13(1):1941711. doi:10.1080/19490976.2021.1941711
63. Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618. doi:10.1016/j.cell.2019.05.004
64. Chen X, Li P, Liu M, et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut. 2020;69(3):513–522. doi:10.1136/gutjnl-2019-319101
65. Terveer EM, Beurden YH, Goorhuis A, et al. How to: establish and run a stool bank. Clin Microbiol Infect. 2017;23(12):924–930. doi:10.1016/j.cmi.2017.05.015
66. Pang WY, Vogensen FK, Nielsen DS, et al. Faecal and caecal microbiota profiles of mice do not cluster in the same way. Lab Anim. 2012;46(3):231–236. doi:10.1258/la.2012.011128
67. Gratton J, Phetcharaburanin J, Mullish BH. Optimized sample handling strategy for metabolic profiling of human feces. Anal Chem. 2016;88(9):4661–4668. doi:10.1021/acs.analchem.5b04159
68. Zeng XJ, Li XQ, Li X, et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood. 2023;141(14):1691–1707. doi:10.1182/blood.2022017514
69. Huang CC, Yi P, Zhu M, et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: an explorer trial. J Autoimmun. 2022;130:102844. doi:10.1016/j.jaut.2022.102844
70. Gulati M, Singh SK, Corrie L, Kaur IP, Chandwani L. Delivery routes for faecal microbiota transplants: available, anticipated and aspired. Pharmacol Res. 2020;159:104954. doi:10.1016/j.phrs.2020.104954
71. Wang JW, Kuo CH, Kuo FC, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. 2019;118(Suppl 1):S23–S31. doi:10.1016/j.jfma.2018.08.011
72. Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012;40(6):643–648. doi:10.1007/s15010-012-0307-9
73. Shi YQ, Dong YW, Huang WH, Zhu DC, Mao H, Su PZ. Fecal microbiota transplantation for ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0157259. doi:10.1371/journal.pone.0157259
74. Zhong SW, Zeng JQ, Deng ZH, et al. Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: a pediatric case report. Ital J Pediatr. 2019;45(1):116. doi:10.1186/s13052-019-0708-9
75. Holvoet T, Joossens M, Vázquez-Castellanos JF, et al. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. 2021;160(1):145–157. doi:10.1053/j.gastro.2020.07.013
76. Jie W, Lv L, Wang CL. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2022;12:827395. doi:10.3389/fcimb.2022.827395
77. El-Salhy M, Winkel R, Casen C, Hausken T, Gilja OH, Hatlebakk JG. Efficacy of fecal microbiota transplantation for patients with irritable bowel syndrome at 3 years after transplantation. Gastroenterology. 2022;163(4):982–994. doi:10.1053/j.gastro.2022.06.020
78. El-Salhy M, Hatlebakk JG, Gilja OH, Kristoffersen AB, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69(5):859–867. doi:10.1136/gutjnl-2019-319630
79. Liu JJ, Zhang X, Ta XJ, Luo MM, Chang XH, Wang H. Fecal microbiome transplantation attenuates manganese-induced neurotoxicity through regulation of the apelin signaling pathway by inhibition of autophagy in mouse brain. Ecotoxicol Environ Saf. 2022;242:113925. doi:10.1016/j.ecoenv.2022.113925
80. Zhou YF, Nie JJ, Shi C, et al. Lysimachia christinae polysaccharide attenuates diet-induced hyperlipidemia via modulating gut microbes-mediated FXR-FGF15 signaling pathway. Int J Biol Macromol. 2023;248:125725. doi:10.1016/j.ijbiomac.2023.125725
81. Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50(5):600–613. PMID: 22330705.
82. Arantes-Rodrigues R, Henriques A, Pinto-Leite R, et al. The effects of repeated oral gavage on the health of male CD-1 mice. Lab Anim. 2012;41(5):129–134. doi:10.1038/laban0512-129
83. Kinder JM, Then JE, Hansel PM, Molinero LL, Bruns HA. Long-term repeated daily use of intragastric gavage hinders induction of oral tolerance to ovalbumin in mice. Comp Med. 2014;64(5):369–376. PMID: 25402177.
84. Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318(20):1985–1993. doi:10.1001/jama.2017.17077
85. Choi HH, Cho YS. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc. 2016;49(3):257–265. doi:10.5946/ce.2015.117
86. Turse EP, Dailey FE, Ghouri YA, Tahan V. Fecal microbiota transplantation donation: the gift that keeps on giving. Curr Opin Pharmacol. 2019;49:24–28. doi:10.1016/j.coph.2019.04.009
87. Groot PD, Scheithauer T, Bakker GJ, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69(3):502–512. doi:10.1136/gutjnl-2019-318320
88. Dailey FE, Turse EP, Daglilar E, Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29–33. doi:10.1016/j.coph.2019.04.008
89. Ghani R, Mullish BH, Roberts LA, Davies FJ, Marchesi JR. The potential utility of fecal (or intestinal) microbiota transplantation in controlling infectious diseases. Gut Microbes. 2022;14(1):2038856. doi:10.1080/19490976.2022.2038856
90. Liu LX, Wang HY, Chen XY, Zhang YD, Zhang HP, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527. doi:10.1016/j.ebiom.2023.104527
91. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi:10.1038/s41598-017-13601-y
92. Shilo S, Godneva A, Rachmiel M, et al. The Gut microbiome of adults with type 1 diabetes and its association with the host glycemic control. Diabetes Care. 2022;45(3):555–563. doi:10.2337/dc21-1656
93. Mancuso C, Santangelo R. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res. 2018;129:329–336. doi:10.1016/j.phrs.2017.12.009
94. Zuo T, Wu XJ, Wen WP, Lan P. Gut microbiome alterations in COVID-19. Genomics Proteomics Bioinf. 2021;19(5):679–688. doi:10.1016/j.gpb.2021.09.004
95. Barrington WT, Lusis AJ. Atherosclerosis: association between the gut microbiome and atherosclerosis. Nat Rev Cardiol. 2017;14(12):699–700. doi:10.1038/nrcardio.2017.169
96. Connell E, Gall GL, Pontifex MG, et al. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol Neurodegener. 2022;17(1):43. doi:10.1186/s13024-022-00548-6
97. Swer NM, Venkidesh BS, Murali TS, Mumbrekar KD. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol Biol Rep. 2023;50(2):1663–1675. doi:10.1007/s11033-022-08038-0
98. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi:10.1038/nature09922
99. Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi:10.1016/j.cell.2015.11.055
100. Jiang S, Shui YJ, Cui Y, et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021;46:102115. doi:10.1016/j.redox.2021.102115
101. Tommaso ND, Santopaolo F, Gasbarrini A, Ponziani FR. The gut-vascular barrier as a new protagonist in intestinal and extraintestinal diseases. Int J Mol Sci. 2023;24(2):1470. doi:10.3390/ijms24021470
102. Wang JC, Zhong Y, Zhu H, et al. Different gender-derived gut microbiota influence stroke outcomes by mitigating inflammation. J Neuroinflammation. 2022;19(1):245. doi:10.1186/s12974-022-02606-8
103. Chen XX, Zhang WL, Lin ZJ, et al. Preliminary evidence for developing safe and efficient fecal microbiota transplantation as potential treatment for aged related cognitive impairments. Front Cell Infect Microbiol. 2023;13:1103189. doi:10.3389/fcimb.2023.1103189
104. Zou YT, Zhou J, Zhu JH, et al. Gut microbiota mediates the protective effects of traditional Chinese medicine formula Qiong-Yu-Gao against cisplatin-induced acute kidney injury. Microbiol Spectr. 2022;10(3):e0075922. doi:10.1128/spectrum.00759-22
105. Zhou HX, Zhao JM, Liu CH, Zhang ZF, Zhang Y, Meng DL. Xanthoceraside exerts anti-Alzheimer’s disease effect by remodeling gut microbiota and modulating microbial-derived metabolites level in rats. Phytomedicine. 2022;98:153937. doi:10.1016/j.phymed.2022.153937
106. Bloom PP, Donlan J, Soto MT, Daidone M, Hohmann E, Chung RT. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatol Commun. 2022;6(8):2079–2089. doi:10.1002/hep4.1950
107. Yu JJ, Meng JH, Qin ZW, et al. Dysbiosis of gut microbiota inhibits NMNAT2 to promote neurobehavioral deficits and oxidative stress response in the 6-OHDA-lesioned rat model of Parkinson’s disease. J Neuroinflammation. 2023;20(1):117. doi:10.1186/s12974-023-02782-1
108. Cai T, Zheng SP, Shi X, et al. Therapeutic effect of fecal microbiota transplantation on chronic unpredictable mild stress-induced depression. Front Cell Infect Microbiol. 2022;12:900652. doi:10.3389/fcimb.2022.900652
109. Wang JF, Cao Y, Hou WL, et al. Fecal microbiota transplantation improves VPA-induced ASD mice by modulating the serotonergic and glutamatergic synapse signaling pathways. Transl Psychiatry. 2023;13(1):17. doi:10.1038/s41398-023-02307-7
110. Wen L, Shi L, Kong XL, et al. Gut microbiota protected against pseudomonas aeruginosa pneumonia via restoring Treg/Th17 balance and metabolism. Front Cell Infect Microbiol. 2022;12:856633. doi:10.3389/fcimb.2022.856633
111. Deng L, Shi YC, Liu P, et al. GeGen QinLian decoction alleviate influenza virus infectious pneumonia through intestinal flora. Biomed Pharmacother. 2021;141:111896. doi:10.1016/j.biopha.2021.111896
112. Chen Z, Yang B, Wang Z, Rong XL, Zhu Q, Guo J. Modulation of the gut microbiota by fufang-zhenzhu-tiaozhi capsule attenuates hypertension induced by a high-fructose and high-salt diet. Front Cell Infect Microbiol. 2022;12:854849. doi:10.3389/fcimb.2022.854849
113. Fang C, Zuo K, Liu Z, et al. Disordered gut microbiota promotes atrial fibrillation by aggravated conduction disturbance and unbalanced linoleic acid/SIRT1 signaling. Biochem Pharmacol. 2023;213:115599. doi:10.1016/j.bcp.2023.115599
114. Hao H, Li Z, Qiao SY, et al. Empagliflozin ameliorates atherosclerosis via regulating the intestinal flora. Atherosclerosis. 2023;371:32–40. doi:10.1016/j.atherosclerosis.2023.03.011
115. Xia YG, Tian Y, Zhou DQ, et al. Gut microbiota involved in spermatogenic function of Sancai Lianmei granules in obese mice. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(1):83–97. doi:10.1007/s00210-022-02296-2
116. Liu JX, Cai JP, Fan P, et al. Salidroside protects mice from high-fat diet-induced obesity by modulating the gut microbiota. Int Immunopharmacol. 2023;120:110278. doi:10.1016/j.intimp.2023.110278
117. He LN, Chen RP, Zhang BZ, et al. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes mellitus. Front Immunol. 2022;13:930872. doi:10.3389/fimmu.2022.930872
118. Chen LJ, Guo L, Feng SS, et al. Fecal microbiota transplantation ameliorates type 2 diabetes via metabolic remodeling of the gut microbiota in db/db mice. BMJ Open Diabetes Res Care. 2023;11(3):e003282. doi:10.1136/bmjdrc-2022-003282
119. Yang ZD, Fu HJ, Su HH, et al. Multi-omics analyses reveal the specific changes in gut metagenome and serum metabolome of patients with polycystic ovary syndrome. Front Microbiol. 2022;13:1017147. doi:10.3389/fmicb.2022.1017147
120. Zhang YW, Cao MM, Li YJ, et al. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Translat. 2022;37:46–60. doi:10.1016/j.jot.2022.08.003
121. Wang ZZ, Qin X, Hu DX, et al. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. 2022;41(13):111890. doi:10.1016/j.celrep.2022.111890
122. Li XX, Zhao WW, Xiao M, et al. Penthorum Chinense Pursh. extract attenuates non-alcholic fatty liver disease by regulating gut microbiota and bile acid metabolism in mice. J Ethnopharmacol. 2022;294:115333. doi:10.1016/j.jep.2022.115333
123. Xue LF, Deng ZL, Luo WH, He XX, Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol. 2022;12:759306. doi:10.3389/fcimb.2022.759306
124. Gharaie S, Lee K, Newman-Rivera AM, et al. Microbiome modulation after severe acute kidney injury accelerates functional recovery and decreases kidney fibrosis. Kidney Int. 2023;104(3):470–491. doi:10.1016/j.kint.2023.03.024
125. Liu XX, Zhang M, Wang XF, et al. Fecal microbiota transplantation restores normal fecal composition and delays malignant development of mild chronic kidney disease in rats. Front Microbiol. 2022;13:1037257. doi:10.3389/fmicb.2022.1037257
126. Zhang HY, Tian JX, Lian FM, et al. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed Pharmacother. 2021;133:110857. doi:10.1016/j.biopha.2020.110857
127. Li QQ, Cao MX, Wei ZJ, et al. The protective effect of Buzhong Yiqi decoction on ischemic stroke mice and the mechanism of gut microbiota. Front Neurosci. 2022;16:956620. doi:10.3389/fnins.2022.956620
128. Wang RQ, Liu MN, Ren GL, et al. Zhilong Huoxue Tongyu Capsules’ effects on ischemic stroke: an assessment using fecal 16S rRNA gene sequencing and untargeted serum metabolomics. Front Pharmacol. 2022;13:1052110. doi:10.3389/fphar.2022.1052110
129. Liu MT, Huang YJ, Zhang TY, et al. Lingguizhugan decoction attenuates diet-induced obesity and hepatosteatosis via gut microbiota. World J Gastroenterol. 2019;25(27):3590–3606. doi:10.3748/wjg.v25.i27.3590
130. Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49. doi:10.1017/S0029665120006916
131. Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407–1421. doi:10.1161/CIRCULATIONAHA.118.036652
132. Glorieux G, Gryp T, Perna A. Gut-derived metabolites and their role in immune dysfunction in chronic kidney disease. Toxins. 2020;12(4):245. doi:10.3390/toxins12040245
133. Skye SM, Hazen SL. Microbial modulation of a uremic toxin. Cell Host Microbe. 2016;20(6):691–692. doi:10.1016/j.chom.2016.11.005
134. Bush KT, Singh P, Nigam SK. Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. JCI Insight. 2020;5(7):e133817. doi:10.1172/jci.insight.133817
135. Veldeman L, Vanmassenhove J, Biesen WV, et al. Evolution of protein-bound uremic toxins indoxyl sulphate and p-cresyl sulphate in acute kidney injury. Int Urol Nephrol. 2019;51(2):293–302. doi:10.1007/s11255-018-2056-x
136. Watanabe H, Miyamoto Y, Honda D, et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83(4):582–592. doi:10.1038/ki.2012.448
137. Poveda J, Sanchez-Niño MD, Glorieux G, et al. p-cresyl sulphate has pro-inflammatory and cytotoxic actions on human proximal tubular epithelial cells. Nephrol Dial Transplant. 2014;29(1):56–64. doi:10.1093/ndt/gft367
138. Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012;7(3):e34026. doi:10.1371/journal.pone.0034026
139. Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81(7):640–650. doi:10.1038/ki.2011.445
140. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(1):9–21. doi:10.1038/nrgastro.2016.169
141. Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995. doi:10.1038/s41467-020-15831-7
142. Wang SQ, Lv D, Jiang SH, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci. 2019;133(17):1857–1870. doi:10.1042/CS20190171
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

