Back to Journals » International Journal of General Medicine » Volume 17
Clinical Value of the Diagonal Earlobe Crease in Patients with Chest Pain for Diagnosing Coronary Heart Disease
Authors Gao J , Dou J, Yang HH, Guo RL, Jiang C, Tse G , Liu T, Liu JW, Luo DL
Received 12 January 2024
Accepted for publication 18 April 2024
Published 23 April 2024 Volume 2024:17 Pages 1557—1569
DOI https://doi.org/10.2147/IJGM.S454888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Redoy Ranjan
Jie Gao,1,* Jie Dou,1,* Hui-Hui Yang,1 Ruo-Ling Guo,1 Chao Jiang,1 Gary Tse,2– 4 Tong Liu,2 Jian-Wei Liu,5 Dong-Lei Luo6
1Chengde Medical University, Chengde, 067000, People’s Republic of China; 2Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Department of Cardiology, Tianjin Institute of Cardiology, Second Hospital of Tianjin Medical University, Tianjin, 300211, People’s Republic of China; 3School of Nursing and Health Studies, Hong Kong Metropolitan University, Hong Kong, 999077, People’s Republic of China; 4Epidemiology Research Unit, Cardiovascular Analytics Group, PowerHealth Limited, Hong Kong, 999077, People’s Republic of China; 5Department of Cardiothoracic Interventional Vascular Surgery, Chengde Central Hospital/Second Clinical College of Chengde Medical University, Chengde, Hebei, 067000, People’s Republic of China; 6Department of Cardiology, Chengde Central Hospital / Second Clinical College of Chengde Medical University, Chengde, 067000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dong-Lei Luo, Department of Cardiology, Chengde Central Hospital / Second Clinical College of Chengde Medical University, Chengde, 067000, People’s Republic of China, Email [email protected] Jian-Wei Liu, Department of Cardiothoracic Interventional Vascular Surgery, Chengde Central Hospital/Second Clinical College of Chengde Medical University, Chengde, Hebei, 067000, People’s Republic of China, Email [email protected]
Purpose: To investigate the clinical application value of diagonal earlobe crease (DELC) in patients with chest pain for the diagnosis of coronary heart disease (CHD) and to construct a risk model by multivariate logistic regression.
Patients and Methods: Our trial enrolled prospectively and consecutively 706 chest pain patients with suspected CHD between January 2021 to June 2023 from Chengde Central Hospital. According to coronary angiography results, they were categorized into the CHD (n=457) and non-CHD groups (n=249).
Results: The trial demonstrated a significant positive relationship between DELC and CHD. Independent risk factors were sex, age, hypertension, diabetes mellitus, LP (a), Cys C, and DELC, whilst HDL-C was a protective factor, for CHD. Patients with-DELC were older than those in the without-DELC arm (P< 0.001) and had a higher proportion of males than females (61.6% vs 50.0%, P=0.026). After multifactorial correction, independent risk factors for CHD included DELC (OR=1.660, 95% CI:1.153 to 2.388, P=0.006), age (OR=1.024, 95% CI:1.002 to 1.045, P=0.030), gender (OR=1.702, 95% CI:1.141 to 2.539, P=0.009), hypertension (OR=1.744, 95% CI:1.226 to 2.482, P=0.002), diabetes mellitus (OR=2.113, 95% CI:1.404 to 3.179, P< 0.001), LP(a) (OR=1.010, 95% CI:1.003 to 1.017, P=0.005), Cys C (OR=3.549, 95% CI:1.605 to 7.846, P=0.002). The Hosmer and Lemeshow (H-L) test (P=0.818) suggests a high goodness of fit, and the area under the ROC curve was calculated to be 0.721 (95% CI:0.682 to 0.760, P< 0.001), which demonstrates that the model has a superior diagnostic value for CHD.
Conclusion: DELC is an independent risk factor for CHD after adjusting for sex, age, hypertension, diabetes mellitus, smoking index, LP (a), Cys C, and HDL-C. Our model can be used clinically for assessing the risk of CHD.
Keywords: coronary heart disease, diagonal earlobe crease, coronary angiography, model
Introduction
Coronary heart disease (CHD), also known as ischemic heart disease (IHD), is characterised by myocardial ischemia, hypoxia, or necrosis due to stenosis or occlusion of the lumen from coronary atherosclerosis.1 CHD is due to a combination of lifestyle, social and environmental factors, and has become a major global public health threat.2–4 The burden of premature mortality from cardiovascular disease (CVD) in China decreased by 19.27% compared with that of 15 years ago in 2020. Still, IHD remains the main cause of increased mortality.5 The prevalence of CHD is increasing, which is due to a combination of an aging population, high work pressure, and unhealthy lifestyles.3
Chest pain is the most frequent presenting complaint in the emergency department and cardiovascular medicine department, amongst which CHD is a frequent cause.6 The 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/ SCMR Guideline for the Evaluation and Diagnosis of Chest Pain defines chest pain as referred pain in the shoulders, arms, jaw, neck, and upper abdomen. To reduce ambiguity, it recommends using “cardiac”, “possible cardiac”, and “non-cardiac” to describe the potential causes of suspected chest pain.6 Amongst patients attending the emergency department with chest pain, non-cardiac chest pain accounted for more than 50%, and CHD or acute coronary syndrome (ACS) accounted for only 5.1%, but chest pain remains the most common symptom of CHD.6,7 Therefore, early diagnosis of CHD in patients experiencing chest pain and the provision of treatment is essential to reduce disability and mortality, thereby improving the quality of life in patients with slow life.
There are several ways to diagnose CHD. Coronary angiography (CAG) has become the gold standard for diagnosing CHD, but it is an invasive procedure that may result in vessel injury and intraoperative infection.8 Besides, the high cost and hospitalization make it difficult for some patients. Additionally, coronary computed tomography angiography (CCTA) is also a valuable adjunct, which make preliminary assessments of CHD severity.9 In comparison to CAG, CCTA does not necessitate a hospital stay and can provide additional information, especially about pericoronary attenuation and calcium score. However, the accuracy of CCTA may be affected by factors, such as patient obesity, high heart rate, or severe coronary artery calcification. It is important to consider that both CAG and CCTA require the use of iodine contrast agents, which may cause allergies in certain patients, and some primary hospitals lack the necessary equipment for differences in medical standards.9 Thus, the American Heart Association/American College of Cardiology (AHA/ACC) and the European Society of Cardiology (ESC) proposed the Duke clinical score (DCS) and the updated Diamond-Forrester model (UDFM), which categorize patients into low, intermediate, and high-risk populations through the estimation of pretest probability (PTP) to guide the decision of the need for CAG.10,11 Yet, several researches have shown that the classical UDFM described above overestimates the likelihood of CHD, thereby reducing diagnostic accuracy.12,13 Japanese scholars have argued that the inclusion of low-probability patients in clinical trials is usually neglected in PTP studies, which would then lead to the overuse of CAGs, and the estimation of PTP and clinical probability of CHD is often underestimated.14
Therefore, searching for fast, safe, convenient, and accurate diagnostic methods more acceptable to patients has a significant clinical application value. In 1973, Frank first discovered the diagonal earlobe crease (DELC) in the New England Journal of Medicine (N Engl J Med) and conducted a detailed analysis of 20 CHD patients with positive signs. And he proposed that DELC was associated with CHD, except that the above relationship needed to be further verified.15 Ancient Chinese physicians observed that the “earlobe crease sign” was associated with the early onset of coronary atherosclerosis and that the DELC was once called the “CHD groove”.16 DELC, also known as Frank’s sign, is characterized by a fold that runs backward from the tragus at a 45⁰ angle across the lobule to the rear edge of the auricle.17 It is present on the skin surface of the earlobe. It is easily observed with the naked eye but has attracted little attention, likely because its clinical use has not been well-defined, so the link between DELC and CHD remains controversial. If its diagnostic precision is adequate to support decision-making, it will contribute to the early diagnosis and prevention of CHD.18 Fewer studies have included DELC in CHD risk models. Building upon the variables identified in the China-PAR study,19 our trial integrated easily observable DELC to model the risk of CHD.
Materials and Methods
Patients and Criteria
This study was a single-center, prospective cohort study, which consecutively included patients with chest pain with CHD suspected who were in the Department of Cardiology of Chengde City Central Hospital from January 2021 to June 2023. Based on CAG, patients who met the criteria were divided into the CHD and non-CHD groups. Incorporation criteria were patients presenting for chest pain, aged 18–80 years, consenting to CAGs, and agreeing to have a DELC taken. Exclusion criteria were a previous definitive diagnosis of CHD or a history of coronary revascularization (including percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG)); non-cardiac chest pain; other cardiac conditions (eg, congenital heart disease, heart valve disease, or large vessel disease); iodine contrast allergy; patients wearing earrings. This study complies with the Declaration of Helsinki. All participants signed an informed consent. This trial is registered at http://www.chictr.org.cn, and the Chinese clinical registration number is ChiCTR2000041499.
Specimen Collection and Processing
The characteristics of all participants were obtained from the Department of Cardiovascular Medicine, Chengde City Central Hospital, Hebei Province, China. The following baseline details were collected. (1) Demographic characteristics: age, sex, height, weight, body mass index (BMI) was calculated- formula by BMI=kg/m2. (2) The serum enzymes and blood cytology indices included white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hb), red cell volume distribution width (RDW), glucose (Glu), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), lipoprotein (a) [LP (a)], and cystatin C (Cys C). (3) Other clinical characteristics include CHD-related risk factors and past history, ECG ST-T and cardiac ultrasound (left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and segmental wall motion abnormalities (SWMA)). The earlobes of the patients were taken for evaluation in sitting or lying position under natural light source, and compliant DELCs were collected for inclusion in the study and examined by two experienced observers who were unaware of the patient’s condition.
The result of CAG is used as the basis for CHD diagnosis. A clinically significant stenosis was defined as stenosis of at least 50% of the vessel diameter.20 The major coronary arteries include the right coronary artery, left main coronary artery, left anterior descending branch, and left circumflex branch. The major branches include the first/second diagonal branch, the obtuse marginal branch, the acute marginal branch, the left ventricular posterior branch, and the posterior descending branch. Experienced cardiologists performed CAGs; the access route was radial or femoral artery, and CAGs were performed using the Judkins method, with at least 4 positions projected in the left coronary artery (left/right anterior oblique plus pedicled, left/right anterior oblique plus cephalic), and at least two right coronary arteries projected in the right coronary artery (left anterior oblique, orthogonal plus cephalic). CAGs were assessed by two independent experts, experienced interventionalists who had no conflict of interest with the study. The decision was made by joint consultation with the third experienced interventionalist in the case of a controversy.
Definition of Nouns
The definition of hypertension is based on repeated office SBP values ≥140 mmHg and/or DBP ≥90 mmHg.21 The definition of diabetes mellitus is based on the diagnostic and categorization criteria proposed by the WHO Expert Committee on Diabetes Mellitus (1999), which are used internationally.22 Overweight: 25kg/m2≤BMI<29.9kg/m2; Obesity: BMI≥30kg/m2.23 Smoking Index = smoking years×daily smoking volume. NLR= Neutrophil-to-Lymphocyte ratio.24 Family history of CHD includes first-degree relatives less than 55 years of age for men or less than 65 years of age for women, the occurrence of angina pectoris, myocardial infarction, sudden cardiac death without apparent cause, previous CABG or PCI.25 DELC is a deep diagonal crease (>1mm) extending at an angle from the ear screen to the outer edge of the earlobe, accounting for more than two-thirds of the length of the earlobe16 (Figure 1). Chest pain can be categorized as typical, atypical, or non-cardiac chest pain2,14 (Table 1).
 |
Table 1 Traditional Clinical Classification of Chest Pain |
 |
Figure 1 Diagonal Earlobe Crease. |
Data Quality and Missing Data
Data were verified for completeness and inconsistencies. All missing values were Missing Completely at Random (MCAR); their percentage was less than 10% of the total cases. Any inconsistencies or incomplete data have been corrected and finalized. Direct deletion was used for cases with large amounts of missing data. The full continuous variables were imputed using stochastic regression imputation; the complete categorical variables were supplemented by mode.
Statistical Analysis
Student’s t-test or Mann–Whitney U-test was used for comparing groups, and continuous variables were presented as mean ± standard deviation or median and interquartile range. The chi-square test compared groups based on counts and percentages (%) expressed as categorical variables. Individual risk factor features were examined using multivariate and univariate logistic regression analysis. By calculating odds ratios (ORs) and 95% confidence intervals (95% CIs), integrated diagnosis model based on sex, age, hypertension, diabetes mellitus, smoking index, LP (a), Cys C, and HDL-C with DELC. Model performance was visualized by plotting CHD receiver operating characteristics (ROC) and area under the curve (AUC). Furthermore, a Hosmer-Lemeshow (H-L) goodness-of-fit statistics was computed. Non-significant H-L statistics indicates that the model fit well. Variance Inflation Factor (VIF) was calculated to examine the multicollinearity between the influencing factors. A two-sided P<0.05 was statistically significant. SPSS version 26.0 (IBM, Corporation, Armonk, NY, USA) was performed for statistical analysis, and graphics drawing used GraphPad Prism 8.0. The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement was followed by our trial, and used as a reporting guideline.26
Results
Characteristics of Study Patients
A total of 1187 patients presenting with chest pain and suspected CHD were collected in this trial, and the inclusion and exclusion criteria were strictly followed: 31 refused angiography 155 cases with missing information, and 295 did not meet the inclusion criteria. Finally, 706 patients were included, with a mean age of (61.03 ± 8.78) years and 377 (53.4%) males. The flow chart of the trial is shown in Figure 2. Based on the diagnostic criteria, they were categorized into non-CHD group (n= 249) and CHD group (n= 457), in which 333 cases of CHD patients with DELC status (Figure 2).
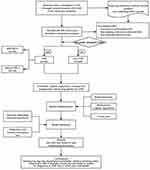 |
Figure 2 Flowchart of the trial. Abbreviations: CAG, Coronary angiography; DELC, Diagonal earlobe crease; CHD, Coronary heart disease. |
The baseline clinical and procedural characteristics for all patients who were eligible for recruitment are listed in Table 2 and Table 3. Differential analysis showed that age, gender, smoking history, hypertension, diabetes mellitus, systolic blood pressure, Glu, HDL-C, LP (a), Cys C, ECG ST-T, and SWMA were statistically different between the non-CHD and CHD groups (P<0.05). Instead, the rest of the indicators were similar (P>0.05).
 |
Table 2 Clinical Comparison of Subjects with and without CHD |
 |
Table 3 Symptomatic and Physical Signs Factors in the Two Groups |
The proportion of typical chest pain was significantly higher in the CHD group than in the non-CHD group (P=0.011). CHD patients were prone to myocardial ischemia during exertion or emotional stress, which induced chest pain (P=0.004). The sample showed that subjects with DELC had a higher prevalence of coronary artery stenosis than those without DELC, with a statistically significant difference between the CHD and non-CHD groups (P<0.001) (Table 3).
Patients diagnosed with CHD were used as study subjects for subgroup analysis. The patients in the CHD arm were categorized into those with-DELC (n= 333) and those without-DELC (n= 124). Those with DELC were older (P<0.001), with a higher proportion of males (P=0.026). NLR and Cys C were higher for the DELC group (P<0.05) (Table 4).
 |
Table 4 Characteristics of the CHD Participants with and without DELC |
Relationships of DELC with the Diagnosis of CHD
Using CHD as the dependent variable, logistic regression was used to evaluate whether the following factors were significant predictors: DELC, sex, hypertension, diabetes mellitus, age, smoking index, LP (a), Cys C, and HDL-C. Multivariate analysis revealed that independent risk factors for CHD included sex, age, hypertension, diabetes, DELC, LP (a) and Cys C. Additionally, the HDL-C emerged as a protective factor against CHD (OR: 0.602, P=0.049), with a 39.8% reduction in CHD risk for every 1 mmol/L increase in HDL-C (Table 5, Figure 3).
 |
Table 5 The Univariate and Multivariate Logistic Regression of the Characteristics of the CHD and Non-CHD |
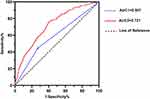
The VIF for each factor was less than 10, and there was no multicollinearity. The AUCs of DELC alone (model 1) and the model for CHD (model 2) are shown in Figure 3. The AUCs of model 1 and 2 were 0.587 (95% CI:0.543 to 0.632, P<0.001) and 0.721 (95% CI: 0.682 to 0.760, P<0.001), respectively, indicating fair discriminatory ability. The sensitivity of model 1 was 72.9%, and the specificity was 45.6%. The sensitivity of model 2 was 59.5%, the specificity was 76.3%, and the Youden index of model 2 was 0.358 for the diagnosis of CHD in this hospital-based population. The H-L goodness-of-fit tests of model 2 were non-significant (P=0.818). Overall, model 2 showed a slightly better performance (Figure 4).
Discussion
The number of cases of CVD has almost doubled from 271 million in 1990 to 523 million in 2019. While CHD is the most prevalent type of CVD, the disability and mortality rates caused by CHD are increasing. This development implies a growing need for the prevention and treatment of CHD in national health systems.3 CAG and CCTA are effective methods for diagnosing CHD, but there are limitations to their application due to patient conditions, technical requirements, and cost issues.8,9,20 Subsequently, the AHA/ACC and ESC proposed the DCS and UDFM models to calculate PTPs for guidance in CAG selection. Still, the above models overestimated the prevalence of CHD.10,12–14 Therefore, a noninvasive, simple, and reliable predictor of atherosclerosis is needed for early diagnosis, prevention, and treatment of CHD to reduce mortality.
Risk factors for CHD are essential for disease risk assessment and diagnosis, as well as for the development of individualized treatment plans. The Global Burden of Cardiovascular Disease and Risk Factors 1990–2019 and the 2019 ESC Guidelines for the Diagnosis and Treatment of Chronic Coronary Syndromes describe several CHD risk factors such as hypertension, diabetes mellitus, dyslipidemia, overweight and obesity, and tobacco.2,3 Different risk prediction models for CHD have been developed.27–29 The first CVD assessment model was introduced by the Framingham Heart Research Center in the United States, and majority of the CVD risk assessment models have since been published for Western countries, including the Q Risk Index model in the United Kingdom and the Systemic Coronary Risk Assessment Model in Europe.28,29 However, the spectrum of CVD and the prevalence of risk factors in China differ enormously from those in Western countries. So, disease prediction models based on Western populations may not apply to CVD risk assessment in China. Fuwai Hospital, Chinese Academy of Medical Sciences, proposed the China-PAR model for the Chinese population. The model is a prospective cohort study in which a Chinese population aged 35–74 years was selected for follow-up tracking, with a mean follow-up time of 12.3 years. Age, systolic blood pressure, total cholesterol, HDL-C, smoking status and diabetes mellitus, geographic location, and family history of CHD were enrolled to construct a CHD prediction model for assessing the risk of CHD.19 In our trial, logistic regression was applied to analyze independent risk factors of CHD and construct a simple CHD model.
The mechanisms underlying the association between DELC and CHD are unclear. DELC is rarely present in infant and adolescent populations, showing that DELC indirectly suggests the process of skin and vascular aging.30 Stoyanov et al31 performed autopsies on 45 adult patients and collected samples from both earlobes and cardiac chambers for histopathological examination. Myocardial morphological changes were found to be significantly associated with the presence of DELC.28 Oda et al32 posited that bilateral DELC may be linked to endothelial dysfunction, an early manifestation of atherosclerosis.29 Lipid metabolism is a key factor in atherosclerosis, and atherosclerosis is the “culprit” in the development of CHD. Based on the subgroup analysis of this study, DELC and dyslipidemia were not significantly correlated (P<0.05), but TG levels were different in the two groups. TG levels in the test were numerically slightly higher than the normal range. It may be related to many factors, such as patients’ dietary structure, used lipid-lowering drugs, lifestyle habits, and exercise intensity. Notably, NLR and Cys C significantly differed in the subgroup analysis (P<0.05). Lymphocytes and neutrophils are involved in the body’s inflammatory response, which may lead to vascular endothelial dysfunction, and their ratio (NLR) is a relatively new indicator used to reflect the body’s inflammatory state.24 NLR has been documented to be significantly associated with coronary artery stenosis.33 Inflammatory mediators are released with vascular endothelial injury, with an imbalance between Cys C and hydrolyzing enzymes, further promoting atherosclerosis.34 Based on the subgroup analysis, the above two indicators may indirectly reflect the close relationship between DELC and inflammation-induced atherosclerosis in patients with CHD.35 Frank’s sign has previously been reported.36 A Japanese study of men with Frank’s sign and metabolic syndrome found that the leukocyte telomere length in subjects with DECL were shortened.37
In our study, DELC was significantly and positively associated with CHD (P=0.006), and it may increase the prevalence of CHD. Subgroup analysis suggested that more males than females with DELC (61.6% vs 50.0%, P=0.026) and patients with DELC were elder than those without DECL (63.47 ± 7.82 vs 58.02 ± 9.34, P<0.001). There was no clear association between DELC and common CHD risk factors, consistent with previous studies reporting.16,38
Wang et al16 enrolled 558 patients (402 males and 156 females) who underwent suspected or confirmed CHD and underwent angiography. Participants were categorized into no DELC, unilateral DELC, and bilateral DELC arms. The area under the ROC curve of DELC for CHD diagnosis was 0.693 (95% CI: 0.636 to 0.750, P<0.001). In this study application value of model 1 was shown AUC1=0.587, suggesting the diagnostic performance of the model was general. However, after adjusting for confounders, the diagnostic model constructed based on sex, age, hypertension, diabetes mellitus, smoking index, LP (a), Cys C, HDL-C, and DELC (model 2) was shown AUC2=0.721 (95% CI: 0.682 to 0.760, P<0.001). The H-L goodness of fit test suggested a high model fit goodness of fit (P=0.818). Therefore, it is important to consider validating this model in clinical practice.
Chest pain is a common reason for seeking medical attention,6 but there are many causes of chest pain, such as CHD, pneumothorax, pulmonary embolism, and acute aortic dissection. Nishiori et al39 reported the association between Frank’s sign and acute aortic dissection. Ramírez Montesinos et al40 described the relationship between DELC and chest pain. DELC formation is a lengthy process and once formed, it is almost irreversible and considered a stable sign.41 For individuals with DELC and chest pain, it is important to consider both aortic and coronary artery disease. The accuracy of disease diagnosis is crucial in medical practice. Our trial findings suggest that individuals with chest discomfort and DELC should be cautious about the possibility of CHD. Predictive diagnosis through DELC and readily available clinical information for chest pain patients can help avoid invasive examinations for suspected CHD, leading to early treatment and optimal use of medical resources.
Our study still has a few limitations. (1) It was a prospective cohort study that required real-time collection of DELCs, and some information bias (including diagnostic suspicion bias and exposure suspicion bias) may exist. (2) This trial was a single-center study; the extensive CHD diagnostic model was not validated, and the generalization performance was unknown. Further validation is needed in the future. (3) The experimental results of this study are only suitable for the physical examination of healthy people or the prevention of patients with low or medium risk of chest pain. For high-risk individuals, it is still recommended to perform CAGs in time to clarify the diagnosis and timely treatment to avoid delaying the timing of treatment. (4) The type (unilateral, bilateral), degree (deep, shallow), and number (single, multiple) of DELC were not meticulously classified. However, this study developed a diagnostic model based on routinely and readily available indicators in clinical practice. The multicollinearity between factors was analyzed, and a visualized forest plot was drawn.
Conclusion
Amongst patients presenting with chest pain, those with DELC had 1.66-fold increased odds of CHD compared to those without DELC. DELC can be used as a sign for early recognition of CHD. Models based on sex, age, hypertension, diabetes mellitus, smoking index, LP (a), Cys C, and HDL-C can be used to predict CHD.
Abbreviations
DELC, Diagonal earlobe crease; CHD, Coronary heart disease; IHD, Ischemic heart disease; CVD, Cardiovascular disease; ACS, Acute coronary syndrome; CAG, Coronary angiography; DCS, Duke clinical score; UDFM, Updated Diamond-Forrester model; PTP, Pretest probability; BMI, Body mass index; LVEF, Left ventricular ejection fraction; LVEDD, Left ventricular end diastolic diameter; SWMA, Segmental wall motion abnormalities; CABG, Coronary artery bypass grafting; PCI, Percutaneous coronary intervention.
Institutional Review Board Statement
The Ethics Committee approved the trial protocol and authorized this trial at Chengde Central Hospital/Second Clinical College of Chengde Medical University. This study complies with the Declaration of Helsinki.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This study was funded by the Medical Science Research Project of Hebei Province (NO. 20200348, 20200236), the S&T Program of Chengde (NO. 202109A019), the Project of Chengde Central Hospital (NO. YJKT20220001) and Hebei Province Master’s Graduate Innovation Ability Training Funding Project (NO. CXZZSS2023147).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Wang J, Wang X, et al. Knockdown of hypoxia-inducible factor 1-alpha (HIF1α) interferes with angiopoietin-like protein 2 (ANGPTL2) to attenuate high glucose-triggered hypoxia/reoxygenation injury in cardiomyocytes. Bioengineered. 2022;13(1):1476–1490. doi:10.1080/21655979.2021.2019874
2. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi:10.1093/eurheartj/ehz425
3. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
4. Tan X. Lifestyle, social environment, physiological environment and cardiovascular disease. Heart and Mind. 2022;6(1):1–2. doi:10.4103/hm.hm_1_22
5. Wang W, Liu Y, Liu J, et al. Mortality and years of life lost of cardiovascular diseases in China, 2005–2020: empirical evidence from national mortality surveillance system. Int J Cardiol. 2021;340:105–112. doi:10.1016/j.ijcard.2021.08.034
6. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/ SCCT/SCMR Guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454.
7. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176(7):1029–1032. doi:10.1001/jamainternmed.2016.2498
8. Xiong F, Mao R, Zhao R, et al. Plasma exosomal S1PR5 and CARNS1 as potential non-invasive screening biomarkers of coronary heart disease. Front Cardiovasc Med. 2022;9:845673. doi:10.3389/fcvm.2022.845673
9. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2015;149(3):e5–e23. doi:10.1016/j.jtcvs.2014.11.002
10. Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118(2):81–90. doi:10.7326/0003-4819-118-2-199301150-00001
11. Genders TS, Steyerberg EW, Alkadhi H, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32(11):1316–1330. doi:10.1093/eurheartj/ehr014
12. Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pre-test probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation. 2011;124(22):2423–2432. doi:10.1161/CIRCULATIONAHA.111.039255
13. Reeh J, Therming CB, Heitmann M, et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J. 2019;40(18):1426–1435. doi:10.1093/eurheartj/ehy806
14. Nakano S, Kohsaka S, Chikamori T, et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882–915. doi:10.1253/circj.CJ-21-1041
15. Frank ST. Aural sign of coronary-artery disease. N Engl J Med. 1973;289:327–328.
16. Wang Y, Mao LH, Jia EZ, et al. Relationship between diagonal earlobe creases and coronary artery disease as determined via angiography. BMJ Open. 2016;6(2):e008558. doi:10.1136/bmjopen-2015-008558
17. Kajihara Y. A diagonal earlobe crease. Eur J Intern Med. 2022;99:96. doi:10.1016/j.ejim.2022.01.017
18. Więckowski K, Gallina T, Surdacki A, et al. Diagonal earlobe crease (Frank’s Sign) for diagnosis of coronary artery disease: a systematic review of diagnostic test accuracy studies. J Clin Med. 2021;10(13):2799. doi:10.3390/jcm10132799
19. Yang X, Li J, Hu D, et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: the China-PAR Project (Prediction for ASCVD Risk in China). Circulation. 2016;134(19):1430–1440. doi:10.1161/CIRCULATIONAHA.116.022367
20. Xu B, Tu S, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398(10317):2149–2159. doi:10.1016/S0140-6736(21)02248-0
21. Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023;41:1874–2071.
22. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
23. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi:10.1161/CIR.0000000000000678
24. Xydonas S, Parissis J, Lioni L, et al. Immunosenescence in patients with chronic systolic heart failure. J Cardiovasc Med. 2016;17(8):624–630. doi:10.2459/JCM.0000000000000372
25. Moonesinghe R, Yang Q, Zhang Z, et al. Prevalence and cardiovascular health impact of family history of premature heart disease in the United States: analysis of the National Health and Nutrition Examination Survey, 2007–2014. J Am Heart Assoc. 2019;8(14):e012364. doi:10.1161/JAHA.119.012364
26. Heus P, Reitsma JB, Collins GS, et al. Transparent reporting of multivariable prediction models in journal and conference abstracts: TRIPOD for abstracts. Ann Intern Med. 2020;173(1):42–47. doi:10.7326/M20-0193
27. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38(1):46–51. doi:10.1016/0002-9149(76)90061-8
28. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi:10.1016/S0195-668X(03)00114-3
29. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335(7611):136. doi:10.1136/bmj.39261.471806.55
30. Pacei F, Bersano A, Brigo F, et al. Diagonal earlobe crease (Frank’s sign) and increased risk of cerebrovascular diseases: review of the literature and implications for clinical practice. Neurol Sci. 2020;41(2):257–262. doi:10.1007/s10072-019-04080-2
31. Stoyanov GS, Dzhenkov D, Petkova L, et al. The Histological Basis of Frank’s Sign. Head Neck Pathol. 2021;15(2):402–407. doi:10.1007/s12105-020-01205-4
32. Oda N, Maruhashi T, Kishimoto S, et al. Relation of the Bilateral Earlobe Crease to Endothelial Dysfunction. Am J Cardiol. 2017;119(12):1983–1988. doi:10.1016/j.amjcard.2017.03.029
33. Bao X, Zhou G, Xu W, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: novel markers for the diagnosis and prognosis in patients with restenosis following CAS. Biomark Med. 2020;14(4):271–282. doi:10.2217/bmm-2019-0155
34. Li W, Sultana N, Siraj N, et al. Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J Cell Mol Med. 2016;20(9):1664–1672. doi:10.1111/jcmm.12859
35. Koyama T, Watanabe H, Ito H. The association of circulating inflammatory and oxidative stress biomarker levels with diagonal earlobe crease in patients with atherosclerotic diseases. J Cardiol. 2016;67(4):347–351. doi:10.1016/j.jjcc.2015.06.002
36. Bazoukis G, Papadatos SS, Varrias D, et al. The importance of inspection in clinical cardiology: frank’s sign. Clin Case Rep. 2022;10(7):e6150. doi:10.1002/ccr3.6150
37. Higuchi Y, Maeda T, Guan JZ, et al. Diagonal earlobe crease are associated with shorter telomere in male Japanese patients with metabolic syndrome. Circ J. 2009;73(2):274–279. doi:10.1253/circj.CJ-08-0267
38. Wu XL, Yang DY, Zhao YS, et al. Diagonal earlobe crease and coronary artery disease in a Chinese population. BMC Cardiovasc Disord. 2014;14(1):43. doi:10.1186/1471-2261-14-43
39. Ono R, Kato K, Saito Y, et al. Frank’s sign with cyanotic cauliflower ear. QJM. 2021;114(3):209. doi:10.1093/qjmed/hcaa332
40. Ramírez Montesinos R, Duran Taberna M, Torres Quilis C. Frank’s sign and chest pain. Med Clin. 2020;154(11):465–466. doi:10.1016/j.medcli.2019.01.027
41. Christoffersen M, Frikke-Schmidt R, Schnohr P, et al. Visible age-related signs and risk of ischemic heart disease in the general population: a prospective cohort study. Circulation. 2014;129(9):990–998. doi:10.1161/CIRCULATIONAHA.113.001696
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.