Back to Journals » OncoTargets and Therapy » Volume 12
Clinicopathological Characteristics And EGFR-TKIs Efficacies In Lung Squamous Cell Carcinoma Patients Harboring An EGFR Sensitizing Mutation
Authors Zhou S , Wang H, Jiang W, Yu Q
Received 2 August 2019
Accepted for publication 16 October 2019
Published 30 October 2019 Volume 2019:12 Pages 8863—8871
DOI https://doi.org/10.2147/OTT.S225760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Shaozhang Zhou, Huilin Wang, Wei Jiang, Qitao Yu
Department of Respiratory Oncology, Guangxi Medical University Affiliated Tumor Hospital, Nanning 530021, People’s Republic of China
Correspondence: Qitao Yu
Department of Respiratory Oncology, Guangxi Medical University Affiliated Tumor Hospital, No.71, Heti Road, Nanning 530021, Guangxi, People’s Republic of China
Tel +86 0771 533 4955
Fax +86 0771 530 0613
Email [email protected]
Objective: This study analyzed the relationship between the clinicopathological features and epidermal growth factor receptor (EGFR) mutation status of squamous cell lung cancer (SqCLC) patients. Mutation status was analyzed by comparing the amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) and next-generation sequencing (NGS). We also assessed the efficacies of EGFR tyrosine kinase inhibitors (TKIs).
Methods: Retrospective analysis was performed for 292 SqCLC patients treated at the Guangxi Medical University Affiliated Tumor Hospital from December 2013 to December 2018. The EGFR mutations in tumor tissues were identified by ARMS-PCR and NGS. The affiliation between EGFR mutation and clinicopathological features was analyzed. Efficacies of EGFR-TKIs and survival were evaluated using the benchmarks of response evaluation criteria in solid tumors 1.1 (RECIST 1.1) and the Kaplan–Meier method, respectively.
Results: Among the 292 SqCLC patients, 24 (8.2%) were identified to have an EGFR-sensitizing mutation. Both ARMS-PCR and NGS were equally effective in detecting EGFR mutations. Females and non-smokers had higher EGFR mutation rates than males and smokers (22.1% vs. 5.1%, P = 0.007 and 16.7% vs. 4.5%, P = 0.001, respectively). EGFR mutation was unrelated to the degree of differentiation, clinical stage, specimen type and level of serum carcino-embryonic antigen (CEA) and squamous cell carcinoma antigen (SCC) (P > 0.05). In the 14 EGFR mutant cases treated with EGFR-TKIs, the objective response rate (ORR) and disease control rate (DCR) were 28.6% and 78.6%, respectively. Median progression-free survival (mPFS) and overall survival (mOS) were 4.9 and 10.75 months, respectively, with fine tolerance and mild side-effects.
Conclusion: EGFR-sensitizing mutations are rare in SqCLC patients with females and non-smokers having a higher risk of harboring them. There was no difference in the detection rates of EGFR for both the ARMS-PCR and NGS methods. EGFR-TKIs showed modest efficacies and low toxicity profiles in EGFR mutant cases.
Keywords: squamous cell lung carcinoma, epidermal growth factor receptor mutation, clinicopathologic features, tyrosine kinase inhibitors
Introduction
Lung cancer remains a prominent cause of cancer-related mortality worldwide. Approximately, 85% of newly diagnosed cases of lung cancer belong to the class of non-small cell lung cancer (NSCLC), among which adenocarcinoma and squamous cell carcinoma account for nearly 50% and 30% of cases, respectively.1 In the past decade, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), as a primary example of targeted therapy, have achieved unprecedented advancement in the treatment of NSCLC patients harboring EGFR-sensitizing mutations.
A substantial amount of data has demonstrated that a variety of demographic and clinicopathological features, such as gender, ethnicity, smoking history and histological type, are closely related to EGFR mutation status. For Caucasians, the frequency of EGFR mutations in NSCLC patients is 12%,2–4 whereas in China, the frequency is substantially higher at 50.2%.5 Due to the higher efficacies, lower classic toxicities and well tolerability, the EGFR-TKIs have become an optimal choice as the first-line of therapy in EGFR mutant NSCLC patients as opposed to chemotherapy.6
The majority of existing clinical data related to EGFR-TKIs mainly focus on lung adenocarcinoma or non-squamous cell carcinoma (non-SCC).2,7–9 However, despite using the platinum-based therapy regimen as a standard of care for the past several decades, squamous cell carcinoma of the lung (SqCLC) is still the second-largest cancer type of NSCLC. The efficacy of chemotherapy in SqCLC patients appears to have reached a plateau with a response rate of only 20–40%, median progression-free survival (mPFS) of 4–6 months, median overall survival (mOS) of 8–10 months and a 1-year survival rate of 30–40%.6,10 As a result, it is of utmost importance to overcome this therapeutic dilemma of SqCLC by exploring new strategies, especially with recent advances in the era of targeted molecular therapy. However, knowledge about the frequency of EGFR mutations in SqCLC, its predominance in a population that is more likely to harbor EGFR mutation in adenocarcinoma patients, and the efficacies of EGFR-TKIs in this subgroup of patients are not yet available.
Very few studies have reported the frequency of EGFR mutation in SqCLC.2,7–9 In addition, non-uniformity in detection methods and scarcity of statistically substantial sample sizes in this field pose a difficulty in data interpretation. Thus, the aim of this study was to analyze the relationship between the clinicopathological features and EGFR mutation status in patients with SqCLC and to observe the efficacies of EGFR-TKIs in these patients to provide clinical information that might be helpful for determining the treatment strategy for such cases.
Materials And Methods
Patients
A total of 272 NSCLC patients admitted to Guangxi Medical University Affiliated Tumor Hospital and subjected to detection of EGFR mutations from December 2013 to December 2018 were selected for the study, which was reviewed and approved by the Institutional Ethics Committee of the hospital. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient. The following criteria were applied for the selection of patients: (1) Restriction of histological type to SqCLC and exclusion of adenosquamous carcinoma and uncertain histological types. The diagnosis of SqCLC was based on the histopathology characteristics or IHC criteria. (2) Information regarding demography, epidemiology, pathology, stage, serum level of carcino-embryonic antigen (CEA) and squamous cell carcinoma antigen (SCC) and clinical efficacies for each patient were mined from the original medical records. (3) Detection of EGFR mutation prior to adjuvant or salvage treatment, including chemotherapy or targeted therapy. Non-smokers were defined as patients who have smoked no more than 100 cigarettes in their lifetime. Eastern Cooperative Oncology Group – performance status (ECOG-PS) was used to evaluate the patients’ physical status. Tumor histology was classified according to the 3rd edition of WHO Classification of Tumours. Tumor stages were determined using the 7th version American Joint Commission on Cancer (AJCC) guidelines. Tumor samples were divided into two types: surgical samples and biopsy samples. The former is referred to as surgical samples, which were obtained from original or metastatic lesions by surgical resection, and the latter is referred to samples obtained by fibro-bronchoscopy or various percutaneous punctures targeting primary or metastatic lesions. Tumor samples were obtained prior to targeted therapy and chemotherapy. Serum levels of CEA and SCC up to 5.0 ng/mL and 1.5 µg/L, respectively, were considered as normal.
Detection Of EGFR Mutation
Amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) and next-generation sequencing technology (NGS) were used to define the EGFR mutation status of tissue samples in our institute. ARMS-PCR, a PCR-based method that can detect previously predefined point mutations, was used to define EGFR gene mutations.11 The ADx EGFR29 Mutation Kit (Amoy Diagnostics, Xiamen, China) covers 29 point mutations of EGFR including exon 18 G719X (G719A, G719, G719C), exon 19 deletions, exon 20 insertions (three types of insertions), exon 20 T790M and S768I, and exon 21 L858R and L861Q mutation, etc. The assay was performed in accordance with manufacturer’s protocol with the MX3000P (Stratagene, La Jolla, USA) real-time PCR system. The 25 μL RT-PCR reaction comprised 0.4 μL template DNA, 3.6 μL deionized water, and 16 μL reaction mix (reaction buffer, dNTPs, specific oligos and probes). PCR was carried out with an initial denaturation at 95°C for 10 mins, followed by 40 cycles of amplification (at 95°C for 30 s and 61°C for 1 min).12 The results were analyzed according to the criteria defined by the manufacturer. Positive results were defined as Ct (sample) – Ct(control)\Ct(cut-off). NSG, as a high-throughput sequencing technology capable of detecting mutations, indels, copy number variations, and genomic rearrangements simultaneously was performed in the American College of Pathologists (CAP) certified labs (Shihe Jiyin Biotech Inc., Nanning, China and Geneplus Technology, Beijing, China) in China.
EGFR-sensitizing activation mutations were defined according to National Comprehensive Cancer Network (NCCN) guidelines, including common exon 19 del and exon 21 L858R mutations and rare exon 18 G719X, exon 20 S768I, exon 20 insertion variant A763_Y764insFQEA and exon 21 L861Q mutations.
Evaluation Of The Efficacies Of EGFR-TKIs Treatment
Patients with SqCLC harboring EGFR mutation were treated with Icotinib 125 mg three times daily or gefitinib 250 mg, erlotinib 150 mg, or afatinib 40 mg once daily until the manifestation of disease progression or intolerable side-effects. Efficacies were initially evaluated through routine CT scanning after EGFR-TKI treatment every 4 weeks. If patients’ disease presented as non-progressed, the duration of the evaluation was adjusted to every 2 months.
The response to EGFR-TKIs was accessed according to the response evaluation criteria in solid tumor (RECIST) criteria version 1.1.13 Evaluations of complete response (CR) or partial response (PR) and stable disease (SD) or progression of disease (PD) were validated 4 weeks later. The objective response rate (ORR) and disease control rate (DCR) were calculated by (CR+PR)/total cases × 100% and (CR+PR+SD)/total cases × 100%, respectively. PFS was calculated from the date of the beginning of EGFR-TKI treatment to the date of tumor progression. Overall survival (OS) was calculated from the date of diagnosis of advanced disease to the date of patient death or last follow-up. Drug toxicity and adverse reactions were evaluated according to the common toxicity criteria (NCI-CTC 3.0) established by the National Cancer Institute of the United States.14
Statistical Analyses
EGFR mutation status and parameters of clinicopathological characteristics were analyzed by Chi-square or Fisher’s exact test. The Kaplan–Meier method was used to estimate the median PFS after TKI therapy. The data were analyzed using SPSS version 21.0 statistical software (SPSS Inc., Chicago, USA), and a P < 0.05 represented statistical significance.
Results
Results Of EGFR Mutation Detection
Frequency Of EGFR Mutation In SqCLC Patients
A total of 292 patients initially diagnosed with SqCLC were selected according to inclusive criteria from December 2013 to December 2018. Twenty-four out of 292 SqCLC patients were identified to have an EGEF activating mutation with a mutation rate of 8.2% (24/292), out of which 14 patients were verified to have an exon 19 del (14/292, 4.8%) (Figure 1), 9 were verified to have an exon 21 L858R mutation (9/292,3.1%) (Figure 2), and one was verified to have an L861Q mutation (1/292, 0.3%). There were no EGFR concurrent mutations, and the rest of the enrolled patients were found to possess the wild type EGFR gene (267/292, 91.8%).
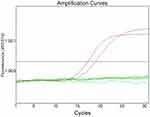 |
Figure 1 Detection of EGFR exon 19 del mutation in an SqCLC tumor tissue specimen by the ARMS-PCR method. |
 |
Figure 2 Detection of EGFR exon 21 L858R mutation in an SqCLC tumor tissue specimen by the NGS method. |
Comparison Between ARMS-PCR And NGS Based Detection Of EGFR Mutations
The results for EGFR mutation rate were 8.7% (19/219) for ARMS-PCR and 7.3% (5/73) for NGS method, respectively. This difference was not statistically significant (P > 0.05).
Relationship Between EGFR Mutation And Clinicopathological Features
Analysis of 24 EGFR positive SqCLC patients revealed that the frequencies of EGFR mutations were significantly higher in patients who had no history of smoking (14.3% vs. 4.8%) and whose gender was female (18.1% vs. 5%) with P = 0.007 and P = 0.001, respectively. However, parameters such as age, degree of differentiation, clinical stages, sample types, values of serum CEA and SCC were not correlated with EGFR mutation status (P > 0.05). The relationship between EGFR mutations and the clinicopathological features is summarized in Table 1.
 |
Table 1 Correlation Between EGFR Mutations And Clinicopathological Features In Patients With SqCLC (n=292) |
Efficacies Of EGFR-TKIs In Patients With EGFR-Sensitizing Mutations
Fourteen out of 25 patients who received EGFR-TKIs as the first- or second-line therapy and had measurable lesions along with follow-up data were included for efficacy assessment. Of these patients, 8 received icotinib, 4 received gefitinib and 2 received afatinib. The number of patients for first-, second- and third-line setting was 10, 3 and 1, respectively. Their retrospective analysis revealed that among them 4 patients had PR, 7 had SD, and 3 had PD. The ORR and DCR were 28.6% and 78.6%, respectively. The median PFS and median OS were 4.7 months and 10.6 months, respectively (Figures 3 and 4). Four patients had mild skin rash and 1 suffered from diarrhea. The toxicities were graded as 1 or 2 indicating well tolerability and mild side-effects. The detailed information of efficacies for individual patient are listed in Table 2.
 |
Table 2 Efficacies Of EGFR-TKIs On SqCLC Patients With EGFR-Sensitizing Mutations |
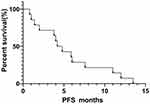 |
Figure 3 Progression-free survival curve of 14 SqCLC patients showing an mPFS of 4.7 months. |
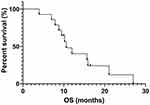 |
Figure 4 Overall survival curve of 12 SqCLC patients showing an mOS of 10.6 months. |
Discussion
The necessity of detecting EGFR mutations and the application of EGFR-TKI therapy in SqCLC patients remains controversial. Guidelines from the European Society for Medical Oncology (ESMO) recommend that EGFR mutations should be detected in cases of non-squamous carcinoma, while the NCCN recommends the detection of EGFR mutations in SqCLC only in cases of non-smokers, small specimen type, or mixed histological types.15,16 The Chinese Society of Clinical Oncology (CSCO) is mostly in accordance with the NCCN on this topic, but certainly points out that patients with squamous carcinoma mixed with adenocarcinoma should undergo EGFR mutation detection. The reason for all these disputes may be attributed to the low frequency and low cost-effectiveness of these strategies in the particular subsets of patients, especially in western countries and non-Asian races.
Additionally, varying frequencies of EGFR mutations in SqCLC patients are reported from different institutes, testing platforms and ethnic groups. Yohei Miyamae et al have reported that the EGFR gene mutation rate is 3.4% (3/87) in Japanese SqCLC patients upon detecting 87 such patients by direct sequencing.17 Another study on Korean SqCLC patients, using the same method, demonstrated an EGFR mutation rate of 8.4% (21/250).18 Akito Hata et al used peptide nucleic acid-locked nucleic acid (PNA-LNA) to detect 249 SqCLC patients, 33 of whom were identified to harbor EGFR mutations with a mutation rate of 13.3%.19 Furthermore, Amit Joshi et al used TaqMan probe real-time fluorescent quantitative PCR in 639 SqCLC patients and found the EGFR mutation rate to be 4.5% (29/639).20 Interestingly, we noted that the EGFR mutation rate mentioned above ranged from 3.4% to 13.3% and the studies were mainly done in Asian patients. However, all these studies failed to further distinguish predominant subgroups that are more likely to harbor EGFR mutations.
A study from Zhang et al revealed that female sex and non-smokers are correlated with higher EGFR mutations by detecting 28 positive cases among a total of 163 SqCLC patients using the ARMS-PCR method.21 Meanwhile, the study also confirmed that age and pathological differentiation seem to be unrelated to EGFR mutations. However, several studies found inconsistent results. A study from Zhang H et al also showed the tendency of higher EGFR detection rate in females and non-smokers by detecting 139 SqCLC patients, but there was no statistical difference.22 Another study from Zhang TT et al identified that the proportion of poor and moderate differentiations was lower in EGFR mutation harboring SqCLC patients compared to wild type patients.23
Our study identified the EGFR mutation rate to be 8.2% in 292 SqCLC patients using the ARMS-PCR method, whose documented sensitivity is 1% and specificity can reach 100%.24 Our results were similar to those of studies by Cho18 and Zhang T23 but lower than those reported by Akito Hata19 and Zhang Q.21 Besides different methods, the different sample sizes and a potential mix-up of SqCLC with adenocarcinoma patients can be attributed, in part, to the reporting of various EGFR mutation detection rates. We also noted that, in our study, the detection rate of the EGFR mutation was 7.3% using NGS. Although there was no statistically significant difference, NGS detected less mutations than ARMS-PCR. There are a few possible explanations: (1) the heterogeneity of tumors. Mutated and wide-type cells generally co-exist in one solid tumor. If the tissue for detection just contained wide-type cells (especially for the biopsy of small samples), that may lead to false-negative results. (2) The small sample size combined with low frequency of EGFR mutation in this study, which may contribute to the discrepancy in the detection rate between the two methods.
Another finding from our study demonstrated that SqCLC patients harboring EGFR mutations may share the same clinicopathological characteristics with adenocarcinoma of lung – higher frequency in females and non-smokers. At the same time, differentiation, staging, specimen type, serum CEA, and SCC levels were unrelated to EGFR mutations, which are consistent with several other studies.21,22
Interestingly, we also verified no significant difference in EGFR mutation rate between surgical and biopsy samples, suggesting that sample type should not be a criterion for EGFR detection. Similar conclusions were arrived at by Kang et al after comparing the detection rate between surgical resection and rebiopsy tissue of SqCLC patients.25 These findings may be due to the high sensitivity and specificity of the ARMS-PCR method, which is capable of detecting less than 1% mutant cells in biopsy tumor tissue. In summary, we strongly recommend that SqCLC patients should be routinely subjected to the detection of an EGFR mutation, especially when patients are female and non-smokers.
As far as efficacies are concerned, there are no powerful data that show clear clinical benefits for SqCLC patients treated with EGFR-TKIs, which in turn may restrict its wide use in those patients. In our study, 10, 3, and 1 patients used EGFR-TKIs as a first-line, second-line and third-line treatment, respectively, which exhibited an ORR of 28.6%, a DCR of 78.6%, a mPFS of 4.7 months, and an mOS of 10.6 months. Arithmetically, the efficacies of EGFR-TKIs seem to be similar with first-line platinum-based doublet regimens but side-effects and tolerability were superior to chemotherapy. Due to the small cohort of treated patients, a firm conclusion is elusive and hence, the results of this study should be interpreted with caution. For further identification of the efficacies of EGFR-TKIs on SqCLC patients, monitoring of the same in a large patient cohort is necessary.
Table 3 summarizes the efficacies of EGFR-TKIs for EGFR-mutated SqCLC or non-adenocarcinoma patients from 4 studies with the ORR ranging from 26.3% to 50%, DCR from 47.3% to 77.3%, and PFS from 3.67 to 5.1 months. All these studies also validate that EGFR-TKIs have some efficacy in general, but this efficacy is still not comparable with that in adenocarcinoma patients harboring EGFR-sensitizing activation mutations.
 |
Table 3 Clinical Efficacies Of EGFR-TKIs For SqCLC Patients With EGFR Mutations |
The modest efficacy of EGFR-TKIs in SqCLC patients may be due to the activation of bypass signaling pathways such as BMP-BMPR-Smad1/5-p70S6K and PI3K-AKT-mTOR, according to some reports, which weaken the dependence of EGFR signaling in cells and subsequently result in low inhibition by EGFR-TKIs.26 In addition, it is reported that EGFR VIII, a variant or subform of EGFR with an incidence rate of 5–8% in SqCLC, seems to play a crucial role in primary resistance against first-generation TKIs.27,28 Promisingly, second-generation EGFR-TKIs, afatinib and dacomtinib are expected to overcome this therapeutic obstacle induced by EGFR VIII.
Moreover, the efficacies of EGFR-TKIs in EGFR-mutated SqCLC patients were also investigated by a few large-scale clinical trials. LUX-Lung829 compared the efficacies between Erlotinib and afatinib in SqCLC patients, showing a DCR, mPFS, and mOS of the afatinib group versus Tarceva group to be 50.5% vs. 39.5%, 2.6 months vs. 1.9 months, and 7.9 months vs. 6.8 months, respectively. These differences were statistically significant. In our study, merely 2 patients received Afatinib as second-line treatment with an mPFS of 4.2 months and 13.5 months, and an mOS of 21.1 months, and unreached until last follow up, showing acceptable clinical benefit.
However, there are certain drawbacks to our study that should not be omitted. First, only 25% (73/292) of patients underwent NGS detection, which is capable of simultaneous detection of mutations, indels, copy number variations, and genomic rearrangements. ARMS-PCR detected only 29 previously identified point mutations in the EGFR gene and may have missed valuable information related to concurrent or accompanying mutations deemed important to broaden insights into the mechanisms of acquired resistance. Second, the frequency rate of the EGFR mutation in SqCLC is quite low and hinders adequate statistical significance to arrive at conclusions due to the small number of patients with mutations. Third, the retrospective design seems to be unconquered with its inner deficiency. Combining these drawbacks together, conclusions from this article must be drawn with caution.
Last but not the least, it has been well established that mutations in PI3KCA, FGFR1, PETN, DDR2, IGF-1R, BRAF, and FGFR2 and amplification of PI3KCA and PDGFRA may be involved in the initialization and development of SqCLC. However, for the remaining 25% of SqCLC patients, the driver mutations are still unclear.30 Finally, with the widespread application of NGS in clinical practices and the increasing number of clinical trials, more driver mutations and drugs in SqCLC beyond EGFR and EGFR-TKIs are to be verified, with potential breakthroughs in this field possible in the near future.
Conclusion
According to our study, we have confirmed that EGFR-sensitizing mutations remain a rare event in SqCLC patients. Both ARMS-PCR and NGS methods showed no difference in the detection of EGFR mutations. Detection of EGFR mutations is recommended especially in female patients or those patients who have no history of smoking. EGFR-TKIs show modest efficacies and low toxicity profiles in EGFR mutant cases.
Acknowledgment
We would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
2. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. doi:10.1097/JTO.0000000000000033
3. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911.
4. Song X. Clinical efficacy evaluation of tyrosine kinase inhibitors for non-adenocarcinoma lung cancer patients harboring EGFR-sensitizing mutations. Onco Targets Ther. 2017;10:3119–3122. doi:10.2147/OTT.S134523
5. Pan Y, Wang R, Ye T, et al. Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component. Chest. 2014;145:473–479. doi:10.1378/chest.12-2679
6. Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. Thorac Oncol. 2017;12(4):612–623. doi:10.1016/j.jtho.2016.12.014
7. Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
8. Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi:10.1200/JCO.2011.36.8456
9. Sequist LV, Yang JCH, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334.
10. Bironzo P, Di Maio M. A review of guidelines for lung cancer. J Thorac Dis. 2018;10:S1556–S1563. doi:10.21037/jtd
11. Newton CR, Graham A, Heptinstall LE, et al. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989;17(7):2503–2516. doi:10.1093/nar/17.7.2503
12. Chu H, Zhong C, Xue G, et al. Direct sequencing and amplification refractory mutation system for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Oncol Rep. 2013;30(5):2311–2315. doi:10.3892/or.2013.2709
13. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
14. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi:10.1016/S1053-4296(03)00031-6
15. Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol. 2014;25(9):1681–1690. doi:10.1093/annonc/mdu145
16. Mascaux C, Tsao MS, Hirsch FR. Genomic testing in lung cancer: past, present, and future. J Natl Compr Canc Netw. 2018;16(3):323–334. doi:10.6004/jnccn.2017.7019
17. Miyamae Y, Shimizu K, Hirato J, et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep. 2011;25:921–928. doi:10.3892/or.2011.1182
18. Cho SH, Park LC, Ji JH, et al. Efficacy of EGFR tyrosine kinase inhibitors for non-adenocarcinoma NSCLC patients with EGFR mutation. Cancer Chemother Pharmacol. 2012;70:315–320. doi:10.1007/s00280-012-1876-0
19. Hata A, Katakami N, Yoshioka H, et al. How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutations? J Thorac Oncol. 2013;8:89–95. doi:10.1097/JTO.0b013e31827690b5
20. Joshi A, Zanwar S, Noronha V, et al. EGFR mutation in squamous cell carcinoma of the lung: does it carry the same connotation as in adenocarcinomas? Onco Targets Ther. 2017;10:1859–1863. doi:10.2147/OTT.S125397
21. Zhang Q, Zhu L. Epidermal growth factor receptor gene mutation status in pure squamous-cell lung cancer in Chinese patients. BMC Cancer. 2015;15:88. doi:10.1186/s12885-015-1056-9
22. Zhang H, Yang X, Qin N, et al. Detection and analysis of EGFR and KRAS mutations in the patients with lung squamous cell carcinomas. Zhongguo Fei Ai Za Zhi. 2015;18:621–625. doi:10.3779/j.issn.1009-3419.2015.10.04
23. Zhang T, Li J. Driven gene in patients with lung squamous cell carcinoma: analysis of clinicopathologic characteristics and prognosis. Zhongguo Fei Ai Za Zhi. 2016;19:648–652. doi:10.3779/j.issn.1009-3419.2016.10.02
24. Liu XP, Lu YC, Zhu GS, et al. The diagnostic accuracy of pleural effusion and plasma samples versus tumour tissue for detection of EGFR mutation in patients with advanced non-small cell lung cancer: comparison of methodologies. J Clin Pathol. 2013;66(12):1065–1069.
25. Kang L, Zheng J, Zhu X. Relationship between EGFR mutations and pathological classification and specimen of lung adenocarcinoma. Zhongguo Fei Ai Za Zhi. 2017;20(6):382–388. doi:10.3779/j.issn.1009-3419.2017.06.03
26. Wang Z, Shen Z, Li Z, et al. Activation of the BMP-BMPR pathway conferred resistance to EGFR-TKIs in lung squamous cell carcinoma patients with EGFR mutations. Proc Natl Acad Sci U S A. 2015;112:9990–9995. doi:10.1073/pnas.1510837112
27. Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 2015;5:390–401. doi:10.1016/j.apsb.2015.07.001
28. Duan J, Wang Z, Bai H, et al. Epidermal growth factor receptor variant III mutation in Chinese patients with squamous cell cancer of the lung. Thorac Cancer. 2015;6:319–326. doi:10.1111/1759-7714.12204
29. Felip E, Hirsh V, Popat S, et al. Symptom and quality of life improvement in LUX-Lung 8, an open-label phase III study of second-line afatinib versus erlotinib in patients with advanced squamous cell carcinoma of the lung after first-line platinum-based chemotherapy. Clin Lung Cancer. 2017;19(1):74–83. doi:10.1016/j.cllc.2017.06.002
30. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550.
31. Liu Y, Zhang Y, Zhang L, et al. Efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors for lung squamous carcinomas harboring EGFR mutation: a multicenter study and pooled analysis of published reports. Oncotarget. 2017;8:49680–49688. doi:10.18632/oncotarget.17915
32. Xu J, Zhang Y, Jin B, et al. Efficacy of EGFR tyrosine kinase inhibitors for non-adenocarcinoma lung cancer patients harboring EGFR-sensitizing mutations in China. J Cancer Res Clin Oncol. 2016;142:1325–1330. doi:10.1007/s00432-016-2133-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
