Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Clinicopathological Risk Factors of Unfavorable Outcomes in Vietnamese Women with Primary Invasive Breast Cancer: A Retrospective Cohort Study
Authors Huynh CG, Huynh NX, Truong BHT, Thai TT , Doan PTT
Received 7 June 2023
Accepted for publication 27 July 2023
Published 1 August 2023 Volume 2023:15 Pages 551—561
DOI https://doi.org/10.2147/BCTT.S422289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Chau Giang Huynh,1 Nghiem Xuan Huynh,1 Bich-Ha Thi Truong,2 Truc Thanh Thai,3 Phuong-Thao Thi Doan4
1Department of Pathology, Hung Vuong Hospital, Ho Chi Minh City, Vietnam; 2Department of Obstetrics and Gynecology, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam; 3Department of Medical Statistics and Informatics, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam; 4Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
Correspondence: Truc Thanh Thai, University of Medicine and Pharmacy at Ho Chi Minh City, 217 Hong Bang, Ward 11, District 5, Ho Chi Minh City, Vietnam, Tel +84 908 381 266, Email [email protected]
Background: The rate of unfavorable outcomes, such as recurrence and death, in women with invasive breast cancer varies widely across countries and populations. Identifying those with high-risk profiles is critical so that early detection, prediction, and intervention can be made to improve their survival rate. Therefore, our study evaluated the rate of unfavorable outcomes and its association with clinicopathological characteristics in Vietnamese women with primary invasive breast cancer.
Methods: A retrospective open cohort study was conducted on Vietnamese women with invasive breast cancer who underwent a mastectomy and were regularly followed up by the hospitals. Kaplan–Meier method was used to estimate the rate of unfavorable outcomes to take into account the follow-up time of each patient. Univariate and multiple Cox regression analyses were conducted to examine the associations between unfavorable outcomes and clinicopathological characteristics.
Results: Among 204 women included in the data analysis, the mean age was 54.4 ± 10.9 years. The majority of patients were diagnosed with early-stage (76.5%) or locally advanced (22.5%) breast cancer. The 5-year rate of unfavorable outcomes was 12.8%, and the 8-year rate was 31.7%. Patients with advanced stages had a higher risk of unfavorable outcomes compared to those with early stages (IA, IIA, T2N1). Patients with lymph node metastases and those with triple-negative molecular classification had significantly higher rates of unfavorable outcomes.
Conclusion: Although Vietnamese women with breast cancer have a relatively low rate of unfavorable outcomes compared to other countries, findings from this study emphasize the importance of early detection and underscore the need for targeted interventions for patients with advanced stages, lymph node metastases, and triple-negative breast cancer to optimize their treatment, outcomes, and overall prognosis.
Keywords: unfavorable outcomes, invasive breast cancer, high-risk profiles, clinicopathological characteristics, Vietnamese women
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide, with about 2.26 million new cases, representing 11.7% of all cancer cases reported in 2020.1 In the United States, breast cancer accounts for almost 29% of all new cancers in women.2 While there has been a decline in breast cancer mortality rates in developed countries thanks to increased screening mammography and advances in adjuvant therapies, unfavorable outcomes remain high in many resource-limited countries. Even with advanced treatment strategies, most breast cancer recurs within the first five years of diagnosis, particularly with hormone receptor-negative or HER2-positive disease. The 5-year survival incidence of women in high-income countries with breast cancer of all cancer stages is 85–90%, lower in black women (80%) than in white women and lower in women with hormone receptor-negative tumors than in those with hormone receptor-positive tumors.3–5 In countries with limited economic and medical resources, 5-year survival rates are relatively low and vary widely across countries. For example, the 5-year survival estimates range from less than 20% survival in Mali to 35–50% in Uganda and 85% in Mauritius.6,7
Because of the high prevalence of breast cancer, identifying those with high-risk profiles is critical so that early detection, prediction, and intervention can be made to improve their survival rate. Many classical risk indicators, including age, grade, tumor size, nodal involvement, invasion, and metastasis, have been reported in previous studies worldwide.8–10 However, these clinical characteristics do not cover tumor biological heterogeneity among populations. Recent scientific evidence has shown the potential of immunohistochemistry factors (ER, PR, HER2, Ki- 67, PD-L1) or on H&E slides (TILs). For example, in a study of over 4000 patients with invasive breast cancer, patients with ER-positive disease had a lower annual risk of recurrence during the first five years after their initial treatment compared with those with ER-negative disease (9.9% versus 11.5%).11 Without systemic therapy, HER2 overexpression is a marker of poor prognosis in pathologically node-positive and node-negative breast cancer patients. In addition, data suggest that HER2 retains prognostic value even in the presence of small tumors (ie, ≤1 cm in size).12,13 Tumor-infiltrating lymphocytes (TILs) are an adverse prognostic factor for survival in luminal-HER2-negative breast cancer.14 In a recent systematic review and meta-analysis, PD-L1 upregulation was associated with worse clinical outcomes in breast cancer patients, emphasizing the significance of PD-L1 as a prognostic marker.15 Therefore, the combination of classical factors and immunohistochemistry factors would be beneficial to personalize and optimize adjuvant therapy and to prevent unnecessary exposure to potentially toxic and expensive medicines.
In Vietnam, the burden of breast cancer remains a major healthcare challenge. Previous studies have indicated that the prevalence of breast cancer in women increased from 13.8 cases per 100,000 in 2000 to 29.9 cases per 100,000 in 2010.16 Another study in Ho Chi Minh City in 2021 revealed a total of 14,222 new cases of breast cancer (13,948 women, or 98%) registered from 1996 to 2015. The age-standardized rate of breast cancer was 107.4 cases per 100,000 women (13,948 women), an increase of 70% compared to the rate from 1996–2000.16 The latest available data from the International Agency for Research on Cancer documented 21,555 new breast cancer cases and 9345 deaths in the country. The age-standardized incidence and death rate of breast cancer in Vietnam is 25.9 per 100,000 population and 11.5 per 100,000 population, respectively. Breast cancer has become the most frequent disease and the fourth leading cause of cancer death among Vietnamese women.1 Several investigations on breast cancer on clinical characteristics, histopathology, and immunohistochemistry in Vietnam have been conducted.17,18 However, studies evaluating the association between unfavorable outcomes with clinicopathological characteristics, ER, PR, HER2, Ki-67, TILs, and PD-L1 expression in Vietnamese with invasive breast cancer is limited.19
Therefore, our study evaluated the rate of unfavorable outcomes and its association with clinicopathological characteristics, ER, PR, HER2, Ki-67, TILs, and PD-L1 in Vietnamese women with primary invasive breast cancer. Findings from this study can provide a deep understanding of clinicopathological risk factors of unfavorable outcomes in Vietnamese women with primary invasive breast cancer.
Materials and Methods
Settings and Participants
We conducted a retrospective open cohort study and collected data from 2014 to 2021 at the Department of Pathology, University of Medicine and Pharmacy, and the Department of Pathology, Hung Vuong Hospital, in Ho Chi Minh City. A total of 263 patients who had mastectomy specimens and were regularly followed up by the hospitals until November 31, 2022, after being diagnosed with invasive breast cancer were included in the study. Patients had to have clinicopathological characteristics, ER, PR, HER2, Ki-67, and paraffin-embedded tissue blocks to be included. Those who underwent preoperative neoadjuvant chemotherapy (n = 11) were excluded because during the study period from 2014 to 2021 neoadjuvant chemotherapy was not a standard of care in Vietnam and thus was not routinely administered in the country. A few hospitals might have some trials to evaluate feasibility and safety of neoadjuvant chemotherapy or were still in search for an optimal neoadjuvant chemotherapy for this population. The patients diagnosed with carcinoma in situ were also excluded. We excluded one patient with no paraffin-embedded tissue block, 12 patients without information about the follow-up as they did not return to the hospital after their diagnosis, and 46 patients with no tumor cells or tissues on the immunohistochemical slides from the data analysis (Figure 1).
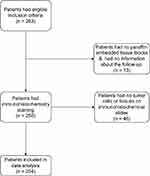 |
Figure 1 Flowchart of patient selection. |
Procedures and Measurements
Mastectomy specimens from patients who did not undergo preoperative chemotherapy were collected for this study. Information on clinicopathological characteristics, estrogen receptor (ER), progesterone receptor (PR), tumor cell proliferation index (Ki67), and human epidermal growth factor receptor 2 (HER2) biomarkers were also obtained from clinical and electronic medical records. ER and PR expression was considered positive if the tumor cell nucleus was stained ≥1%. HER2 expression was positive if the number of tumor cells with complete and strong circumferential membranes stained was >10%. Ki-67 expression was calculated as the percentage (%) of tumor cell nucleus staining, and a cutoff of ≥14% was used to identify high expression.
The number and percentage of TILs were evaluated by two trained pathologists using H&E slides, and a cutoff 11% was used. PD-L1 expression was evaluated using the IHC 22C3 pharmDx antibody by immunohistochemistry with Autostainer Link 48. A Combined Positive Score (CPS) was calculated by dividing the number of tumor cells, lymphocytes, and macrophages stained by PD-L1 by the total viable tumor cells multiplied by 100. PD-L1 protein expression was considered positive if CPS ≥1. Disagreements were assessed by a third experienced pathologist. Time to unfavorable outcomes was calculated from the date of operation to the first occurrence of 1) ipsilateral locoregional invasive breast tumor recurrence, 2) contralateral invasive breast cancer, 3) distant disease recurrence, or 4) death from any cause.
Ethical Approval
In accordance with standard procedure, all paraffin-embedded tissue block samples from women with invasive breast cancer were stored at the hospitals for up to 15–20 years. This study was approved by the Medical Ethics Committee at Hung Vuong Hospital, Ho Chi Minh City (141/HDDD-BVHV, dated January 12th, 2022) and was conducted in accordance with the Declaration of Helsinki. Given that all data used in this study were obtained from medical records and the stored tissue block samples, and no direct contact was established between the researchers and the patients, the requirement for written informed consent was waived by the ethics committee.
Data Analysis
The data were recorded and stored in EpiData software, version 4.6.0.4, and statistical analyses were performed using Stata version 16.0. Quantitative variables were described using mean and standard deviation. Categorical variables were described using frequency and percentage. Because each patient was followed up for a different duration, the rate of unfavorable outcomes was estimated using the Kaplan–Meier method to take into account the follow-up time. Univariable Cox regression analyses were conducted to examine the associations between unfavorable outcomes and clinicopathological characteristics, ER, PR, HER2, Ki-67, TILs, and PD-L1 expression. The results were presented as Hazard Ratio with its corresponding 95% confidence interval. Potential predicting factors with a p-value less than 0.2 in the previous univariable analysis were further evaluated in the multivariable Cox regression analysis using a stepwise backward approach. All statistical tests were two-sided, and a p-value less than 0.05 was considered statistically significant.
Results
The data analysis included a total of 204 women, with a median follow-up duration of 44.5 months (range: 5.2–100.7 months) (Figure 2). Of these women, 12.7% (n = 26) experienced at least one unfavorable outcome during the follow-up period, including ipsilateral locoregional invasive breast tumor recurrence (n = 10), contralateral invasive breast cancer (n = 4), distant disease recurrence (n = 16) and death (n = 10). The rate of unfavorable outcomes over the follow-up period is presented in Figure 3. The 5-year cumulative rate of unfavorable outcomes was 12.8%, and the 8-year cumulative rate was 31.7%.
 |
Figure 2 Distribution of follow-up duration in Vietnamese women with invasive breast cancer. |
 |
Figure 3 Probability of unfavorable outcome in Vietnamese women with invasive breast cancer. |
The mean age of the participants was 54.4 ± 10.9 years, with the majority of women (64.2%) being 50 years or older and almost half of them being postmenopausal. Most of the women (75%) had two or fewer children, and the majority of tumors (69.1%) were found in the upper and outer positions of the breast, with 43.1% of women discovering the tumors themselves. Approximately one-fourth (24%) of the women had a body mass index of 25 kg/m2 or greater. In addition, 18.6% of women had a history of diabetes, and 6.4% had a history of benign breast disease. However, the rate of unfavorable outcomes was not significantly different across these characteristics, as shown in Table 1.
 |
Table 1 The Association Between Clinical Characteristics and Unfavorable Outcomes Among Vietnamese Women with Invasive Breast Cancer |
In our study, the majority of patients were diagnosed with early-stage (76.5%) or locally advanced (22.5%) breast cancer, while only a small proportion (1%) were diagnosed with advanced-stage cancer. Almost all patients (94.6%) had a tumor diameter of 5 cm or less. Lymph node metastasis was present in almost half (47.1%) of the patients at diagnosis. The most common histological type observed was invasive ductal carcinoma, accounting for 91.2% (n=186) of cases. The majority of patients had grade 1 or grade 2 histological grades (77%), and peritumoral vascular invasion was found in 10.8% (n=22) of patients. The prevalence of ER, PR, and Ki67 expressions were 72.1% (n=147), 63.7% (n=130), and 76% (n=155), respectively. However, HER2 expression was positive in only 30.9% (n=63) of patients. The luminal molecular subtype accounted for 74.5% of the total patients, while the percentages of triple-negative and HER-enriched subtypes were 9.8% and 15.7%, respectively. Tumor-infiltrating lymphocytes (TILs) and PD-L1 were expressed in 36.3% and 31.4% of patients, respectively (Table 2).
 |
Table 2 The Association Between Pathological Characteristics and Unfavorable Outcomes Among Vietnamese Women with Invasive Breast Cancer |
There were significant differences in the rate of unfavorable outcomes among patients with advanced tumor stages, lymph node metastases, and molecular classifications. Patients with advanced stages had a higher risk of unfavorable outcomes (HR = 10.26, 95% CI 2.32–45.28, p = 0.002) compared to those with early stages (IA, IIA, T2N1). Additionally, patients with lymph node metastases and those with triple-negative molecular classification had significantly higher rates of unfavorable outcomes (HR = 2.84, 95% CI 1.23–6.54, p = 0.014, and HR = 2.67, 95% CI 1.13–6.29, p = 0.025) (Table 2). These findings remained unchanged in the multivariable Cox regression analysis (Figure 4).
 |
Figure 4 Final Cox regression model to identify factors associated with unfavorable outcomes among Vietnamese women with invasive breast cancer. |
Discussion
Identifying high-risk patients with invasive breast cancer for recurrence is crucial since the majority of recurrences occur within the first five years of diagnosis, especially in patients with hormone receptor-negative disease.11 Our study is among the first to explore the association between unfavorable outcomes and clinicopathological factors in Vietnamese women with invasive breast cancer. Our results found a relatively low rate of unfavorable outcomes during the follow-up and significant risk factors, including tumor stage, lymph node metastases, and molecular classification.
The relatively low rate of unfavorable outcomes found in our study is consistent with previous studies in Asia and Europe.1 For example, in a study of 4105 patients with operable breast cancer treated on International Breast Cancer Study Group clinical trials I to V, the annual risk of recurrence was highest during the first five years (10.4%), with a peak between years 1 and 2 (15.2%).11 In another study of 13,722 patients in Scotland with stage I, II, or III breast cancer, the recurrence risks in the subsequent 5 and 10 years were 11% and 19%, respectively.20 The low rate of unfavorable outcomes in our study may be attributed to the fact that a significant proportion of patients enrolled were likely screened, diagnosed, and treated at early stages (76.5%) and had a tumor size of 5 cm or less (94.6%). However, our results are different from some studies in Africa, where the 5-year age-standardized relative survival was low and varied significantly (12% in Uganda (Kyadondo) and 20% to 60% in South Africa (Eastern Cape), Kenya (Eldoret), and Zimbabwe (Harare).7 Differences in the rate of unfavorable outcomes between different geographical regions may be due to variations in the quality of health services, awareness, human resources, and the effectiveness of breast cancer screening programs. A recent study conducted in five sub-Saharan African countries estimated that 28% to 37% of breast cancer deaths in these countries could be prevented through earlier diagnosis of symptomatic disease and adequate treatment. While the low rate of unfavorable outcome found in our study presents a positive indication in Vietnam, it is important to note that the results were obtained from a specific group of patients in two major hospitals. This rate is likely to be higher in regions and medical facilities with limited resources and low patient’s awareness about the disease. This underscores the importance of enhanced breast cancer attention and clinical breast examination by qualified health providers, followed by timely and proper treatment, to improve both the overall survival rate and the event-free survival rate in this vulnerable population.1,21,22
Our findings on the association between unfavorable outcomes and advanced stage (stage IV), lymph node metastases, and triple-negative molecular subtypes are consistent with previous studies. In a meta-analysis of 88 trials involving 62,923 women with ER-positive breast cancer who were recurrence-free after five years of endocrine therapy, those with T1 tumors had a distant recurrence risk of 13% in the absence of lymph node involvement, 20% with one to three involved lymph nodes, and 34% with four to nine involved nodes.20 In clinical practice, the stage is a prognostic factor determined by multiple characteristics such as tumor size, lymph node involvement, and metastatic disease. In the American Joint Committee on Cancer (AJCC) eighth edition staging system, five-year disease-free survival (DFS) rates varied between 98–100% for stage I disease, 85–98% for stage II, and 70–95% for stage III.23 Additionally, a study on 1118 patients receiving neoadjuvant chemotherapy for stage I–III breast cancer found that the risk of distant recurrence and death was highest in triple-negative breast cancer three years after diagnosis but declined rapidly after that.24 In another study comparing 12,902 women with hormone receptor-positive and HER2-negative breast cancer, women with TNBC had worse breast cancer-specific survival (HR=2.99, 95% CI 2.59–3.45), worse overall survival (HR=2.72, 95% CI 2.39–3.10), and a significant increase in mortality rate within two years of diagnosis (HR=6.10, 95% CI 4.81–7.74).25
However, our study did not find statistically significant associations between several potential clinical and immunohistochemical factors and unfavorable outcomes in Vietnamese breast cancer patients. These included histologic grade, Ki-67, HER2 expression, PDL1 expression, and TILs expression. However, the inconsistency of the association between these factors and survival rate in breast cancer women has also been reported in previous studies. For example, a similar study in 2019 among 248 Vietnamese women with breast cancer at the National Cancer Hospital in Hanoi found that prognostic factors such as age, menopause status, nodal status, tumor grade, ER status, and HER2 status were not associated with the survival incidence.19 In contrast, previous studies have shown that features predicting a high rate of distant recurrence include tumor size, positive margins, high nuclear grade, age <35 years old, and HER2 positive or HR negative status, as well as TILs and PD-L1.26,27 For instance, HER2-positive status provides prognostic information in node-negative breast cancer and predictive information in selecting targeted and systemic therapy.28–30 One possible explanation from our study was the small number of patients with unfavorable results (n=26), which might limit our statistical power in detecting these associations. Further research with a larger sample size and the number of outcomes is needed.
Some limitations in our study should be considered when interpreting the study findings. First, the follow-up period was relatively short compared to other studies worldwide, so we could only estimate unfavorable outcomes up to 8 years after the diagnosis. Second, due to resources and personnel constraints, as well as the unavailability of data, we could not investigate all the potential factors that may contribute to unfavorable outcomes in patients such as their treatment and adherence during the follow-up period. Third, although we recruited all eligible patients at two major hospitals in the biggest city in Vietnam over a relatively long period from 2014 to 2021, it is essential to note that the sample size was relatively small compared to many other studies around the world. This small sample size was likely to affect the statistical power in our study. Therefore, conducting further research, particularly prospective studies, is crucial to gain a more comprehensive understanding of the critical factors that influence the outcomes of Vietnamese women with invasive breast cancer.
Conclusions
Although Vietnamese women with primary invasive breast cancer have a relatively low rate of unfavorable outcomes compared to other countries, effective breast cancer screening programs for early-stage detection and timely and appropriate interventions are still necessary to reduce the recurrence rate and increase disease-free survival and overall survival. Proper interventions should be based on risk profiles, such as advanced stage, lymph node metastasis, and triple-negative subtype. Further studies are needed, especially on tumor grade, ER expression, Ki-67 expression, HER2-enriched breast cancer, breast cancer history, TILs, and PD-L1 expression, to optimize treatment.
Acknowledgments
The authors thank Chau Minh Man, Bui The Long, Duong Minh Thu, Hoang Le Quynh Anh, Vo My Thien An, Le Thi Kim Hanh, Ha Pham Yen Vy, Duong Ngoc Thien Huong, and Nguyen Thi Hoang An for their help during the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Disclosure
The authors declare no conflicts of interest to declare that are relevant to the content of this article.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. doi:10.3322/caac.21320
3. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (Concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi:10.1016/s0140-6736(17)33326-3
4. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi:10.1001/jama.295.21.2492
5. Miller JW, Smith JL, Ryerson AB, Tucker TC, Allemani C. Disparities in breast cancer survival in the United States (2001–2009): findings from the Concord-2 study. Cancer. 2017;123(Suppl 24):5100–5118. doi:10.1002/cncr.30988
6. Sankaranarayanan R. Cancer survival in Africa, Asia, the Caribbean and Central America. Introduction. IARC Sci Publ. 2011;162:1–5.
7. Joko-Fru WY, Miranda-Filho A, Soerjomataram I, et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: a population-based registry study. Int J Cancer. 2020;146(5):1208–1218. doi:10.1002/ijc.32406
8. Gasparini G, Pozza F, Harris AL. Evaluating the potential usefulness of new prognostic and predictive indicators in node-negative breast cancer patients. J Natl Cancer Inst. 1993;85(15):1206–1219. doi:10.1093/jnci/85.15.1206
9. Hayes DF, Trock B, Harris AL. Assessing the clinical impact of prognostic factors: when is “statistically significant” clinically useful? Breast Cancer Res Treat. 1998;52(1–3):305–319. doi:10.1023/a:1006197805041
10. Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi:10.1016/s0140-6736(11)61625-5
11. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016;34(9):927–935. doi:10.1200/jco.2015.62.3504
12. Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989;7(8):1120–1128. doi:10.1200/jco.1989.7.8.1120
13. Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10(7):1049–1056. doi:10.1200/jco.1992.10.7.1049
14. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi:10.1016/s1470-2045(17)30904-x
15. Zhang Y, Tian J, Qu C, et al. Prognostic value of programmed cell death ligand-1 expression in breast cancer: a meta-analysis. Medicine. 2020;99(49):e23359. doi:10.1097/md.0000000000023359
16. Pham DX, Ho TH, Bui TD, Ho-Pham LT, Nguyen TV. Trends in breast cancer incidence in Ho Chi Minh City 1996–2015: a registry-based study. PLoS One. 2021;16(2):e0246800. doi:10.1371/journal.pone.0246800
17. Nguyen SM, Nguyen QT, Nguyen LM, et al. Delay in the diagnosis and treatment of breast cancer in Vietnam. Cancer Med. 2021;10(21):7683–7691. doi:10.1002/cam4.4244
18. Trieu PD, Mello-Thoms C, Brennan PC. Female breast cancer in Vietnam: a comparison across Asian specific regions. Cancer Biol Med. 2015;12(3):238–245. doi:10.7497/j.issn.2095-3941.2015.0034
19. Vu Hong T, Nguyen Ba D, Skoog L, Ta Thanh V, Tani E. Breast cancer survival defined by biological receptor and menopausal status in Vietnamese women. Cancer Control. 2019;26(1):1073274819865279. doi:10.1177/1073274819865279
20. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi:10.1056/NEJMoa1701830
21. McCormack V, McKenzie F, Foerster M, et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. Lancet Glob Health. 2020;8(9):e1203–e1212. doi:10.1016/s2214-109x(20)30261-8
22. Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11(2):165–173. doi:10.1016/s1470-2045(09)70335-3
23. Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–1785. doi:10.1245/s10434-018-6486-6
24. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi:10.1200/jco.2007.14.4147
25. Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the national comprehensive cancer network. Cancer. 2012;118(22):5463–5472. doi:10.1002/cncr.27581
26. Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295(14):1658–1667. doi:10.1001/jama.295.14.1658
27. Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19(6):1688–1697. doi:10.1200/jco.2001.19.6.1688
28. Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332(14):901–906. doi:10.1056/nejm199504063321401
29. Cirqueira MB, Mendonça CR, Noll M, et al. Prognostic role of PD-L1 expression in invasive breast cancer: a systematic review and meta-analysis. Cancers. 2021;13(23):6090. doi:10.3390/cancers13236090
30. Cooke T, Reeves J, Lanigan A, Stanton P. HER2 as a prognostic and predictive marker for breast cancer. Ann Oncol. 2001;12 Suppl 1:S23–S28. doi:10.1093/annonc/12.suppl_1.s23
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
