Back to Journals » Infection and Drug Resistance » Volume 16
Clostridium ramosum Bacteremia in an Immunocompetent Patient with SARS-CoV-2 Infection: A Case Report
Authors Bao D, Xu X, Wang Y, Zhu F, Wu Y, Li H
Received 15 May 2023
Accepted for publication 1 July 2023
Published 8 July 2023 Volume 2023:16 Pages 4455—4461
DOI https://doi.org/10.2147/IDR.S421409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Danni Bao,1 Xiaohong Xu,1 Yizhang Wang,1 Fengjiao Zhu,1 Yanhong Wu,1 Hongzhang Li2
1Department of Clinical Laboratory, Sanmen People’s Hospital, Taizhou, Zhejiang, People’s Republic of China; 2Department of Gastroenterology, Sanmen People’s Hospital, Taizhou, Zhejiang, People’s Republic of China
Correspondence: Hongzhang Li, Department of Gastroenterology, Sanmen People’s Hospital, Taizhou, Zhejiang, People’s Republic of China, Email [email protected]
Abstract: We report a case of Clostridium ramosum bacteremia in a 73-year-old patient with SARS-CoV-2 infection and right lower abdominal tenderness in China. The microbiological features and genomic epidemiological characteristics of C. ramosum worldwide were investigated to identify the possible sources of infection. Whole-genome sequencing of C. ramosum WD-I2 was performed using an Illumina NovaSeq 6000 platform. Phylogenetic analysis of C. ramosum WD-I2 and other publicly available C. ramosum isolates was performed and visualized using the interactive Tree of Life (iTOL) web server. The resistome of C. ramosum WD-I2 consists of two antimicrobial resistance genes (tetM and ermB), which explains the antimicrobial resistance trait to tetracycline and macrolides. Phylogenetic analysis showed that the strain closest to our isolated strain WD-I2 was SUG1069, recovered from a pig feces sample from Canada, which differed by 589 SNPs. To our knowledge, this is the first report of C. ramosum bacteremia in China. Our findings highlight the potential risk of invasive C. ramosum infections during the COVID-19 pandemic.
Keywords: Clostridium ramosum, bacteremia, antimicrobial resistance, SARS-CoV-2, whole genome sequencing
Introduction
Clostridium ramosum was first identified and characterized in 1898 following its isolation from a patient with pulmonary gangrene and appendicitis.1 This species, initially identified as Bacillus ramosum and later referred to as C. ramosum, was renamed Erysipelatoclostridium ramosum in 2013.2 However, its taxonomic name has not been legally published according to the International Code of Nomenclature of Bacteria.3 C. ramosum is a gram-positive, anaerobic, non-motile Bacillus species capable of forming spores. It is commonly found as part of the normal intestinal flora in humans, with over 83% of adult patients having it present in their feces. This high prevalence makes it one of the most widespread commensal clostridial species in humans.4 Although C. ramosum can be isolated from clinical specimens, it is rarely identified as the primary cause of infection; therefore, its pathogenicity has been largely underestimated.5 Infections caused by C. ramosum have been predominantly reported in young children (under 5 years old) with inner ear infections and immunocompromised elderly individuals.6 However, in certain cases, C. ramosum has been reported to cause infections in healthy individuals. These infections are usually opportunistic and occur under certain circumstances such as trauma, surgery, or in the presence of other underlying medical conditions.7–9
In certain instances, C. ramosum causes bacteremia, which has been associated with a higher mortality rate, particularly in immunocompromised hospitalized patients.6 The predominant source of bloodstream infection is the translocation of strains from the digestive system to cutaneous decubitus.5 Although rifampin resistance was discovered in 1971, tetracycline, aminoglycosides, and fluoroquinolone resistance were also noted; C. ramosum did not show multidrug resistance to most antimicrobial agents.6,10
Increasing evidence suggests that SARS-CoV-2 infection can compromise the integrity of the intestinal barrier by adversely affecting all layers of defense, including biological, mechanical, and immune barriers.11 This broken gut barrier allows luminal bacteria, fungi, and endotoxins to spread to typically sterile extraintestinal locations and the bloodstream, thereby promoting systemic inflammation and immune activation.11 Patients with COVID-19 have been found to have microbiota that are more enriched in virulent bacteria such as enterococci, as well as opportunistic pathogens such as Clostridium hathewayi, Clostridium ramosum, Actinomyces viscosus, Streptococcus spp., and Rothia spp., when compared to healthy individuals.12–14 Here, we report a case of septic shock associated with C. ramosum bacteremia in a patient with SARS-CoV-2 infection and its genomic characterization.
Case Report
A 73-year-old female patient sought medical attention during the COVID-19 pandemic in China (December 2022) due to persistent right lower limb pain resulting from a fall four days earlier. Upon admission, the patient was in a frail state, exhibited tenderness in the right lower abdominal area, and tested positive for COVID-19. Upon admission, the patient had a normal body temperature. However, the patient presented with symptoms such as chills, a mild cough with phlegm, frequent urination, and two episodes of diarrhea prior to admission.
Laboratory testing showed a white blood cell count of 3.9×109/L (94.7% neutrophils), platelets 131×109/L, hemoglobin 110g/L, pH value (T) 7.54, carbon dioxide partial pressure (T) 22.4 mmHg, partial pressure of oxygen (T) 186 mmHg, lactic acid 4.7 mmol/L, glutamic pyruvic transaminase 49 U/L, glutamic oxaloacetic transaminase 159 U/L, albumin 22.3 g/L, potassium 3.08mmol/L, urea 14.52 mmol/L, creatine kinase 2885 U/L, creatine kinase isoenzyme 58 U/L, lactate dehydrogenase 519 U/L, and high sensitivity C-reactive protein 319.9 mg/L. The patient was treated with imipenem 0.50g q6h and vancomycin 0.5 q6h for two days. Unfortunately, the patient was discharged on the third day after admission and died. Her laboratory test results were notable for a white blood cell count of 11.9×109/L (89.2% neutrophils), platelets 11×109/L, high sensitivity C-reactive protein >370mg/L, IL-6 956.65 pg/mL, and lactic acid concentration 6.5 mmol/L. Abdominal computed tomography (CT) revealed free air around the right retroperitoneal area (Figure 1), which was diagnosed as intestinal perforation.
 |
Figure 1 Images of abdomen computed tomography (CT). Free air was observed around the right retroperitoneal area (indicated by red arrows). |
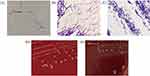
Blood specimen from the patient was injected into conventional aerobic and anaerobic bioM’erieux BacT/Alert bottles. One of the anaerobic bottles (bottle 1) became positive after 57.4 hours of incubation, and the other anaerobic bottle (bottle 2) became positive after 68.5 hours of incubation. Gram staining revealed a weakly gram-positive rod (Figure 2A). After 24 h of anaerobic incubation, the organism produced a pure culture on blood agar but did not grow aerobically in subculture. A pure culture of tiny, grayish, smooth, spherical, and non-hemolytic colonies with intact edges was obtained after 24 h (Figure 2D). The colonies of C. ramosum grown in blood agar for 48 h turned egg-shaped, and the color turned light brown (Figure 2E). Gram staining showed gram-variable/negative rods after 24 h (Figure 2B), which became more strongly gram-positive later in culture (Figure 2C). C. ramosum isolate WD-I2 was first identified using matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS VITEK, bioMérieux) and then verified using 16S rRNA gene sequencing. Antimicrobial susceptibility testing for the following antimicrobial agents: penicillin, ampicillin, ceftriaxone, tetracycline, meropenem, imipenem, clindamycin, chloramphenicol, sulfamethoxazole and metronidazole was performed by broth microdilution method and interpreted using the Clinical and Laboratory Standards Institute (CLSI) guidelines (M100-S32 and M11-A8), except for vancomycin, erythromycin, gentamicin, and ciprofloxacin, which does not have a valid breakpoint.
Whole-genome sequencing of C. ramosum WD-I2 was performed using an Illumina NovaSeq 6000 platform (Illumina Inc., San Diego, CA, USA). A total of 98 C. ramosum strains were retrieved from the NCBI GenBank database to obtain all publicly available C. ramosum genomes on January 8th, 2023. Phylogenetic analysis of C. ramosum WD-I2 and other publicly available C. ramosum isolates was performed using the Snippy software (https://github.com/tseemann/snippy). The phylogenetic tree was visualized using the interactive Tree of Life (iTOL) V5 web server.15 The genome sequence of C. ramosum WD-I2 has been deposited in NCBI GenBank under the accession number JAJQMP000000000.
The genome of C. ramosum strain WD-I2 comprises 3,580,417 bp and exhibits a G +C content of 31.5%. The strain was predicted to have 77 contigs, 3536 coding sequences (CDSs), and 68 RNAs (62 tRNA, 3 rRNA, and 3 ncRNA) genes. The resistome of C. ramosum strain WD-I2 consists of two antimicrobial resistance genes (tetM and ermB). The tet(M) gene encodes an efflux pump that actively expels tetracycline and related antibiotics from the bacterial cell, thereby conferring resistance. The ermB gene modifies the 23S rRNA component of the bacterial ribosome through methylation, reducing the binding affinity of clindamycin and leading to resistance. Antimicrobial susceptibility testing showed that the isolate was resistant to clindamycin (MIC > 16 μg/mL) and tetracycline (MIC = 16 μg/mL), but still susceptible to a varieties of antimicrobial agents, including penicillin (MIC = 0.25 μg/mL), ampicillin (MIC < 0.25 μg/mL), ceftriaxone (MIC < 0.5 μg/mL), meropenem (MIC < 0.125 μg/mL), imipenem (MIC < 0.125 μg/mL), chloramphenicol (MIC = 1 μg/mL), sulfamethoxazole (MIC < 0.125 μg/mL) and metronidazole (MIC = 2 μg/mL). The MIC of vancomycin, erythromycin, gentamicin, and ciprofloxacin were 1 μg/mL, >16 μg/mL, 16 μg/mL and 4 μg/mL, respectively. These findings explain the antimicrobial resistance of WD-I2 to tetracycline and macrolides. Notably, the C. ramosum strain WD-I2 carries multiple virulence genes, including adherence (fbpA/fbp68, groEL, lap, flmH, and plr/gapA), regulation (LisR/LisK), toxin (nagH, nagK, and cylR2), antiphagocytosis (mlB and cpsA), enzyme (eno), immune evasion (cps2K, cpsY, galE, acpXL, gtaB), iron uptake (hemL), and serum resistance (rmlA), which may be related to the poor prognosis of infection. Among these, groEL is a heat shock protein that serves an adhesive function in Clostridium spp.16 and MU-toxins (nagH and nagK) are modular hydrolytic enzymes that contribute to their pathogenicity.17 The virulence gene cylR2 encodes an exotoxin, which is lethal for a wide range of gram-positive bacteria and plays key roles in the host-pathogen dialog.18
The phylogenetic relationship between C. ramosum WD-I2 and 97 C. ramosum strains currently deposited in the NCBI GenBank database was investigated to evaluate the genomic epidemiological features of C. ramosum strains in a global context (Figure 3). These isolates were mainly distributed in USA (71, 72.45%), and the host of isolation mainly distributed in humans (92, 93.88%), which were recovered from the fecal and mucosal tissues with Crohn’s disease, except for the isolate collected from a blood sample in this study. To our knowledge, this is the first report of C. ramosum bacteremia in China. Phylogenetic analysis showed that the strain closest to our isolated strain WD-I2 was SUG1069, recovered from a pig feces sample from Canada, which differed by 589 SNPs.
Discussion
C. ramosum is a gram-positive anaerobic enteric bacterium that typically exists in the human gut microbiota. It is a member of the RIC group, which also includes Clostridium innocuum and Clostridium clostridioforme. C. ramosum rarely affects children (under 5 years old) with inner ear infections or elderly individuals with impaired immune systems.6,9 The production of IgA proteases by C. ramosum may facilitate its penetration of intestinal mucosal defenses, especially in patients who are particularly vulnerable to infection.19,20 Previous studies have demonstrated that C. ramosum can cause a variety of life-threatening conditions, including but not limited to bacteremia, osteomyelitis, septic arthritis, mastoiditis, spondylodiscitis, otitis media, pyelonephritis, septic arterial emboli, endocarditis, gas gangrene, septic pseudoarthrosis, peritoneal dialysis-related peritonitis, and liver abscess.10,21 The majority of C. ramosum isolates have been identified in blood cultures,21,22 which is one of the three species with the highest case fatality rates in Clostridium bacteremia.23 Adults with underlying cancer or immunosuppression and patients with bowel perforation and abscess formation are particularly vulnerable to C. ramosum infections.8 The source of the bacteremia was probably translocation from the gastrointestinal tract and soft tissue infections.5 Moreover, the incidence of C. ramosum infections is likely underestimated, as Clostridium spp. may be unrecognized in cases of polymicrobial bacteremia due to its long incubation periods. Therefore, accurate processing of blood cultures and careful analysis of gram stains are crucial for bacteriological diagnosis of invasive C. ramosum infections.
Currently, there are limited studies on bacteremia during the COVID-19 pandemic in China. However, previous reports have indicated that bacteremia is more common in COVID-19 patients than in non-COVID-19 patients.24–27 The compromised integrity of the intestinal barrier due to SARS-CoV-2 infection and the higher prevalence of C. ramosum in the microbiota of COVID-19 patients may contribute to the increased incidence of bacteremia. Additionally, the production of IgA protease by C. ramosum may aid in the breach of intestinal mucosal barriers and cause bacteremia.19,20 It is worth noting that glucocorticoids, which have potent anti-inflammatory actions, can have negative effects on gut barrier function.28
Conclusions
This study presents the first report on the clinical manifestations, antimicrobial resistance mechanisms and genomic features of C. ramosum bacteremia recovered from an immunocompetent patient in China with the aim of exploring the potential relationship between C. ramosum and COVID-19 infection. Continuous monitoring of C. ramosum infections using conventional and whole-genome sequencing techniques is essential for a better understanding of the mechanisms of resistance and transmission dynamics of this emerging bacterial pathogen.
Ethical Approval
The study was approved by the Ethics Committee of Sanmen People’s Hospital, Taizhou, China.
Patient Consent for Publication
The patient provided written informed consent for the publication of case details and accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Zhejiang Provincial Medical and Health Science and Technology Plan (2023KY414).
Disclosure
We declare that we have no conflicts of interest in connection with this paper, and that we received no payment or services from a third party in relation to this study.
References
1. Tally FP, Armfield AY, Dowell VR, et al. Susceptibility of Clostridium ramosum to antimicrobial agents. Antimicrob Agents Chemother. 1974;5(6):589–593. doi:10.1128/AAC.5.6.589
2. Yutin N, Galperin MY. A genomic update on clostridial phylogeny: gram-negative spore formers and other misplaced clostridia. Environ Microbiol. 2013;15(10):2631–2641. doi:10.1111/1462-2920.12173
3. Meier-Kolthoff JP, Carbasse JS, Peinado-Olarte RL, et al. TYGS and LPSN: a database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022;50(D1):D801–d807. doi:10.1093/nar/gkab902
4. Senda S, Fujiyama Y, Ushijima T, et al. Clostridium ramosum, an IgA protease-producing species and its ecology in the human intestinal tract. Microbiol Immunol. 1985;29(11):1019–1028. doi:10.1111/j.1348-0421.1985.tb00892.x
5. Shinzato T, Yonaha T, Oshiro Y, et al. Clostridium ramosum bacteremia: a case series at a general acute care hospital. J Infect Chemother. 2023;29(1):78–81. doi:10.1016/j.jiac.2022.09.009
6. Forrester JD, Spain DA. Clostridium ramosum bacteremia: case report and literature review. Surg Infect (Larchmt). 2014;15(3):343–346. doi:10.1089/sur.2012.240
7. Lavigne JP, Bouziges N, Sotto A, et al. Spondylodiscitis due to clostridium ramosum infection in an immunocompetent elderly patient. J Clin Microbiol. 2003;41(5):2223–2226. doi:10.1128/JCM.41.5.2223-2226.2003
8. Dahya V, Ramgopal M, Collin B, et al. Clostridium ramosum osteomyelitis in an immunocompetent patient after traumatic injury. Infect Dis Clin Pract. 2015;23:102–104. doi:10.1097/IPC.0000000000000209
9. Zakham F, Pillonel T, Brunel AS, et al. Molecular diagnosis and enrichment culture identified a septic pseudoarthrosis due to an infection with Erysipelatoclostridium ramosum. Int J Infect Dis. 2019;81:167–169. doi:10.1016/j.ijid.2019.02.001
10. Milosavljevic MN, Kostic M, Milovanovic J, et al. Antimicrobial treatment of Erysipelatoclostridium ramosum invasive infections: a systematic review. Rev Inst Med Trop Sao Paulo. 2021;63:e30. doi:10.1590/s1678-9946202163030
11. Assimakopoulos SF, Eleftheriotis G, Lagadinou M, et al. SARS CoV-2-induced viral sepsis: the role of gut barrier dysfunction. Microorganisms. 2022;10(5):1050. doi:10.3390/microorganisms10051050
12. Cao J, Wang C, Zhang Y, et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2021.1887722
13. Zuo T, Zhang F, Lui GCY, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi:10.1053/j.gastro.2020.05.048
14. Gaibani P, D’Amico F, Bartoletti M, et al. The gut microbiota of critically ill patients with COVID-19. Front Cell Infect Microbiol. 2021;11:670424. doi:10.3389/fcimb.2021.670424
15. Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–w296. doi:10.1093/nar/gkab301
16. Hennequin C, Collignon A, Karjalainen T. Analysis of expression of GroEL (Hsp60) of Clostridium difficile in response to stress. Microb Pathog. 2001;31(5):255–260. doi:10.1006/mpat.2001.0468
17. Chitayat S, Adams JJ, Smith SP. NMR assignment of backbone and side chain resonances for a dockerin-containing C-terminal fragment of the putative mu-toxin from Clostridium perfringens. Biomol NMR Assign. 2007;1(1):13–15. doi:10.1007/s12104-007-9002-7
18. Rumpel S, Razeto A, Pillar CM, et al. Structure and DNA-binding properties of the cytolysin regulator CylR2 from Enterococcus faecalis. EMBO j. 2004;23(18):3632–3642. doi:10.1038/sj.emboj.7600367
19. Rechner PM, Agger WA, Mruz K, et al. Clinical features of clostridial bacteremia: a review from a rural area. Clin Infect Dis. 2001;33(3):349–353. doi:10.1086/321883
20. Kosowska K, Reinholdt J, Rasmussen LK, et al. The Clostridium ramosum IgA proteinase represents a novel type of metalloendopeptidase. J Biol Chem. 2002;277(14):11987–11994. doi:10.1074/jbc.M110883200
21. Gollapudi LA, Narurkar R, Wang G, et al. Clostridium ramosum (C. ramosum) Bacteremia: single-center Study. Open Forum Infect Dis. 2017;4(suppl_1):S556–S556. doi:10.1093/ofid/ofx163.1446
22. Legaria MC, García SD, Tudanca V, et al. Clostridium ramosum rapidly identified by MALDI-TOF MS. A rare gram-variable agent of bacteraemia. Access Microbiol. 2020;2(8):acmi000137. doi:10.1099/acmi.0.000137
23. Leal J, Gregson DB, Ross T, et al. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000–2006. J Infect. 2008;57(3):198–203. doi:10.1016/j.jinf.2008.06.018
24. Mormeneo Bayo S, Palacián Ruíz MP, Moreno Hijazo M, et al. Bacteremia during COVID-19 pandemic in a tertiary hospital in Spain. Enferm Infecc Microbiol Clin. 2021;40(4):183–186. doi:10.1016/j.eimc.2021.01.015
25. Sturm LK, Saake K, Roberts PB, et al. Impact of COVID-19 pandemic on hospital onset bloodstream infections (HOBSI) at a large health system. Am J Infect Control. 2022;50(3):245–249. doi:10.1016/j.ajic.2021.12.018
26. Bauer KA, Puzniak LA, Yu KC, et al. Epidemiology and outcomes of culture-positive bloodstream pathogens prior to and during the SARS-CoV-2 pandemic: a multicenter evaluation. BMC Infect Dis. 2022;22(1):841. doi:10.1186/s12879-022-07810-8
27. Pérez-Granda MJ, Carrillo CS, Rabadán PM, et al. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: a plea for control. J Hosp Infect. 2022;119:149–154. doi:10.1016/j.jhin.2021.09.020
28. Tena-Garitaonaindia M, Arredondo-Amador M, Mascaraque C, et al. Modulation of intestinal barrier function by glucocorticoids: lessons from preclinical models. Pharmacol Res. 2022;177:106056. doi:10.1016/j.phrs.2022.106056
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.