Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Clinicopathological Features of Hepatocellular Carcinoma with Metabolic Risk Factors
Authors Sun L, Zhao H, Ding XY , Yang K, Wang GS, Chen JM, Han XY, Wan G, Zhang L, Zhou XG, Chen XM, Wang P, Xie W
Received 4 April 2023
Accepted for publication 31 May 2023
Published 5 June 2023 Volume 2023:10 Pages 833—846
DOI https://doi.org/10.2147/JHC.S412129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr David Gerber
Lei Sun,1 Hong Zhao,2 Xiao-Yan Ding,3 Kun Yang,1 Gui-Shuang Wang,4 Jia-Min Chen,1 Xiao-Yi Han,1 Gang Wan,5 Liang Zhang,1 Xin-Gang Zhou,1 Xiang-Mei Chen,1 Peng Wang,1 Wen Xie2
1Department of Pathology, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 2Center of Liver Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 3Department of Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 4Center of Medical Insurance, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 5Department of Medical Records and Statistics, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
Correspondence: Wen Xie, Center of Liver Diseases, Beijing Ditan Hospital, Capital Medical University, No. 8 Jing Shun East Street, Chaoyang District, Beijing, 100015, People’s Republic of China, Tel +86-10-84322818, Email [email protected] Lei Sun, Department of Pathology, Beijing Ditan Hospital, Capital Medical University, No. 8 Jing Shun East Street, Chaoyang District, Beijing, 100015, People’s Republic of China, Tel +86-10-84322536, Email [email protected]
Objective: This study aims to explore the pathological characteristics of metabolic-related hepatocellular carcinoma (HCC) and its correlation with metabolic factors.
Methods: Fifty-one patients with liver cancer of unknown causes were enrolled. Biopsy of the liver and staining of the liver tissues with hematoxylin–eosin as well as special and immunohistochemical stains were performed. The histological subtypes of HCC were diagnosed based on the WHO Classification of Malignant Hepatocellular Tumors. The NAFLD activity score system was adopted for assessing the surrounding non-neoplastic liver tissues.
Results: Of the total, 42 (82.4%) patients were diagnosed with HCC, 32 had metabolic risk factors, 20 patients met the diagnostic criteria of the metabolic-associated fatty liver disease (MAFLD)-related HCC, and 40.6% (13/32) had liver cirrhosis. The incidence of cirrhosis (p = 0.033) and diabetes mellitus type 2 (p = 0.036) in patients with MAFLD-related HCC was notably higher than that in HCC patients with only metabolic risk factors. Among the 32 HCC cases with metabolic risk factors, trabecular type was the most prevalent, followed by steatohepatitis type, scirrhous type, solid type, pseudoglandular type, clear-cell type, and macrotrabecular type. The degree of tumor cells’ swelling and ballooning was found to be positively related to the degree of fibrosis in the surrounding liver tissues (p = 0.011) as well as the proportion of cirrhosis (p = 0.004). Moreover, the degree of fibrosis in the surrounding liver tissues showed a negative correlation with the levels of serum cholesterol (p = 0.002), low-density lipoprotein (p = 0.002), ApoA1 (p = 0.009), ApoB (p = 0.022), total protein (p = 0.015), WBC count (p = 0.006), and PLT count (p = 0.015).
Conclusion: Pathological characteristics of the tumor and adjacent non-neoplastic liver tissues of HCC with metabolic risk factors were found to be correlated with metabolic abnormalities.
Keywords: metabolic-associated fatty liver disease, MAFLD, hepatocellular carcinoma, metabolic risk factors, pathology
Introduction
Liver cancer is one of the most common malignancies globally and the most common cause of death in patients with chronic liver disease.1,2 In 2020, the primary liver cancer in China ranked fifth in terms of the incidence rate among malignant tumors and second among the causes of death due to cancer.1 Hepatocellular carcinoma (HCC) is the most common primary liver cancer and has multiple risk factors. One of these risk factors in developed countries is metabolic-associated fatty liver disease (MAFLD), which has been projected as the major reason for cirrhosis and HCC.3,4 MAFLD is a chronic liver disease having an association with obesity, type 2 diabetes mellitus (T2DM), and hyperlipidemia. In addition, the epidemic of obesity and DM2 has fueled an elevating prevalence of MAFLD, rendered it a societal health problem with the worldwide prevalence of 24%.5
However, MAFLD-related HCC has been relatively poorly characterized. Unlike HCC resulting from other causes, the etiology and pathogenesis of MAFLD-HCC remain unclear. Many factors contribute to the pathogenesis of MAFLD, with metabolic disorders being the primary cause of its development and progression. Owing to the close relationship between MAFLD and metabolic syndrome and the systemic metabolic disorder of liver manifestation, several studies have indicated that metabolic features, particularly disease-related risk factors such as hypertension, obesity, dyslipidemia, and T2DM, are closely associated with the incidence of MAFLD-HCC.4,6
Abnormal laboratory parameters are frequently reported in HCC cases. Because most HCC patients with metabolic risk factors have underlying fibrosis or liver steatosis, the adjacent unaffected liver function has been recognized as an important factor for determining HCC manifestations. The liver plays an important role in lipid metabolism; hence, the pathological and functional changes in the liver have been associated with circulating lipid modification. However, the relationship between clinicopathological features of MAFLD-HCC or HCC with metabolic risk factors and laboratory indicators reflecting liver function has rarely been reported.
Herein, a retrospective analysis was conducted in patients with metabolic risk factors admitted to the Beijing Ditan Hospital, Capital Medical University, and their metabolic risk factors related to HCC were examined. In this study, the etiology and pathogenesis of MAFLD-related HCC and HCC with metabolic risk factors were examined by investigating the pathological characteristics of HCC with metabolic risk factors, clinicopathological and metabolic characteristics of MAFLD-related HCC and HCC with only metabolic risk factors, and the correlations between the pathological characteristics and laboratory indicators of HCC with metabolic risk factors.
Methods
Study Design
The study had a single-center retrospective design. Patients diagnosed as having liver cancer including HCC and intrahepatic cholangiocarcinoma admitted to Beijing Ditan Hospital, Capital Medical University, from January 1, 2011, to December 31, 2020 were included. The diagnosis in all cases was confirmed through surgical biopsy or percutaneous needle biopsy of the tumor. Basic information and medical history, laboratory examination results, and imaging examination results of the enrolled patients were recorded. Patients diagnosed with liver cancer from an unknown cause were included, whereas those diagnosed with liver cancer of known etiologies such as viral hepatitis, alcoholic liver disease, autoimmune liver diseases, drug-induced liver injury, and hepatic metastatic carcinoma were excluded.
Patients
A total of 51 patients who were admitted to the Beijing Ditan Hospital from January 1, 2011 to December 31, 2020 were diagnosed with liver cancer by surgical biopsy or percutaneous needle biopsy of the tumor. Patients were grouped into the HCC group with metabolic risk factors and the group without metabolic risk factors according to whether they met at least one of the following three criteria:3 overweight/obesity (body mass index [BMI] ≥ 25 kg/m2 in Caucasians or BMI ≥ 23 kg/m2 in Asians), type 2 diabetes mellitus (T2DM), or two or more of four metabolic-related factors (ie, hypertension, blood triglyceride level of ≥1.70 mmol/L, high-density lipoprotein cholesterol [HDL-C] levels of <1.0 mmol/L in men and <1.3 mmol/L in women, and prediabetes). Patients who had liver steatosis in biopsy or had fatty liver on imaging were considered to meet the diagnostic criteria for MAFLD (Figure 1). Clinical information including the demographic profile and serological test results was acquired from the patients’ charts in the electronic medical record system. Biochemical indicators including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), γ-glutamyl transpeptidase (GGT), total bilirubin (TBiL), direct bilirubin (DBiL), total bile acid (TBA), total cholesterol (TCHO), triglycerides (TG), high-density liptein cholesterol (HDL-C), low-density liptein cholesterol (LDL-C), apolipoprotein A1 (APO-A1), apolipoprotein B (ApoB), lipoprotein(a) (LPa), albumin (ALB), and fasting blood glucose (GLU) were retrospectively analyzed. The BMI was calculated using the following formula: weight/height in meters squared (kg/m2).
 |
Figure 1 Flow chart of the study participants. |
The written informed consent was acquired from the participants by physicians. In addition, the approval of the study protocol was obtained from the Ethics Committee of Beijing Ditan Hospital, Capital Medical University. However, all procedures used in this research involving human participants were performed based on the ethical standards and in accordance with the 1964 Helsinki declaration as well as the later amendments or comparable ethical standards.
Histopathological Measurements
In this study, paraffin-embedded blocks of the liver tissues were sectioned and then stained with hematoxylin and eosin, as well as with special stains including Masson trichrome, reticulin, and Periodic Acid Schiff with diastase (D-PAS). The immunohistochemical stains of HepPar-1, GPC-3, GS, HSP70, CK7, CK19, CD34, and CD10 were performed for the biopsy specimens in the pathology laboratory. Embedded sections were deparaffinized with xylene, followed by an alcohol gradient and water rinses, and incubated with 0.3% hydrogen peroxide for 10 min at room temperature to eliminate endogenous peroxidase activity. After antigen retrieval under high pressure with a citrate buffer, individual slides were incubated at 4 °C overnight with primary antibodies. Slides were then washed three times with phosphate-buffered saline (PBS), followed by incubation with horseradish peroxidase-labeled secondary antibodies at 37 °C for 30 min. Subsequently, the slides were washed and developed with DAB, hematoxylin counterstained, and mounted. PBS diluent was used in place of antibodies as negative controls.7
The grades and histological subtypes of HCC were diagnosed according to the WHO Classification of Malignant Hepatocellular Tumors (5th edition). The surrounding adjacent nontumoral liver tissues were evaluated by the NAFLD activity score (NAS) for inflammatory activity and a separate fibrosis staging system in conjunction with NAS for the extent of fibrosis.8 All sections were evaluated by two experienced pathologists.
Statistical Analyses
SPSS 20.0 (IBM Statistics, SPSS, Chicago, IL) was used to perform all statistical analyses. Continuous values are denoted by the mean ± standard deviation, and categorical variables are indicated as frequencies or ratios. Nonparametric variables are denoted as the median with interquartile range (IQR). Normally-distributed data were analyzed using Student’s t-test. For abnormally distributed continuous or ordinal dependent variables, differences between two groups were explored by using Mann–Whitney U-test. To explore differences in the categorical variables, the Chi-square test was employed. In addition, a p value of <0.05 was considered to indicate statistical significance. Spearman correlation coefficient was calculated to determine the correlation between the laboratory parameters and pathological features. The correlations were considered significant at p ≤ 0.05.
Results
Patient Characteristics
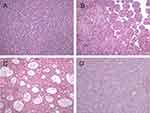
A total of 51 patients were diagnosed with liver cancer from an unknown cause, which included 42 (82.4%) cases of HCC. In addition, 32 cases exhibited metabolic risk factors and 20 cases met the diagnostic criteria for MAFLD, with the peripheral liver tissue steatosis accounting for >5% of hepatocytes. Steatosis was typically macrovesicular, but the degree of steatosis in all cases was <30% (Figure 2). The average age of the patients with HCC with metabolic risk factors was 63.2 ± 11.4 years, and the patient cohort comprised 21 (65.6%) men and 11 (52.4%) women.
A total of 51 cases of liver cancer from an unknown cause included 9 (17.6%) cases of intrahepatic cholangiocarcinoma (ICC). Of these 9 cases, 7 involved metabolic risk factors and 3 met the diagnostic criteria for MAFLD. The average age of patients with ICC associated with the metabolic risk factors was 62.6 ±7.3 years, and of these patients, 3 (42.9%) were men and 4 (57.1%) were women.
No evident differences were noted in the age and gender between the HCC and the ICC groups. However, the proportion of liver cirrhosis in the HCC group with metabolic risk factors was notably higher than that in the ICC group with metabolic risk factors (p = 0.039).
Comparison Between HCC with and without Metabolic Risk Factors
The mean age of HCC patients with metabolic risk factors (32 cases) was obviously higher than that of HCC patients without metabolic risk factors (10 cases). Among the 32 HCC patients with metabolic risk factors, 8 (25%) were obese, 25 (78.1%) had T2DM, 23 (71.9%) had hypertension, 10 (31.3%) had blood triglyceride level ≥1.70 mmol/L, and 24 (75%) had HDL-C levels lower than the standard level.
The proportion of patients with T2DM, hypertension, blood triglyceride level ≥1.70 mmol/L, serum TG, ApoB, and GLU levels was notably higher in the HCC group with metabolic risk factors (n = 32) than in the group without metabolic risk factors (n = 10). In addition, the proportion of patients with macrotrabecular-type HCC was significantly lower than that of HCC patients without metabolic risk factors (Table 1).
 |
Table 1 Comparison Between Different Groups |
Comparison Between MAFLD-Related HCC and HCC with Only Metabolic Risk Factors
The incidence of liver cirrhosis and T2DM in patients with MAFLD-related HCC (20 cases) was notably higher than that in HCC patients with only metabolic risk factors (12 cases), whereas the proportion of patients with scirrhous-type HCC was notably lower than that of HCC patients with only metabolic risk factors (Table 1).
Pathological Characteristics of HCC with Metabolic Risk Factors
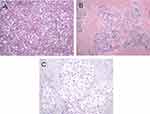
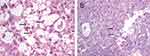
Among the 32 cases of HCC with metabolic risk factors, based on the pathological types, 13 (40.6%) cases were of the trabecular type, 9 (28.1%) of the steatohepatitis type, 4 (12.5%) of the scirrhous type, 2 (6.3%) of the solid type, another 2 (6.3%) of the pseudoglandular type, 1 (3.1%) of the clear-cell type, and the remaining 1 (3.1%) case was of the macrotrabecular type (Figures 3 and 4). Tumor cells in 19 cases were accompanied by different degrees of swelling and ballooning because of inflammation or glycogen accumulation, which accounted for 59.4% of the total cases. Mallory–Denk bodies were detected in 17 (53.1%) cases, and glycogenated nuclei were detected in 12 (37.5%) cases (Figure 5).
The degree of inflammation in the surrounding liver tissues (p = 0.037) and the levels of serum cholesterol (p = 0.050) were higher in the trabecular-type HCC than in the other types. However, the degree of tumor cell swelling and ballooning (p = 0.007) and the proportion positive of Mallory–Denk bodies (p = 0.005) in the trabecular-type HCC were markedly lower than those in the other types. The degree of fibrosis in the surrounding liver tissues (p = 0.010), the proportion of tumor cell swelling and ballooning (p = 0.000), and the proportion positive of Mallory–Denk bodies (p = 0.001) in the steatohepatitic-type HCC were notably higher than those in the other types, whereas the peripheral blood WBC count (p = 0.017), RBC count (p = 0.012), and Hb content (p = 0.026) were significantly lower in steatohepatitic-type HCC than in the other types. The degree of steatosis in the surrounding liver tissues (p = 0.031) in the scirrhous-type HCC was significantly lower than that in the other types (Table 2).
 |
Table 2 Pathological Characteristics and Laboratory Indicators Between Different Pathological Types of HCC with Metabolic Risk Factors |
Correlational Analysis Between the Pathological Characteristics and Laboratory Indicators of HCC with Metabolic Risk Factors (Table 3)
The lower the differentiation of HCC with metabolic risk factors, the stronger the expression of CK19, the higher the proliferation index of ki-67 (p = 0.009), and the lower the expression of Hep Par 1. The degree of swelling and ballooning of tumor cells exhibited a positive association with the degree of fibrosis in the surrounding liver tissues (p = 0.011) as well as the proportion of liver cirrhosis (p = 0.004), whereas it showed a negative association with the expression of CK8 (p = 0.015); serum cholesterol, ApoA1, ApoB, total protein, albumin levels; WBC count, RBC count, and PLT count; and Hb level. The expression of GPC3 in the tumor cells was positively related to the serum AFP level and negatively associated with the serum LPa level, GLU level, and PLT count. The expression of HSP70 in the tumor cells exhibited a positive association with the levels of serum ALT, TBiL, and DBiL and a negative relationship with the serum cholesterol and ApoB levels.
 |
Table 3 Correlation Analysis Between Pathological Characteristics and Laboratory Indicators of HCC with Metabolic Risk Factors |
The degree of steatosis in the surrounding liver tissues was negatively correlated with the levels of serum AKP, GGT, and LPa. The degree of inflammation in the surrounding liver tissues was negatively correlated with the decreased HDL-C levels and positively related to the serum LPa levels. Additionally, the degree of fibrosis in the surrounding liver tissues was negatively correlated with the levels of serum cholesterol, LDL-C, ApoA1, ApoB, and total protein as well as WBC and PLT counts.
The occurrence of cirrhosis was positively associated with the degree of swelling and ballooning of tumor cells (p = 0.004), the proportion positive of Mallory–Denk bodies (p = 0.003), glycogenated nuclei (p = 0.022), GPC3 expression (p = 0.005), serum TBiL (p = 0.028), and DBiL (p = 0.025) levels; however, it was negatively associated with the elevated blood triglyceride level, serum cholesterol level, LDL-C level, ApoB level, the total protein level, WBC count, and PLT count.
Comparison Between the Liver Cirrhosis and Non-Cirrhosis Groups
Among the 32 patients with HCC involving metabolic risk factors, 13 (40.6%) had liver cirrhosis and 19 (59.4%) did not have liver cirrhosis. In addition, the degree of swelling and ballooning of tumor cells, proportion positive of Mallory–Denk bodies and glycogenated nuclei, GPC3 expression, and the serum TBiL level in the liver cirrhosis group were notably higher than those in the non-cirrhosis group. On the other hand, the incidence of elevated blood triglyceride level, serum cholesterol, triglycerides, LDL-C, ApoB, WBC count, and PLT count in the cirrhosis group were notably lower than those in the non-cirrhosis group (Table 4).
 |
Table 4 Comparison of Liver Cirrhosis and Non-Cirrhosis Patients with HCC with Metabolic Risk Factors |
Discussion
MAFLD, also called non-alcoholic fatty liver disease (NAFLD), represents the metabolic syndrome-related chronic fatty liver disease. So far, liver histology has been identified as the precise method for diagnosing MAFLD, albeit it is invasive in nature. Steatosis usually occurs around the central veins, mostly within zones 2 and 3. As numerous liver disorders display similar histological manifestations, >5% of hepatocytes with fatty alteration should be diagnosed as MAFLD.9 In this study, 20 of the 32 cases of HCC with metabolic risk factors met the diagnostic criteria for MAFLD, but the degree of steatosis in all cases was <30%. A total of 12 cases exhibited no fatty liver on imaging or pathology, and whether they had a history of fatty liver is unknown; however, the presence of metabolic abnormality-related factors was noted. Accordingly, HCC with metabolic risk factors was diagnosed in these patients. The reason for this diagnosis may be that hepatic steatosis had “burned out” and was no longer evident in the advanced stage owing to the energy consumption by the tumor. Another reason may be that the liver needle aspiration specimen was small and did not adequately reflect the overall changes.
The age of patients has been identified as the critical risk factor for HCC, and the association is influenced by the etiology of liver disease. In the case of HBV, the mean age at the time of HCC diagnosis is 50 years. On the contrary, the average age for NAFLD-related HCC diagnosis is 70 years.10 Our results confirm that the mean age of HCC patients with metabolic risk factors was remarkably higher than that of HCC patients with no metabolic risk factors. This observation suggests that MAFLD has a slower progression. HCC is more commonly recorded among men, with a male-to-female ratio in Asian countries of around 2:3.1 Similarly, in the present study, among HCC patients with metabolic risk factors, 21 were men and 11 were women. Male dominance herein can be attributed to different lifestyles, body fat distributions, body compositions, as well as the metabolism of sex hormones in men.11 MAFLD has a higher prevalence and a potentially greater severity among men than in women; typically, estrogen can protect against MAFLD occurrence.11
Several studies have verified the close relationship between metabolic features and HCC occurrence. Particularly, for non-cirrhosis cases, T2DM and obesity have been identified to independently predict the HCC risk.12 T2DM has been closely related to hepatic steatosis, and it may lead to an increased risk of NAFLD/MAFLD development.13 Based on our results, the incidence of T2DM in patients with MAFLD-related HCC was notably higher than that in HCC patients with only metabolic risk factors. Typically, the MAFLD definition considers these results and the close relationship between metabolic syndrome and metabolic dysfunction with liver steatosis.
Microscopically, HCC cells comprise hepatocytes such as the cells producing macrotrabecular, trabecular, and solid growth, as well as pseudoglandular/pseudoacinar patterns. The trabecular structure is the most common growth pattern. Our study recorded the same results, with the trabecular type accounting for 40.6% of all HCC cases involving metabolic risk factors. Moreover, HCC exhibited extensive morphological manifestations, including morphological subtypes accounting for approximately 35% of all HCC cases.14 In decreasing order, the HCC subtypes were as follows: steatohepatitic, clear-cell, macrotrabecular-massive (MTM), scirrhous, chromophobe, fibrolamellar, neutrophil-rich, and lymphocyte-rich subtypes.
Steatohepatitic HCC displays manifestations similar to those of steatohepatitis, including steatosis, Mallory–Denk bodies, cancer cell ballooning, pericellular fibrosis, and inflammation. It is the most common HCC variant reported in the literature,14 representing 5%–20% of all HCC cases.15 Steatohepatitic HCC accounted for 28.1% of HCC cases with metabolic risk factors in our study. However, steatohepatitic HCC can also occur without any background metabolic syndrome or fatty liver disease.16 We also found two cases of steatohepatitic HCC among 10 cases of HCC without any metabolic risk factors. Therefore, the correlation between steatohepatitic HCC and the metabolic risk factors needs to be further analyzed. As reported previously, steatohepatitic HCC is more likely to occur in patients with advanced fibrosis.17 We also observed markedly increased fibrosis of the adjacent liver tissues in patients with steatohepatitic-type HCC.
Hepatocellular swelling and ballooning are considered the types of hepatocellular injury that are commonly but not exclusively reported in NASH and are also common in HCC. The factors that may contribute to ballooning include excess fatty acid oxidation because of the increased fat contents in the cells, which may cause metabolic stress, ultimately leading to the degeneration and apoptosis of lipotoxic cells.18 Ballooning cells frequently contain Mallory–Denk bodies. Identifying Mallory–Denk bodies as well as the ballooning degeneration through liver biopsy is of great significance because these features are related to fibrosis development.19 We also found that the degree of swelling and the proportion of ballooning tumor cells were positively correlated with the fibrosis degree within the adjacent liver tissues, and the occurrence of cirrhosis was positively associated with the degree of swelling and ballooning of tumor cells, as well as the proportion positive of Mallory–Denk bodies and glycogenated nuclei.
Scirrhous HCC represents an uncommon HCC subtype. Its histomorphology includes fibrosis within the tumor as well as non-cirrhotic background liver. The incidence of scirrhous HCC has been reported to be approximately 4.0%,20 albeit it was 12.5% in the present study. There may have been a selection bias in study because not all cases require a biopsy to differentiate cholangiocarcinoma from fibrolamellar HCC. Scirrhous HCC was not observed in the MAFLD-related HCC group in this study, and the proportion of patients with scirrhous HCC markedly decreased compared with that of HCC patients with only metabolic risk factors. We also observed that the degree of steatosis in the surrounding liver tissues in the scirrhous-type HCC was significantly lower than that in the other types. Therefore, we speculated that MAFLD or NAFLD cannot easily progress to scirrhous HCC.
Immunohistochemistry (IHC) plays an important role in evaluating HCC. A panel of markers can be employed to confirm malignancy and distinguish it from non-hepatocellular mass lesions. Typically, glutamine synthetase (GS), glypican-3 (GPC-3), HepPar-1, heat shock protein 70 (HSP70), Ki-67, CD34, CK7, and CK19 are the frequently used markers. GPC-3 represents the cell-surface heparan sulfate proteoglycan, and its expression can be considered an indicator to predict malignant HCC. In this study, GPC3 expression within cancer cells exhibited a positive correlation with the serum AFP level, consistent with the elevated serum AFP level in HCC. HepPar-1 is highly sensitive and specific for HCC (as high as 80%). Nonetheless, its level is <50% for poorly differentiated cancers.14 CK19 expression is related to the aggressiveness of tumors, while GPC-3 and CK19 dual-expression predicts poor prognostic outcomes.21 Our research indicated similar results. The lower the differentiation of HCC with metabolic risk factors, the stronger the expression of CK19, the higher the proliferation index of ki-67, and the lower the expression of HepPar-1.
As previously reported, liver lipoprotein and cholesterol production reduced with cirrhosis progression.22 In addition, compared with non-advanced fibrosis cases, advanced fibrosis cases were associated with markedly decreased LDL-C, cholesterol, and serum Lp(a) levels.23 Moreover, we noted that the degree of fibrosis in the surrounding liver tissues was negatively correlated with the serum cholesterol, LDL-C, ApoA1, and ApoB levels. The occurrence of cirrhosis was negatively correlated with the elevated blood triglyceride levels as well as serum cholesterol, LDL-C, and ApoB levels. Lp(a), mostly produced within the liver, is recognized to be a critical factor for atherosclerotic plaque formation.24 We noted that the serum Lp(a) expression was negatively related to the degree of steatosis in the surrounding liver tissues, with a positive relation to the degree of inflammation in the surrounding liver tissues. Consistent with these results, a study reported that liver inflammation, and not steatosis, affected the serum Lp(a) level.25
Furthermore, we observed that the degree of steatosis in the surrounding liver tissues was negatively related to the serum GGT and AKP levels, which may be attributed to tumor progression. HCC cells have been suggested to produce GGT.26 Another study reported that the AKP level markedly increased in the HCC group relative to that in the cirrhosis group; moreover, the GGT and AKP levels elevated as the tumor progressed, thereby affecting HCC genesis and development.27 With the progression of MAFLD-related HCC, energy consumption increases; therefore, steatosis in the surrounding liver tissues decreases or even disappears with the increase in GGT and AKP levels.
PLT count remarkably decreases in patients with cirrhosis because of portal hypertension.28 According to an observational study, thrombocytopenia represents an important risk factor for cirrhosis occurrence.29 We also found that fibrosis severity within the adjacent liver and the occurrence of cirrhosis were all negatively correlated with the PLT count. PLT has complex effects on HCC, and it has been reported that thrombocytopenia significantly predicted dismal HCC survival and prognosis.30 Furthermore, GPC-3 is highly sensitive to lowly-differentiated HCC but lowly sensitive to highly-differentiated HCC.31 Therefore, GPC3 expression within cancer cells showed a negative correlation with PLT count in the present study.
In several countries, MAFLD is the critical etiology of HCC. In previous studies, the HCC risk was suggested to be restricted to liver cirrhosis; however, at present, HCC-related fatty liver diseases have been suggested to be related to non-cirrhotic liver or liver with mild fibrosis.14 According to our results, there were 59.4% of cases without cirrhosis among the HCC cases having metabolic risk factors. Piscaglia et al reported that 46.2% of NAFLD-related HCCs occurred on a non-cirrhotic background, whereas 97.2% of HCV-related HCCs occurred in cirrhotic livers.32 Another study reported that the cases demonstrating underlying metabolic syndrome as the only risk factor for liver disease are associated with a 5-fold increased risk of HCC without cirrhosis relative to the HCV-related HCC cases.33 Multiple logistic regression was conducted to compare HCC cases with and without cirrhosis; as a result, non-cirrhosis HCC cases were mostly recorded in men, with increased dyslipidemia and larger tumor rates.34 We also noted that the levels of blood triglycerides, serum cholesterol, triglycerides, ApoB, and LDL-C in the non-cirrhosis group were markedly higher than those in the liver cirrhosis group.
Certain limitations of the present study should be acknowledged. First, this is a single-center preliminary study; therefore, multicenter studies should be conducted to validate the present results. Second, the study has a retrospective design with a small sample size; hence, prospective studies with a larger sample size are necessary to analyze the clinicopathological features of HCC with MAFLD or metabolic risk factors. Finally, our study did not consider the treatment methods or patient prognosis; therefore, a long-term follow-up is required to observe the prognosis and outcome of these patients.
Conclusions
The pathological characteristics of HCC with metabolic risk factors were correlated with metabolic abnormalities. Trabecular structure is the most common growth pattern. It is important to identify ballooning degeneration and Mallory–Denk bodies in liver biopsies because these pathological features were positively correlated with the occurrence of cirrhosis. HCC patients with metabolic risk factors were more likely to have no cirrhosis.
Abbreviations
HCC, hepatocellular carcinoma; ICC, cholangiocarcinoma; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic associated fatty liver disease; T2DM, type 2 diabetes mellitus; CRP, C-reactive protein; AFP, Alpha-FetoProtein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AKP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; TBiL, total bilirubin; DBiL, direct bilirubin; TBA, total bile acid; TCHO, total cholesterol; TG, triglycerides; HDL-C, high-density liptein cholesterol; LDL-C, low-density liptein cholesterol; APO-A1, apolipoprotein A1; ApoB, apolipoprotein B; LPa, lipoprotein(a); GLU, Glucose; TP, Total Protein; ALB, albumin; GLO, Globularproteins; WBC, White blood cell; RBC, Red blood cell; Hb, Hemoglobin; PLT, platelet; HE, hematoxylin and eosin; D-PAS, Periodic Acid Schiff with diastase; GS, glutamine synthetase; GPC-3, glypican-3; HSP70, heat shock protein 70.
Funding
This work was supported by Beijing Municipal Administration of Hospitals Incubating Program (PX2022072), Capital‘s Funds for Health Improvement and Research (2022-2-2174) and Research Project of Beijing Municipal Science and Technology Commission (Z191100007619037).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview[J]. Int J Cancer. 2021;149:778–789. doi:10.1002/ijc.33588
3. Eslam M, Newsome PN, Anstee QM, et al. A new definition for metabolic dysfunctionassociated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi:10.1016/j.jhep.2020.03.039
4. Chen VI, Yeh ML, Yang JD. Effects of cirrhosis and diagnosis scenario in metabolic-associated fatty liver disease-related hepatocellular carcinoma. Hepatol Commun. 2020;5(1):122–132. doi:10.1002/hep4.1606
5. Guo F, Estévez-Vázquez O, Benedé-Ubieto R, et al. A Shortcut from Metabolic-Associated Fatty Liver Disease(MAFLD) to Hepatocellular Carcinoma (HCC): c-MYC a Promising Target for Preventative Strategies and Individualized Therapy. Cancers. 2022;14(1):192. doi:10.3390/cancers14010192
6. Xie XC, Zheng MY, Guo WB, et al. Correlation analysis of metabolic characteristics and the risk of metabolic‑associated fatty liver disease-related hepatocellular carcinoma. Sci Rep. 2022;12(1):13969. doi:10.1038/s41598-022-18197-6
7. Sun L, Jia-min C, Yang K, et al. Cytomegalovirus cell tropism and clinicopathological characteristics in gastrointestinal tract of patients with HIV/ AIDS. Diagn Pathol. 2022;17:9. doi:10.1186/s13000-022-01193-9
8. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi:10.1002/hep.20701
9. Won Bae SD, George J, Qiao L. From MAFLD to hepatocellular carcinoma and everything in between. Chin Med J. 2022;135(5):547–556. doi:10.1097/CM9.0000000000002089
10. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi:10.1002/hep.28123
11. Hörist-Kollmann S, Strametz-Juranek J. Female dietary patterns and the pathogenesis of NAFLD. Gend Genome. 2018;2:49–55. doi:10.1177/2470289718787091
12. Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61(5):1234–1245. doi:10.1007/s10620-016-4085-6
13. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
14. Karadag Soylu N. Update on hepatocellular carcinoma: a brief review from pathologist standpoint. J Gastrointest Cancer. 2020;51(4):1176–1186. doi:10.1007/s12029-020-00499-5
15. Torbenson MS, Iol N, Park YN, et al. Hepatocellular carcinoma. WHO Classification of Tumours Editorial Board, editor. Digestive System Tumours. WHO Classification of Tumours Series.
16. Yeh MM, Liu Y, Torbenson M. Steatohepatitic variant of hepatocellular carcinoma in the absence of metabolic syndrome or background steatosis: a clinical, pathological, and genetic study. Hum Pathol. 2015;46:1769–1775. doi:10.1016/j.humpath.2015.07.018
17. Yamaoka K, Saitoh S, Kinowaki K, et al. Clinicopathological assessment of steatohepatitic hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2022;46(8):101799. doi:10.1016/j.clinre.2021.101799
18. Fujiwara N, Nakagawa H, Enooku K, et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67(8):1493–1504. doi:10.1136/gutjnl-2017-315193
19. Mostafa M, Abdelkader A, Evans JJ, et al. Fatty liver disease: a practical approach. Arch Pathol Lab Med. 2020;144:62–70. doi:10.5858/arpa.2019-0341-RA
20. Vyas M, Zhang X. Hepatocellular Carcinoma: role of Pathology in the Era of Precision Medicine. Clin Liver Dis. 2020;24(4):591–610. doi:10.1016/j.cld.2020.07.010
21. Feng J, Zhu R, Chang C, et al. CK19 and glypican 3 expression profiling in the prognostic indication for patients with HCC after surgical resection. PLoS One. 2016;11(3):e0151501. doi:10.1371/journal.pone.0151501
22. Chrostek L, Supronowicz L, Panasiuk A, et al. The effect of the severity of liver cirrhosis on the level of lipids and lipoproteins. Clin Exp Med. 2014;14:417–421. doi:10.1007/s10238-013-0262-5
23. Konishi K, Miyake T, Furukawa S, et al. Advanced fibrosis of non-alcoholic steatohepatitis affects the significance of lipoprotein(a) as a cardiovascular risk factor. Atherosclerosis. 2020;299:32–37. doi:10.1016/j.atherosclerosis.2020.02.026
24. Rehberger Likozar A, Zavrtanik M, Šebeštjen M. Lipoprotein(a) in atherosclerosis: from pathophysiology to clinical relevance and treatment options. Ann Med. 2020;52(5):162–177. doi:10.1080/07853890.2020.1775287
25. Wu T, Ye J, Shao C, et al. Varied Relationship of Lipid and Lipoprotein Profiles to Liver Fat Content in Phenotypes of Metabolic Associated Fatty Liver Disease. Front Endocrinol (Lausanne). 2021;12:691556. doi:10.3389/fendo.2021.691556
26. Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta. 2013;1830:2481–2495. doi:10.1016/j.bbagen.2012.11.008
27. Carr BI, Guerra V. A hepatocellular carcinoma aggressiveness index and its relationship to liver enzyme levels. Oncology. 2016;90:215–220. doi:10.1159/000444394
28. Hayashi H, Beppu T, Shirabe K, et al. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol. 2014;20:2595–2605. doi:10.3748/wjg.v20.i10.2595
29. Carr BI, Guerra V. Hepatocellular carcinoma size: platelets, γ-glutamyl transpeptidase, and alkaline phosphatase. Oncology. 2013;85:153–159. doi:10.1159/000354416
30. Pang Q, Liu S, Wang L, et al. The Significance of Platelet-Albumin-Bilirubin (PALBI) Grade in Hepatocellular Carcinoma Patients Stratified According to Platelet Count. Cancer Manag Res. 2020;12:12811–12822. doi:10.2147/CMAR.S277013
31. Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011–1018. doi:10.1038/modpathol.2008.85
32. Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63(3):827–838. doi:10.1002/hep.28368
33. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131. doi:10.1016/j.cgh.2015.07.019
34. Tobari M, Hashimoto E, Taniai M, et al. The characteristics and risk factors of hepatocellular carcinoma in non-alcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35(5):862–869. doi:10.1111/jgh.14867
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.