Back to Journals » Cancer Management and Research » Volume 15
Clinical Value of Combined Detection of Serum sTim-3 and CEA or CA19-9 for Postoperative Recurrence of Colorectal Cancer Diagnosis
Authors Hong J, Chen X, Chen L, Wang Y , Huang B, Fang H
Received 21 February 2023
Accepted for publication 28 June 2023
Published 3 July 2023 Volume 2023:15 Pages 563—572
DOI https://doi.org/10.2147/CMAR.S407930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Jianfeng Hong,1 Xindong Chen,2 Lingli Chen,2 Yigang Wang,2 Biao Huang,2 Hongming Fang1
1Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, People’s Republic of China; 2Immunological Analysis Laboratory of Academy of Life Sciences, Zhejiang Sci-Tech University, Hangzhou, People’s Republic of China
Correspondence: Hongming Fang, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, 310016, People’s Republic of China, Tel + 86 0571-83865858, Email [email protected] Biao Huang, Immunological Analysis Laboratory of Academy of Life Sciences, Zhejiang Sci-Tech University, Hangzhou, 310016, People’s Republic of China, Tel +86 0571-86843187, Email [email protected]
Purpose: The present study aimed to evaluate the clinical value of Combined Detection of serum soluble T-cell immunoglobulin 3 (sTim-3) with carcinoembryonic antigen (CEA) or glycotype antigen 19– 9 (CA19-9) for Postoperative Recurrence of Colorectal Cancer (CRC) Diagnosis.
Patients and Methods: The serum sTim-3 was measured by highly sensitivity TRFIA, and serum CEA and CA19-9 were obtained through the collection of clinical data. Quantitative detection of serum sTim-3, CEA, CA19-9 in 90 patients after the CRC surgery (52 postoperative recurrence and 38 no-postoperative recurrence), 21 patients with colorectal benign tumors, and 67 healthy controls. To analyze the clinical diagnostic value of combined detection of sTim-3 with CEA or CA19-9 to test whether patients have recurrence after CRC surgery.
Results: The sTim-3 (15.94± 11.24ng/mL) in patients after CRC surgery was significantly higher than in healthy controls (8.95± 3.34ng/mL) and colorectal benign tumors (8.39± 2.28ng/mL) (P < 0.05), and sTim-3 (20.33± 13.04ng/mL) in CRC postoperative recurrent group was significantly higher than in the group without recurrence after CRC surgery (9.94± 2.36ng/mL) (P < 0.05). In terms of detecting postoperative recurrence after CRC surgery, combined detection of sTim-3 and CEA (AUC: 0.819, sensitivity: 80.77%, specificity: 65.79%), sTim-3 and CA19-9 test (AUC: 0.813, sensitivity: 69.23%, specificity: 97.30%) was significantly better than the CEA single test (AUC: 0.547, sensitivity: 63.16%, specificity: 48.08%) and CA19-9 single test (AUC: 0.675 sensitivity: 65.38%, specificity: 67.57%), Delong test P < 0.05.
Conclusion: The efficacy of CEA and CA19-9 single test was not optimal, and the combination of sTim-3 in serum could significantly improve the sensitivity and specificity of detecting patient recurrence after CRC surgery.
Keywords: serum tumor markers, Tim-3, time-resolved fluorescence immunoassay, colorectal cancer recurrence
Introduction
Colorectal malignancies (colorectal cancer, CRC) are one of the most common human malignancies,1 including colon malignancy and rectal malignancy. In recent years, the incidence of CRC (mainly colon malignancy) has been increasing year by year worldwide. In China, the increasing incidence trend of CRC is also very obvious. Most CRC radical cure requires surgical treatment, but according to the current known results,2,3 many patients after surgery metastasis and recurrence, about 75% of these people, about 1/3 for local recurrence, have the opportunity to surgery treatment, the other 2/3 of patients with local recurrence with multiple site metastasis have lost the opportunity of surgery. Therefore, early detection of metastasis and recurrence is the key to surgery, which mainly depends on regular follow-up. Therefore, a follow-up system for relevant cases should be established after colorectal cancer surgery. For example, in addition to the electronic colonoscopy, X-ray and CT scan every 3–6 months at the initial stage of surgery, some tumor markers should be carried out for the convenience of follow-up.
For postoperative follow-up monitoring of colorectal cancer recurrence and metastasis, such as CEA, CA19-9, CA-50, CA72-4, CA242,3 has been widely used in the monitoring of postoperative efficacy and prognosis evaluation of colorectal malignancies; however, their test efficacy in the evaluation of postoperative recurrence evaluation of colorectal malignancies is not ideal.4,5 At present, with the development and progress of related research on the combined detection of various serum tumor markers and the application practice in clinical adjuvant diagnosis, the clinical value of the combined detection of serum tumor markers for the adjuvant diagnosis of recurrence after CRC is gradually improving.6–9 Therefore, we need to explore biomarkers with better test efficacy to help evaluate the risk of postoperative recurrence and even death in patients after colorectal cancer surgery.
T cell immunoglobulin mucin molecule-3 (Tim-3) is a member of the TIM family, whose main physiological function is negative immune regulation. Some studies10 have shown that high levels of Tim-3 expression are present in many types of malignancies, so Tim-3 may play a very important role in the occurrence, development and outcome of malignant tumors.11–13 This study aims to evaluate the clinical value of serum sTim-3 for postoperative recurrence of colorectal malignancy by studying the combination test with CEA or CA19-9.
Research Methods
Study Subjects
Serum samples were selected from patients with malignant tumors treated in Zhejiang Xiaoshan Hospital from September 1, 2020 to December 31, 2022, including 90 patients after CRC surgery (52 patients with postoperative recurrence and 38 patients without postoperative recurrence) and 21 patients with benign colorectal tumors (all patients with colorectal polyps).67 healthy control cases, as the blank control group, were all healthy examination adults in the physical examination center of Zhejiang Xiaoshan Hospital.
Inclusion criteria for the experimental group were: (1) age ≥18 years; (2) clinical, pathological and gastrointestinal diagnosis, postoperative patients underwent radical tumor resection (1–7 years before blood collection); (3) postoperative group never received treatment before surgery (such as radiotherapy and chemotherapy, targeted, immunotherapy, etc.); (4) good hepatic and renal function and general condition.
Exclusion criteria of the experimental group: (1) patients with personal history of other malignant tumors except digestive tract malignancy; (2) patients with poor general condition and severe organ dysfunction (severe liver, kidney and cardiopulmonary dysfunction); (3) patients with personal history of severe neurological or mental diseases or coagulation or blood disorders and unable to cooperate with blood collection; (4) patients with may interfere with the level of target biological indicators: such as patients with severe infection, patients with autoimmune-related diseases, etc.
In the experimental group, the clinical diagnosis criteria for recurrence and metastasis were: (1) the pathological biopsy confirmed recurrence or metastasis; (2) during the follow-up review, the patients had progressive gastrointestinal related symptoms and systemic symptoms, and the imaging diagnosis had two positive evidence in CT, MRI, ultrasound, PET-CT and other examinations.
In the experimental group, there was no recurrence and metastasis: (1) pathological biopsy confirmed no recurrence or metastasis; (2) during follow-up review, the patients had no progressive gastrointestinal symptoms and CT, MRI, ultrasound and PECT-CT.
Enrollment criteria for blank control group: (1) age 18 years; (2) all tumor markers such as serum CEA, CA12-5, CA19-9, CA 12–5 and CA72-4 were negative; (3) no history of digestive tract related diseases; (4) good bone marrow, kidney and liver function; (5) complete clinical relevant data.
This study was conducted strictly and properly based on the Declaration of Helsinki and approved by the Ethics Committee of Zhejiang Xiaoshan Hospital affiliated to Hangzhou Normal University (approval number 2021–011). Informed consent was obtained from all registered subjects before participating in the experiment.
Specimen Collection and Preservation
The study subjects were identified strictly according to the inclusion and exclusion criteria, and the relevant serum samples were collected, and the general conditions of the patients included gender, age and other blood test results. For study subjects, 5mL venous blood was collected after 8–10 hours, then centrifuged at 4000rpm for 5min, and then serum was collected as serum samples for the study and stored in a-80°C refrigerator until use.
Experimental Methods
The TRFIA test methods of sTim-3 and TAT-2 have been established in the cooperative laboratory of my research group, and have certain test efficiency.14,15
- The coating process of antibody: the capture antibody was diluted to 2 ug/mL in coated buffer, and then added to the 96-well plate according to 100 uL/well, placed under 4°C condition overnight, then washed once, 150 uL blocking solution was added to each well, let and closed for two hours, pour the blocking solution, dry the plate surface, and stored in-20°C.
- Labeling process of antibody: place 300 uL of antibody to be labeled in the ultrafiltration tube, then add 200 uL of labeling buffer, centrifuge at 10,000 rpm in centrifuge, repeat this process for 8 times. Next, the antibody specimens were collected by adding 50 uL of labeling buffer and inverted the centrifuge tube to centrifugation for 1 min at 3000 rpm. Then, the collected antibodies were mixed with 60 ug of diethylamine triamine triacetic acid (DTTA) -Eu3+ for one night at 30°C. Next, the labeled antibodies were purity purified using Sephadex-G50 and, after collection, were stored at-20°C.
- The sTim-3 antigen was diluted to different concentrations of the standard product with the assay buffer, where 0 points is the assay buffer.
- Test the concentration of sTim-3 in serum: 100 uL standard or serum samples were added to 96-well plates coated with Tim-3 capture antibody, incubated at 37°C and shock conditions for 1 hour, and eluted twice with the plate washing machine.100 uL 1:1000-fold diluted Eu 3 + labeled Anti-Tim-3 McAb/Anti-TAT-2 McAb was added to each well, incubated at 37°C, shock conditions for 2 hours, and washed six times with a plate washing machine. Finally, 100 uL of enhancement solution was added to each well to detect the fluorescence value for 3min.
- According to the measured fluorescence value, draw the standard curve and calculate the content of sample sTim-3.
Statistical Treatment
Statistical processing was performed by using the SPSS 26.0 software. Quantitative data are expressed as the mean±standard deviation (SD), using the binary logistic regression, and the fitted probability variable was used to construct the receiver operating characteristic curve (ROC) curve for the combined test, and to further evaluate the validity of the test. ANOVA or T-test was used to analyze differences between groups. Statistical analyses were performed and graphs were performed using GraphPad Prism 8. The area under the curve (AUC) of the ROC chart describes the accuracy of the detection index: 1 indicates 100% sensitivity and specificity, and 0.5 indicates no discrimination. Using Delong test for hypothesis testing to compare the AUC differences of ROC models between groups. P < 0.05 was considered as a significant difference for all statistical results.
Results
General Situation
The inclusion criteria and exclusion criteria included 90 patients after the CRC surgery (52 patients with postoperative recurrence and 38 patients without postoperative recurrence) and 21 patients with benign colorectal tumors (all patients with colorectal polyps). And 67 healthy controls, which served as blank controls. Relevant data statistics are shown in Table 1.
 |
Table 1 Serum Indices of Control, Benign Colorectal Tumors, After Surgery for CRC (Including Recurrence and No Recurrence) |
Quantitative data were presented as mean±standard deviation, and differences were analyzed by analysis of variance and multiple comparison test. Each experimental group and control group, p < 0.05; CRC postoperative recurrence-free group, CRC postoperative recurrence group and colorectal benign tumor group, p < 0.05.
Assessment of the TRFIA Method
The TRFIA test method of sTim-3 has been established in the cooperative laboratory of my research group, and has a certain test efficiency.14,15 The parameters are as follows: the TRFIA method of sTim-3, detection sensitivity of 0.66 ng/mL, intra-batch coefficient of variation of 1.64%—4.68%, and between-batch coefficient of variation of 5.72%—9.32%.
Statistical results
sTim-3 and CEA and CA19-9 Content in Serum Samples of Each Group
As shown in Figure 1, the serum sTim-3 (15.94±11.24ng/mL) level in the postoperative CRC group was significantly higher than that of healthy controls (8.95±3.34ng/mL) and colorectal benign tumors (8.39±2.28ng/mL) (P < 0.05), and the serum sTim-3 (20.33±13.04ng/mL) level in the group with recurrence after CRC surgery was significantly higher than that in the group without recurrence after CRC surgery (9.94±2.36ng/mL) (P < 0.05).
 |
Figure 1 Serum sTim-3 levels in each group. |
Evaluation of Single Test and Combined Test Efficacy of sTim-3 and CEA or CA19-9
Based on Figure 2, statistical analysis was performed using the GraphPad Prism 8 tool to calculate the area under the curve (AUC) of the ROC map and to describe the sensitivity and specificity of the test index. By the calculation, the test efficacy of serum sTim-3 in each experimental group was respectively: colorectal benign tumor group (AUC: 0.541, Sensitivity: 58.71%, Specificity: 37.31%), postoperative group for colorectal cancer (AUC: 0.745, Sensitivity: 77.78%, Specificity: 56.72%), CRC postoperative recurrence group (AUC: 0.988, Sensitivity: 97.22%, Specificity: 97.01%), CRC postoperative recurrence-free group (AUC: 0.608, Sensitivity: 86.84%, Specificity: 37.31%).
 |
Figure 2 The ROC curve of a single test of serum sTim-3 in each group. |
It can be seen that the single test of serum sTim-3 has a good detection efficiency in patients with personal history of colorectal cancer (after colorectal cancer surgery), and the detection efficiency of postoperative recurrence diagnosis of colorectal cancer can reach a quite high level (AUC: 0.988, sensitivity: 97.22%, specificity: 97.01%).
As shown in Figure 3, the test efficacy of serum CEA in detecting postoperative recurrence of CRC (AUC: 0.547, sensitivity: 63.16%, specificity: 48.08%). It is not difficult to find that CEA, as a widely used serum tumor marker, CEA has very limited sensitivity and specificity, even for patients with recurrence after CRC, its sensitivity is only 63.16% and its specificity is only 48.08%.
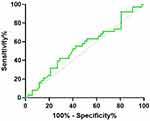 |
Figure 3 The ROC curve of a single test of serum CEA in detecting postoperative recurrence of CRC (Postoperative recurrence vs Postoperative recurrence-free). |
As shown in Figure 4, the test efficacy of serum CA19-9 in detecting postoperative recurrence of CRC (AUC: 0.675, sensitivity: 65.38%, specificity: 67.57%). Similarly, the sensitivity and specificity of CA19-9 are not high, although in patients with recurrence after CRC, its specificity is very low and has limited clinical reference value.
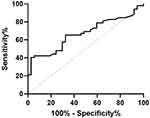 |
Figure 4 The ROC curve of a single test of serum CA19-9 in detecting postoperative recurrence of CRC (Postoperative recurrence vs Postoperative recurrence-free). |
The joint detection index uses binary logistic regression to fit the data and then plot the ROC curve. As shown in Figure 5, the combined test of serum sTim-3 and CEA in diagnosing recurrence after CRC surgery (AUC: 0.819, sensitivity: 80.77%, specificity: 65.79%). Using Delong test to test the hypothesis of AUC in two ROC models, we found significant differences between the two group, the AUC of Joint group was significantly higher than in the single CEA group, P<0.001 (α=0.05, two-sides, P = 0.00018). And we can find that, in contrast to the CEA single test (AUC:0.547, sensitivity:63.16%, specificity: 48.08%), the combination test of serum sTim-3 with CEA could significantly improve the detection efficiency of postoperative recurrence of CRC, particularly for sensitivity.
 |
Figure 5 Comparison of the combination of serum sTim-3 with CEA and a single CEA test in diagnosing recurrence after CRC surgery. |
The same as above, the joint detection index uses binary logistic regression to fit the data and then plot the ROC curve. As shown in Figure 6, the combined testing efficacy of serum sTim-3 with CA19-9 in postoperative recurrence group (AUC: 0.813, sensitivity: 69.23%, specificity: 97.3%). Using Delong test to test the hypothesis of AUC in two ROC models, we found significant differences between the two groups, the AUC of Joint group was significantly higher than in the single CA19-9 group, P<0.05 (α=0.05, two-sides, P = 0.034). The efficacy of sTim-3 and CA19-9 combined testing to detect recurrence after CRC surgery (AUC: 0.813, sensitivity: 69.23%, specificity: 97.3%) was compared with the combination of sTim-3 and CEA (AUC: 0.819, sensitivity: 80.77%, specificity: 65.79%). Among them, the specificity is significantly improved, while its sensitivity is too low. However, the higher sensitivity of the combined serum sTim-3 and CEA test was just complementary. When the two combined tests were performed for the same population, its test efficiency will achieve better results.
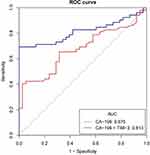 |
Figure 6 Comparison of the combination of serum sTim-3 with CA19-9 and a single CA19-9 test in diagnosing recurrence after CRC surgery. |
Discussion
Postoperative recurrence of CRC (colorectal malignancy) refers to the recurrence of secondary malignant tumors associated with other organs, including liver, lung, bone and other organs and (or) local recurrence. Local recurrence refers to the recurrence of organs or lymph nodes in the abdominal and pelvic areas except for distant organ metastasis. The postoperative recurrence of CRC will appear many clinical manifestations, such as pelvic floor area discomfort, changed stool habits, black stool, progressive wasting, progressive abdominal mass, etc. Therefore, regular follow-up review is particularly important for patients after colorectal cancer surgery. Epidemiological statistics suggest that most of the people at high risk of colorectal malignancy recurrence have recurrence within 2 years after surgery. Therefore, it is often recommended that patients after CRC surgery should be reviewed every 3 months within 2 years after surgery to evaluate the disease and recurrence of.16
However, the risk of recurrence after radical surgery in CRC is high. Overall, the recurrence and metastasis rate of postoperative CRC were relatively high. Therefore, for the patients after CRC surgery, especially in the high-risk group of postoperative recurrence, the early detection of malignant tumor recurrence and metastasis is of great significance to improve the long-term prognosis of patients. During the regular follow-up review of patients after CRC radical resection, the review of blood tumor markers has the advantages of convenience, non-invasive, and low technical requirements. In multiple clinical practical application of CRC postoperative follow-up guidelines or diagnosis and treatment specification are mentioned in CRC patients after postoperative and clinical follow-up review after radiotherapy and chemotherapy treatment, regular, regular review of serum tumor markers, to evaluate lesion recurrence or metastasis, including the serum CEA is the most widely used and clinical research suggest more accurate.16–18 This also demonstrates the importance of serum CEA for the assessment of recurrence in patients after CRC surgery. However, due to the high heterogeneity of colorectal malignancy, the efficacy of individual tumor markers for postoperative CRC is suboptimal.19 However, multiple tumor markers can be evaluated by combined detection to obtain more appropriate detection sensitivity and specificity, which may provide more valuable auxiliary diagnostic results for people with high risk of recurrence after colorectal cancer surgery.
CRC is also a common type of digestive tract tumors. Studies have shown that Tim-3 expression at the cell level, has a high level in colorectal malignant tumors, immune-related T cells. In addition, the higher expression of Tim-3 in helper T cells, the wider the tumor invasion, especially the deeper the infiltrating tissue depth, the greater the possibility of local and regional lymph node metastasis, and the higher the tumor grade and grade, suggesting the more serious the tumor progression. High expression of Tim-3 levels on the surface of T cells can weaken the immune response of related immune cells (especially helper T cells) and show negative regulation. The relevant inhibitory effect of Tim-3 on immunity indicates to some extent that Tim-3 levels are associated with the prognosis of the CRC tumor population, that is, the higher the level of Tim-3 expression, the worse the prognosis of the colorectal malignancy population.20–23 Therefore, the serum expression level of Tim-3 may also provide a reference for recurrence in patients after CRC surgery.24,25
Tumor markers mainly refer to the substances secreted or shed by tumor cells into body fluids or tissues. A single tumor marker has limited test efficacy in the diagnosis of gastrointestinal malignancy and the evaluation of postoperative recurrence, and its test sensitivity and (or) specificity are often not ideal. This is also consistent with our experimental results. Therefore, this study aims to provide a new method for the adjuvant diagnosis of gastrointestinal malignant tumor and postoperative recurrence through the combined detection of serum TIM-3, CEA and CA19-9, etc.
To evaluate the clinical value of serum sTim-3 and CEA or CA19-9 combined detection in the adjuvant diagnosis of colorectal cancer and postoperative recurrence. Based on the experimental data, We found that the serum sTim-3 (15.94±11.24ng/mL) level in patients after CRC surgery was significantly higher than that in healthy controls (8.95±3.34ng/mL) and patients with benign colorectal tumors (8.39±2.28ng/mL) (P < 0.05). This indicates that serum sTim-3 is of clinical significance in distinguishing between patients with a personal history of colorectal malignancy, healthy population and patients with benign colorectal tumors.
At the same time, we found that the serum sTim-3 (20.33±13.04ng/mL) level in the group with recurrence after CRC surgery was significantly higher than that in the group without recurrence after CRC surgery (9.94±2.36ng/mL) (P < 0.05), which also suggested that serum sTim-3 can be used as a monitoring indicator of recurrence after CRC surgery.
Besides, for the combined detection of serum tumor markers. CEA single testing the postoperative recurrence group of CRC (AUC: 0.547, sensitivity: 63.16%, specificity: 48.08%), has poor detection sensitivity and specificity. In the combination of serum sTim-3 and CEA for the postoperative recurrence group of CRC (AUC: 0.819, sensitivity: 80.77%, specificity: 65.79%) to test the efficacy at the level of sensitivity and specificity. All were superior to the CEA single detection.
The efficacy of sTim-3 and CA19-9 combined testing to detect recurrence after CRC surgery (AUC: 0.813, sensitivity: 69.23%, specificity: 97.3%) was compared with the combination of sTim-3 and CEA (AUC: 0.819, sensitivity: 80.77%, specificity: 65.79%). Among them, the specificity is significantly improved, while its sensitivity is too low. However, the higher sensitivity of the combined serum sTim-3 and CEA test was just complementary. When the two combined tests were performed for the same population, its test efficiency will achieve better results. It can provide new ideas for evaluating the combined detection of serum tumor markers for recurrence after CRC surgery.
However, the main limitation of current research work is that the sample size still needs to be further expanded. We will continue to collect experimental specimens and data to enrich research results. In order to incorporate sTim-3 into routine clinical applications, on the one hand, we will further promote the application of high-throughput TRFIA detection reagents to improve detection efficiency and popularity, and on the other hand, we will continue to track the subsequent recurrence of non recurrent CRC patients in the experimental group. Furthermore, we will verify our experimental results that the level of Tim-3 in postoperative recurrence of CRC is positively correlated with disease progression.
Conclusion
The serum level of sTim-3 in patients after CRC surgery was significantly higher than that of the blank control group and colorectal benign tumor group. Meanwhile, the serum level of sTim-3 in patients with a recurrence after CRC surgery was significantly higher than that of the postoperative recurrence-free group of CRC. The serum level of sTim-3 could predict the recurrence of patients after CRC surgery.
The single CEA and CA19-9 tests were not very effective, and the combined serum sTim-3 test could significantly improve the sensitivity and specificity of patient recurrence after CRC surgery. In addition, the combination of sTim-3 and CEA or sTim-3 and CA19-9 complementary to each other in the sensitivity and specificity, which can provide a new idea for evaluating the combination detection of serum tumor markers in auxiliary diagnosis of postoperative recurrence of CRC.
Funding
Funding was provided by the Social Development Fund of Zhejiang Province (No. LGF20H200008), the Key Research and Development Program of Zhejiang Province (No. 2020C03066), Xiaoshan District Major Science and Technology Plan Project of Zhejiang Province (No. 2020207).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Richman DM, Tirumani SH, Hornick JL, et al. Beyond gastric adenocarcinoma: multimodality assessment of common and uncommon gastric neoplasms. Abdominal Radiology. 2017;42(1):124–140. doi:10.1007/s00261-016-0901-x
2. Chunxiao W, Kai G, Yangming G, et al. Analysis of the onset and death of colorectal cancer in China in 2015. Chin J Cancer. 2020;30(4):241–245.
3. Yachong J, Xuchu Z, Jiajie W, Dezhi K, Bin Z. Clinical diagnostic value of combined serum tumor marker measurement for nodal recurrence and metastasis detection. Mark Immun Clin. 2021;28(07):1106–1109.
4. Hao Y, Jiaming L, Kun Z, Xin Z, Kun H. The clinical value of postoperative serum CEA with CA19-9-9, YKL-40, CRP and IL-6 in predicting recurrence and survival of colorectal cancer. Mod Med. 2022;50(09):1143–1150.
5. Konishi T, Shimada Y, Hsu M. et al.Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018;4(3):309–315. doi:10.1001/jamaoncol.2017.4420
6. Sjin JK, Kim HC, Lee WY, et al. High preoperative serum CA 19-9 levels can predict poor oncologic outcomes in colorectal cancer patients on propensity score analysis. Ann Surg Treat Res. 2019;96(3):107–115. doi:10.4174/astr.2019.96.3.107
7. Song Z, Yuan HL, Yeqiang Q, Yunfei C, Xiaoxia Z, Huan Z. Diagnostic value of the combined detection of different serum tumor markers in digestive tract malignancies. Pharmaceut Oncol. 2014;4(04):282–285.
8. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi:10.1016/S0140-6736(20)31288-5
9. Wang H. Significance of combined detection of tumor markers in the diagnosis of cancer. J Basic Clin Oncol. 2013;26(4):357–358.
10. Juan Z, Zhenghao Z, Nannan P, Jialin Z, Jianhua Q. Progress in studying the expression and mechanism of Tim-3 in malignant tumors. J Pract Med. 2017;33(21):3667–3670.
11. Xu B, Yuan L, Gao Q, et al. Circulating and tumor⁃infiltrating Tim- 3 in patients with colorectal cancer. Oncotarget. 2015;6(24):20592–20603. doi:10.18632/oncotarget.4112
12. Zhou E, Huang Q, Wang J, et al. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol. 2015;8(7):8018–8027.
13. Huang YH, Zhu C, Kondo Y, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386–390. doi:10.1038/nature13848
14. Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011;71(21):6567–6571. doi:10.1158/0008-5472.CAN-11-1487
15. Chen M, Wang L, Wang Y, et al. Soluble Tim3 detection by time-resolved fluorescence immunoassay and its application in membranous nephropathy. J Clin Lab Anal. 2020;34(6):23248.
16. Difu P, Lamei G, Anming F, Kean Z. Report of 60 cases of reoperation of colorectal cancer. Chin J General Surg. 2007;09:849–851.
17. The National Health Commission, PRC. China code for diagnosis and treatment of colorectal cancer (2020 edition). Chin J Surg. 2020;58(8):561–585.
18. Zhang Y, Cai P, Liang T, et al. TIM-3 is a potential prognostic marker for patients with solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(19):31705–31713. doi:10.18632/oncotarget.15954
19. Mokhles S, Macbeth F, Farewell V, et al. Meta- analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg. 2016;103(10):1259–1268. doi:10.1002/bjs.10233
20. You YN, Hardiman KM, Bafford A, et al. The American society of colon and rectal surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum. 2020;63(9):1191–1222. doi:10.1097/DCR.0000000000001762
21. Lech G, Slotwinski R, Slodkowski M, et al. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastro Enterol. 2016;22(5):1745–1755. doi:10.3748/wjg.v22.i5.1745
22. Kotzev A. CA19-9 in follow-up of patients with colorectal cancer: Should it stay or should it go. J Buon. 2019;24(3):1310–1311.
23. Papagrigoriadis S. Follow-up of patients with colorectal cancer: the evidence is in favour but we are still in need of a protocol. Int J Surg. 2007;5(2):120–128. doi:10.1016/j.ijsu.2006.04.004
24. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
25. Huang YK, Yu JC, Kang WM, et al. Significance of serum pepsinogens as a biomarker for gastric cancer and atrophic gastritis screening: a systematic ic review and meta-analysis. PLoS One. 2015;10(11):14208.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
