Back to Journals » Cancer Management and Research » Volume 13
Clinicopathological Features and Prognosis of Primary Mediastinal Malignant Germ Cell Tumors: A Retrospective Single-Institution Analysis
Authors Zhang J , Chen Y , Liu L, Zhou M, Huang C, Guo C , Li S
Received 1 July 2021
Accepted for publication 29 October 2021
Published 13 November 2021 Volume 2021:13 Pages 8527—8534
DOI https://doi.org/10.2147/CMAR.S327342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Jiaqi Zhang, Yeye Chen, Lei Liu, Mengxin Zhou, Cheng Huang, Chao Guo, Shanqing Li
Department of Thoracic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China
Correspondence: Shanqing Li
Department of Thoracic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1, Shuaifuyuan, Dongcheng District, Beijing, 100730, People’s Republic of China
Tel +86 13801388072
Fax +86 1069152632
Email [email protected]
Background: Given the lack of evidence-supported guidance for therapeutic recommendations of primary mediastinal malignant germ cell tumors (PMMGCTs), our study aimed to investigate the clinicopathological features, treatment strategies, and prognostic factors of PMMGCTs.
Methods: We carried out a consecutive retrospective evaluation on a series of patients diagnosed with PMMGCTs in Peking Union Medical College Hospital from January 2000 to August 2020.
Results: A total of 58 patients were eligible, consisting of 51 males and seven females. There were 15 patients with seminomas, 39 with nonseminomatous germ cell tumors (NSGCTs), and four with mixed germ cell tumors (GCTs). The 5-year overall survival was 45%, with a median survival time of 32.37 months. Except for the lost follow-up of seven patients, a univariate analysis of overall survival on the remaining patients showed significant differences in pathological type (mixed GCTs were regarded as NSGCTs) (p=0.036), tumor size (> 11cm) (p=0.006), and other sites metastases (OSM) (p=0.001), respectively. Multivariate Cox regression analysis revealed that OSM and surgical resection were independently associated with overall survival in all kinds of PMMGCTs.
Conclusion: OSM was an independent risk factor for patients with PMMGCTs. Surgery was proved to contribute to long-term survival. More in-depth clinical evidence is urgently needed to guide the treatment of PMMGCTs.
Keywords: primary mediastinal malignant germ cell tumors, surgery, prognosis
Introduction
As a set of rare diseases, primary mediastinal germ cell tumors (PMGCTs) comprise a heterogeneous group of neoplasms, accounting for 1–3% of germ cell tumors (GCTs) and 15% of adult anterior mediastinal masses.1,2 PMGCTs usually show more aggressive behavior and poorer prognosis than GCTs in gonadal counterpart.3 PMGCTs are divided into seminomas, nonseminomatous GCTs (NSGCTs) (including yolk sac tumor, embryonal carcinoma, and choriocarcinoma), and teratomas according to histological differences.4 NSGCTs are often associated with increased serum alpha-fetoprotein (AFP) and/or beta-human chorionic gonadotropin (β-HCG), which contributes to disease diagnosis and treatment surveillance.5 In clinical practice, we usually define mature teratoma as a benign disease, and other PMGCTs are considered as the malignant kinds. The treatment strategy for NSGCTs is a multimodal approach which includes cisplatin-based chemotherapy followed by surgical resection of residual tumors, and radiotherapy could be considered in the case of seminoma for their high sensitivity.1 In general, seminomas have better prognosis than NSGCTs.6
However, no randomized trials have ever been carried out to guide treatment in patients with PMGCT due to its rarity and complexity. Current supportive evidence for therapeutic recommendations are based on observational series and experiences of GCTs arising in gonads. Thus, our study aimed to introduce our therapeutic strategies and investigate the associated prognostic factors for patients with primary mediastinal malignant germ cell tumors (PMMGCTs).
Materials and Methods
Patients and Therapeutic Regimen
We consecutively evaluated a series of patients who were pathologically diagnosed with PMMGCTs in Peking Union Medical College Hospital (PUMCH) from January 2000 to August 2020. This study was approved by The Institutional Review Board of PUMCH. Written informed consent was obtained from eligible patients. This study was conducted in accordance with the Declaration of Helsinki. Patients’ data including basic information and schemes for diagnosis and treatment was retrospectively collected through the electronic medical record system.
All patients were managed in a multidisciplinary team including senior surgeons and physicians in the Department of Thoracic Surgery and Department of Oncology. Patients were systemically evaluated to exclude metastatic or multiple primary lesions in other sites. Patients with highly suspected malignant GCTs or with a definite diagnosis (based on radiological findings, aspiration cytology and levels of tumor markers) were treated with neoadjuvant chemotherapy as a priority, whereas surgery was performed when a complete response was not achieved. Patients with indeterminate diagnosis were recommended to accept surgical explorations for biopsy and resection. Postoperative chemotherapy or radiotherapy was selectively conducted depending on their pathology findings, imaging findings, and levels of tumor markers. Chemotherapy or radiotherapy was applied to patients with inoperable tumors or with contraindication for surgery.
Statistical Analysis
Statistical analysis was performed by SPSS 23.0 software (IBM Corp., Armonk, NY). Measurement data conforming to normal distribution was presented as 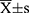 , while the non-normal distribution data was displayed by median (range). Counting data was exhibited as numbers (percentage). Kaplan-Meier method was applied to survival analysis in which the starting point was set on the date of the detection of PMMGCTs and the ending point was set on the date of death or the date of follow-up. Log rank test and Cox regression risk model were performed to analyze prognostic factors. The difference was considered as statistically significant when p<0.05.
, while the non-normal distribution data was displayed by median (range). Counting data was exhibited as numbers (percentage). Kaplan-Meier method was applied to survival analysis in which the starting point was set on the date of the detection of PMMGCTs and the ending point was set on the date of death or the date of follow-up. Log rank test and Cox regression risk model were performed to analyze prognostic factors. The difference was considered as statistically significant when p<0.05.
Results
Patients’ Characteristics
A total of 60 patients diagnosed with PMMGCTs during the last 20 years were retrieved, where two patients without detailed information of treatment were eliminated from the study. The demographics and clinicopathological characteristics of 58 patients with PMMGCTs are shown in Table 1.
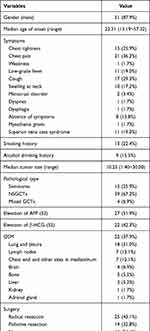 |
Table 1 Patient Demographics and Clinicopathological Characteristics |
There were 51 male patients, accounting for 87.9% of all eligible patients. The median age of onset was 23.31 years (13.19~57.32). The common symptoms included chest pain (36.2%), cough (29.3%), chest tightness (25.9%), low-grade fever (19.0%), and swelling at the neck (17.2%). Patients were characterized by superior vena cava syndrome and myasthenia gravis at the rate of 19.0% (11 patients) and 1.7% (one patient), respectively, while 13.8% of them were asymptomatic.
All PMMGCTs were located in the anterior mediastinum, the median size was 10.25 cm (1.40~30.00). Among all the patients, 15 of them (25.9%) were pathologically confirmed as seminomas, 39 (67.2%) were diagnosed as NSGCTs (one of yolk sac tumor, embryonal carcinoma and choriocarcinoma, or mix of the three types), another four cases (6.9%) were classified into mixed GCTs (defined as GCTs combining seminoma/teratomas and NSGCT-components). Tumor markers test was applied to 52 cases, including 14 with seminomas, 35 with NSGCTs, and another three with mixed GCTs. Increased AFP was detected in 27 patients (51.9%) (26 with NSGCTs and one with mixed GCTs). Rise of β-HCG was found in 22 patients (42.3%), among which 18 cases were NSGCTs, two cases were mixed GCTs, and another two cases were seminomas (<1,000 IU/L).
Although there was no official recommendation of staging system for PMMGCT, we applied the Masaoka-Koga staging system for thymic malignancies to PMMGCT. The corresponding numbers of patients who were classified into stage I, II, III, and IV when diagnosed with PMMGCTs were 5, 10, 30, and 13, respectively.
Malignancies in blood system were detected in three patients (5.2%) during the period of follow-up (chronic myelomonocytic leukemia, myelodysplastic syndromes, and not special leukemia, respectively). Other sites metastases (OSM) occurred in 22 cases (37.9%) during the courses of disease. Lung and pleura (31.0%), lymph nodes (12.1%), chest wall, and other sites in mediastinum (12.1%) were the commonest metastatic sites, followed by brain (6.9%), bone (5.2%), and liver (5.2%).
Treatment
There were 14 patients (24.1%) who received no surgery, and three of them did not receive any treatment because of rapid progression and death before therapy, while another 11 patients received both chemotherapy for extensive disease and radiotherapy for regional disease. There were 44 patients (75.9%) who underwent surgery, of which 25 patients (43.1%) underwent radical resection and 19 patients (32.8%) received palliative resection. Among the 44 patients, 19 of them received preoperative neoadjuvant chemotherapy, while 33 of them received postoperative adjuvant chemotherapy. Radiotherapy was applied to 26 of the patients (44.8%).
The chemotherapy protocols including BEP regimen (bleomycin, etoposide, and cisplatin), EP regimen (etoposide and cisplatin) and VIP regimen (etoposide, ifosfamide, and cisplatin) were selectively applied according to patients’ performance status, pathology, bleomycin associated pulmonary toxicity, and their further therapeutic strategies. EMA-CO regimen (Etoposide, methotrexate, and dactinomycin followed by cyclophosphamide and vincristine) was provided to patients diagnosed with mediastinal chorioepithelioma. Conducting three to four cycles of neoadjuvant chemotherapy was the commonest therapeutic scheme.
Here, we reported a clinical case presenting our routine diagnosis and treatment strategies. A male patient, aged 28, was detected to have an anterior mass with a maximum diameter of 7.9 cm by computed tomography (CT) scanning during health examination in June 2020. After CT-guided transthoracic fine needle aspiration cytology (FNAC) was performed, the pathology result showed immature teratoma. The tumor markers test suggested significant elevation of both AFP (2,572.0 ng/mL) and β-HCG (959.89 IU/L). The patient was admitted to the Department of Oncology and received three cycles of chemotherapy of VIP regimen, after which the levels of AFP and β-HCG fell down to the normal range, and CT examination after two cycles neoadjuvant chemotherapy indicated partial response of tumor. Then median sternotomy was performed. The result of postoperative pathology indicated GCTs (consisting of 20% yolk sac tumor and 80% mature teratoma) with visceral pleura involvement and negative lymph nodes, and the ki-67 index was 70~75% (yolk sac tumor). After three cycles of VIP regimen postoperative adjuvant chemotherapy, unfortunately, the disease progressed, followed by four cycles of BEP regimen salvage chemotherapy, which had a partial response. The CT images during the period of neoadjuvant chemotherapy are shown in Figure 1.
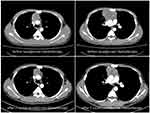 |
Figure 1 CT images showed partial tumor response to neoadjuvant chemotherapy. |
Survival and Prognosis
Among 58 patients in this study, the median follow-up time was 20.60 months. By the end of follow-up, the track of seven patients were lost (including two seminomas, four NSGCTs, and one mixed GCT); 26 patients died and 25 patients survived. The 5-year overall survival (OS) was 45%, with a median survival time of 32.37 months. The OS curve of the whole population is shown in Figure 2.
 |
Figure 2 Overall survival curve of 51 patients with PMMGCTs. |
In the univariate analysis of OS on 51 patients, pathological type (mixed GCTs were regarded as NSGCTs here) (p=0.036), tumor size (>11 cm, dichotomized around the median) (p=0.006), and OSM (p=0.001) showed significant differences in OS. Surgical resection (p=0.062), level of AFP (p=0.054), and Masaoka-Koga staging (p=0.072) indicated potential influences on OS. OS curves in univariate analysis are displayed in Figure 3. However, no statistical difference was found in gender (p=0.757), age at onset (p=0.554), adjacent structure resection (p=0.556), radiotherapy (p=0.905), chemotherapy (p=0.411), preoperative neoadjuvant chemotherapy (p=0.695), and postoperative adjuvant chemotherapy (p=0.780).
Multivariate Cox regression analysis showed that OSM (HR=4.437, 95% CI=1.530~12.352, p=0.006) and surgical resection (HR=0.242, 95% CI=0.082~0.715, p=0.010) were independently associated with OS (Table 2).
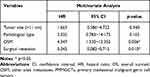 |
Table 2 Multivariate Cox Regression Analysis of OS in 51 Patients with PMMGCTs |
Considering that mixed GCTs possess the same aggressive biology and prognosis to NSGCTs, we re-analyzed the prognostic factors in the reorganized cohort of patients including both pure NSGCTs and mixed GCTs. Excluding five patients lost to follow-up, a total of 38 patients were enrolled. Univariate and multivariate analysis results are shown in Figure 4 and Table 3.
 |
Table 3 Multivariate Cox Regression Analysis of OS in 38 Patients with NSGCTs and Mixed GCTs |
Similarly, OSM (HR=13.825, 95% CI=3.199~59.742, p=0.000) and surgical resection (HR=0.089, 95% CI=0.021~0.376, p=0.001) showed significant differences in OS.
Discussion
As a series of rare diseases, PMMGCTs are lack of specificity in symptoms, signs, and clinicoradiological characteristics, which results in difficulty of clinical diagnosis. Although Marcus et al7 evaluated FNAC of mediastinal masses and concluded that the sensitivity and specificity of FNAC for GCT were 100% and 99%, respectively, though only two cases with GCTs were enrolled. In our study, there were 37 patients who received aspiration cytology examination, and 31 of them were considered as GCTs or malignant GCTs (not mentioned in Table 1), despite the difficulty in obtaining specific pathological classification for each specimen. Considering the specificity of AFP and β-HCG discussed in our study, we recommended aspiration cytology combined with tumor marker testing as an effective diagnostic strategy, especially when it’s difficult to promote our therapeutic decision-making.
Cisplatin-based chemotherapy followed by surgical resection of the residual tumor has been reported as a standard treatment protocol for patients with PMMGCTs.1,6,8,9 However, it’s hard to completely conform to this protocol because of the complexity in realistic situations, therefore, there is no optimal treatment pattern that can be applied to all patients. In our single center, there were many more patients characterized by more advanced and complex diseases, and some patients were transferred from local hospitals after receiving initial therapeutic intervention which caused complications and inconsistency of therapeutic protocols. The results of this retrospective study revealed the obvious advantage of surgical resection to prolong OS of patients with PMMGCTs. According to statistical analysis, radiotherapy and chemotherapy in our study did not exert positive influences on OS, perhaps because the majority of patients in our center received more aggressive and inconsistent adjuvant treatments. All in all, we still recommended this multimodal aggressive approach to obtain more chances of long-term survival.
Furthermore, OSM was proved to be an independent risk factor for OS in our study. In our study, 22 patients had OSM during courses of disease. Though most of them died of metastases, five patients with lung and lymph node metastases were still alive after active treatment. The management of patients with advanced stage diseases is always a thorny problem, especially for patients with PMMGCTs. Clinical scientists have tried approaches to overcome some difficulties, such as modifying chemotherapy regimen10 and changing non-surgical treatment patterns.11 As suggested in other reviews and reports,5,12 aggressive surgery combined with chemotherapy and radiotherapy has shown potential benefits to long-term survival for some selected patients. Patients with limited lung and pleura metastases may gain potential long-term survival after receiving active treatment. In recent years, Necchi et al13 performed genomic profiling on 44 primary mediastinal NSGCTs and found a slight difference in genomic alteration, but evidence for precision treatment was not sufficient. Zschäbitz et al14 analyzed patients with platinum refractory GCTs who were treated with checkpoint inhibitors, including two patients with PMMGCTs (with negative expression of PD-L1), unfortunately, none of them showed an obvious survival benefit. Clinical investigation of new therapeutic options is necessary for these patients.
Prognostic classification for GCTs from the International Germ Cell Cancer Consensus Group is the most widely used strategy in clinical practice.15,16 There is no official staging system for PMMGCTs. Considering that PMMGCTs have developed from the anterior mediastinum, we tried to apply the Masaoka-Koga staging system for thymic malignancies to PMMGCTs. Our results revealed that Masaoka-Koga staging system showed ordered appropriateness in OS curves for PMMGCTs, thus we considered it reasonable to apply the Masaoka-Koga staging system to PMMGCTs, although the statistical difference was not significant.
To our knowledge, this was the fourth largest single-center study of PMMGCTs to date, following reports from Russia,17 China,18 and Peru.19 We reported more cases with advanced-stage PMMGCTs. However, this study had several limitations. First, it was a retrospective study which lasted for 20 years, which affected the consistency of the patients’ treatment due to medical levels and limitations in the past. Second, some patients were transferred from local hospitals after initial treatment, which caused complexity in their diseases and treatment regimens. Finally, cases in our study contained less early-stage tumors, which might affect the overall assessment of PMMGCTs.
Conclusions
PMMGCT is a rare and aggressive disease, which lacks clinical experience. Aspiration cytology combined with tumor markers testing was an effective diagnostic strategy. Patients with seminomas had better prognosis than patients with NSGCTs. OSM was an independent risk factor for patients with PMMGCTs. Surgery, as a part of a multimodal aggressive treatment approach, contributed to long-term survival. The Masaoka-Koga staging system could be considered to predict the clinical prognosis for patients with PMMGCTs. More robust and in-depth clinical evidence is needed for the development of precise and effective therapeutic strategies for PMMGCTs.
Abbreviations
AFP, alpha-fetoprotein; β-HCG, beta-human chorionic gonadotropin; CT, computed tomography; FNAC, fine needle aspiration cytology; GCTs, germ cell tumors; NSGCTs, nonseminomatous germ cell tumors; OS, overall survival; OSM, other sites metastases; PUMCH, Peking Union Medical College Hospital; PMGCTs, primary mediastinal germ cell tumors; PMMGCTs, primary mediastinal malignant germ cell tumors.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by The Institutional Review Board of PUMCH. Written informed consent was obtained from eligible patients.
Acknowledgments
We would like to give our sincere thanks to Professor Zhiyong Zhang, Hongsheng Liu, Yushang Cui, Naixin Liang, Zhijun Han, Li Li, Qi Miao and other medical staffs in our hospital during the last 20 years for their prominent works in this rare disease.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Rosti G, Secondino S, Necchi A, Fornarini G, Pedrazzoli P. Primary mediastinal germ cell tumors. Semin Oncol. 2019;46(2):107–111. doi:10.1053/j.seminoncol.2019.04.001
2. van Leeuwen MT, Gurney H, Turner JJ, et al. Patterns and trends in the incidence of paediatric and adult germ cell tumours in Australia, 1982–2011. Cancer Epidemiol. 2016;43:15–21. doi:10.1016/j.canep.2016.05.006
3. Adra N, Althouse SK, Liu H, et al. Prognostic factors in patients with poor-risk germ-cell tumors: a retrospective analysis of the Indiana University experience from 1990 to 2014. Ann Oncol. 2016;27(5):875–879. doi:10.1093/annonc/mdw045
4. Roden AC. Mediastinal germ cell tumors. In: Nogales FF, Jimenez RE, editors. Pathology and Biology of Human Germ Cell Tumors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2017:327–364.
5. Stram AR, Kesler KA. Mediastinal germ cell tumors: updates in diagnosis and management. Surg Oncol Clin N Am. 2020;29(4):571–579. doi:10.1016/j.soc.2020.06.005
6. Liu Y, Wang Z, Peng ZM, Yu Y. Management of the primary malignant mediastinal germ cell tumors: experience with 54 patients. Diagn Pathol. 2014;9:33. doi:10.1186/1746-1596-9-33
7. Marcus A, Narula N, Kamel MK, et al. Sensitivity and specificity of fine needle aspiration for the diagnosis of mediastinal lesions. Ann Diagn Pathol. 2019;39:69–73. doi:10.1016/j.anndiagpath.2019.02.011
8. Dechaphunkul A, Sakdejayont S, Sathitruangsak C, et al. Clinical characteristics and treatment outcomes of patients with primary mediastinal germ cell tumors: 10-years’ experience at a single institution with a bleomycin-containing regimen. Oncol Res Treat. 2016;39(11):688–694. doi:10.1159/000452259
9. Grabski DF, Pappo AS, Krasin MJ, Davidoff AM, Rao BN, Fernandez-Pineda I. Long-term outcomes of pediatric and adolescent mediastinal germ cell tumors: a single pediatric oncology institutional experience. Pediatr Surg Int. 2017;33(2):235–244. doi:10.1007/s00383-016-4020-0
10. Shamash J, Mee M, Sarker SJ, et al. Dose-dense chemotherapy for untreated poor-prognosis and relapsed germ-cell tumours: an 18-year experience with GAMEC chemotherapy. BJU Int. 2020;125(6):843–852. doi:10.1111/bju.14947
11. Adra N, Abonour R, Althouse SK, Albany C, Hanna NH, Einhorn LH. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: the Indiana University experience. J Clin Oncol. 2017;35(10):1096–1102. doi:10.1200/JCO.2016.69.5395
12. Kuwano H, Tsuchiya T, Murayama T, et al. Outcomes of combined modality therapy for patients with stage III or IV mediastinal malignant germ cell tumors. Surg Today. 2014;44(3):499–504. doi:10.1007/s00595-013-0562-0
13. Necchi A, Bratslavsky G, Chung J, et al. Genomic features for therapeutic insights of chemotherapy-resistant, primary mediastinal nonseminomatous germ cell tumors and comparison with gonadal counterpart. Oncologist. 2019;24(4):e142–e145. doi:10.1634/theoncologist.2018-0430
14. Zschabitz S, Lasitschka F, Hadaschik B, et al. Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer. 2017;76:1–7. doi:10.1016/j.ejca.2017.01.033
15. International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594–603. doi:10.1200/JCO.1997.15.2.594
16. Kollmannsberger C, Nichols C, Meisner C, Mayer F, Kanz L, Bokemeyer C. Identification of prognostic subgroups among patients with metastatic ‘IGCCCG poor-prognosis’ germ-cell cancer: an explorative analysis using cart modeling. Ann Oncol. 2000;11(9):1115–1120. doi:10.1023/A:1008333229936
17. Fedyanin M, Tryakin A, Mosyakova Y, et al. Prognostic factors and efficacy of different chemotherapeutic regimens in patients with mediastinal nonseminomatous germ cell tumors. J Cancer Res Clin Oncol. 2014;140(2):311–318. doi:10.1007/s00432-013-1567-1
18. Wang J, Bi N, Wang X, et al. Role of radiotherapy in treating patients with primary malignant mediastinal non-seminomatous germ cell tumor: a 21-year experience at a single institution. Thorac Cancer. 2015;6(4):399–406. doi:10.1111/1759-7714.12190
19. Alcarraz CE, Morante Z, Mejia G, et al. Clinicopathological features and management of patients with primary malignant mediastinal germ cell tumor: 10 year’s experience in Peruvian patients. J Clin Oncol. 2016;34(15_suppl):e13088–e13088. doi:10.1200/JCO.2016.34.15_suppl.e13088
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


