Back to Journals » Journal of Inflammation Research » Volume 16
Clinical Utility of the Neutrophil-to-Bilirubin Ratio in the Detection of Disease Activity in Ulcerative Colitis
Authors Huang X, Pan Y, Liu Y, Zhou Z, Zhang Y, Gao C, He C
Received 5 April 2023
Accepted for publication 11 June 2023
Published 16 June 2023 Volume 2023:16 Pages 2549—2559
DOI https://doi.org/10.2147/JIR.S413644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xijing Huang,1,* Yan Pan,1,* Ya Liu,1,* Zhou Zhou,1 Yinghui Zhang,1 Caiping Gao,1 Chong He1,2
1Department of Gastroenterology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 2Clinical Immunology Translational Medicine Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chong He, Email [email protected]
Background: Ulcerative colitis (UC) is a chronic relapsing remitting form of inflammatory bowel disease (IBD). Current disease monitoring includes evaluation of symptoms, fecal calprotectin, and colonoscopy. Due to limited availability of the latter two modalities in China, we sought a readily available, inexpensive, disease monitoring laboratory assessment. We recently identified a novel serological index (the neutrophil-to-bilirubin ratio, NBR) for monitoring disease activity in Crohn’s disease. However, the clinical significance has not been evaluated in UC. Here, we aimed to verify the hypothesis that NBR might be useful in monitoring clinical and endoscopic activity in patients with UC.
Methods: To test our hypothesis, we conducted a single-center, retrospective study including a total of 188 patients with UC and 145 non-IBD controls. NBR was calculated to determine its practical value in monitoring disease activity (including clinical and endoscopic activity). Disease activity of UC was determined by the partial Mayo score and the Mayo endoscopic score (MES) system.
Results: NBR was significantly higher in patients with UC than that in controls (12.10, IQR: 9.85– 16.69 versus 5.06, IQR: 3.94– 6.55; p < 0.001) and showed positive correlations with clinical and endoscopic disease activity in UC. Additionally, NBR was significantly lower in patients with endoscopic mucosal healing (MH) than that in those without endoscopic MH (8.81, IQR: 6.67– 11.67 versus 13.51, IQR: 11.04– 18.71; p < 0.001). Serial evaluation of NBR in a subset of patients demonstrated that NBR was significantly decreased during the MH stage compared with that during the endoscopically active stage.
Conclusion: Our study suggests that NBR may be a promising candidate for assessing disease activity in UC, with potential for widespread clinical use and significant clinical implications.
Keywords: ulcerative colitis, bilirubin, neutrophil-to-bilirubin ratio, mucosal healing
Introduction
Ulcerative colitis (UC) is a chronic and disabling inflammatory bowel disease (IBD) characterized by an inflammatory condition of the colon and rectum. The prevalence of UC is rapidly growing worldwide and rising health expenditures,1–3 leading to a substantial healthcare burden. It costs approximately three-fold more in patients with IBD than non-IBD controls regarding both direct and out-of-pocket costs of care,3 which in turn limits their access to further testing and treatment, particularly in low- and lower-middle-income countries.4
Endoscopic examinations remain the diagnostic gold standard for the assessment of endoscopic and histological activity in IBD. However, endoscopy is invasive, expensive and time-consuming, thereby limiting access and utilization.5 Endoscopy has also been reported to be a high-risk procedure for SARS-CoV-2 transmission.6 The rate of endoscopic examination has been greatly decreased for patients with IBD since the COVID-19 outbreak.7 Given the advantage of noninvasiveness, serological and fecal biomarkers that can accurately reflect the endoscopic and histological activity in IBD have become a resurgent interest in the IBD area.
To date, the diagnosis and management of IBD have received increasing attention worldwide. The identification of non-invasive, sensitive, disease-specific, and affordable biomarkers for the diagnosis and activity assessment of IBD, as well as individualized therapies, is of great significance.8 Fecal calprotectin (FCP) has been reported to be useful in various situations such as diagnosis of IBD, correlation with endoscopic severity, assessment of therapeutic effect, and prediction of relapse.9 However, since an FCP test costs 10 times more than a blood cell test, and 6 times more than a serum biochemical test in China, its application is relatively limited, especially in underdeveloped areas, such as Sichuan province. In addition, serological markers are widely used in the clinical management of IBD. The most intensively evaluated serum-based marker was C-reactive protein (CRP) and frequently done as a reference for novel potential markers. However, it has been mentioned that CRP lacks diagnostic accuracy needed for clinical decision-making in patients with UC.
Recently, we identified a novel serological index, the neutrophil-to-bilirubin ratio (NBR), which was effective in monitoring disease activity and predicting response to infliximab (IFX) treatment in patients with Crohn’s disease (CD).10 However, the use of NBR in UC has not yet been reported. We thus hypothesized that NBR might be useful in monitoring clinical and endoscopic activity in patients with UC.
To test our hypothesis, we conducted a single-center, retrospective study that included patients with UC. We analyzed NBR levels and evaluated their correlation with clinical and endoscopic activity. Our study has demonstrated a significant correlation between NBR and both clinical and endoscopic disease activity in patients with ulcerative colitis (UC), which highlights its potential clinical utility for monitoring disease progression. Moreover, NBR may be a non-invasive, cost-effective, easily measurable index for personalized treatment and long-term outcome prediction in UC patients.
Materials and Methods
Participants
The study protocol was approved by the Ethics Committee of the Sichuan Provincial People’s Hospital, Chengdu, China (No.201685, 2020204), and it conformed to the principles of the Declaration of Helsinki. The study aimed to investigate the potential role of NBR for assessing disease activity during the disease course in UC patients. We collected patients’ clinical and laboratory characteristics via reviewing their medical records. The study included 188 patients with UC from the Department of Gastroenterology, Sichuan Provincial People’s Hospital, Chengdu, China, between January 2018 and May 2022. The inclusion criteria for UC patients were a confirmed diagnosis of UC based on conventional criteria, including clinical signs, endoscopic findings, and histological criteria.11 Exclusion criteria for UC patients included the presence of other gastrointestinal diseases, cancers, infections, other systemic autoimmune diseases, hypertension, diabetes, hematopoietic system disease, hepatobiliary disease, and coagulation abnormalities. Additionally, to evaluate the potential usefulness of NBR in UC patients, we also retrospectively included 145 non-IBD controls who underwent routine physical examinations in the hospital during the study period. The inclusion criteria for controls were being age- and gender-matched with UC patients, and the exclusion criteria for controls were excessive alcohol consumption, hematopoietic system disease, hepatobiliary disease, coagulation abnormalities, hypertension, diabetes, infections, other systemic autoimmune diseases, other gastrointestinal diseases, and cancers. The collection and analysis of data were conducted in a blinded and randomized manner to reduce possible bias and ensure the integrity of the study.
Evaluation of Disease Activity of UC
The disease extent in patients with UC was determined based on the Montreal classification.12 The partial Mayo score system (p-Mayo) was employed to evaluate clinical disease activity of UC.13 Clinical activity of UC was defined as follows: remission, a p-Mayo score of ≤2; mild, a p-Mayo score of ≥3 and <5; moderate, a p-Mayo score of ≥5 and <7; severe, a p-Mayo score of ≥7. The evaluation of endoscopic activity of UC was performed by experienced gastroenterologists who had been practicing endoscopy for more than 5 years and had previous experience with various IBD scores. All the gastroenterologists belonged to our clinical center, the Department of Gastroenterology at Sichuan Provincial People’s Hospital, which specializes in IBD management. They were well versed in clinical trials that included endoscopic evaluations. All the gastroenterologists underwent a training session in which they reviewed the endoscopic scoring system used in the study and received guidelines and instructions on the methodology of the scoring system to standardize the interpretation of endoscopic images and the assignment of scores. The Mayo endoscopic score (MES) system was employed to assess the endoscopic appearance in patients with UC, which was based on mucosal erythema, vascular pattern, friability, and ulceration.14 The MES ranges from 0 to 3, with higher scores indicating more severe disease activity. A score of 0 indicates a normal mucosal appearance, while a score of 3 indicates severe disease with ulcers, spontaneous bleeding, and loss of vascular pattern. Endoscopic MH was defined as a MES of 0 or 1, and endoscopic complete MH was defined as a MES of 0.15
Statistical Analysis
All statistical analysis was performed using a Prism software Version 8.4 (GraphPad Software, San Diego, California, USA). Kolmogorov–Smirnov test was performed for checking data normality. NBR was defined as the ratio of blood neutrophil percentage-to-serum bilirubin level. Differences of NBR between groups were examined by the Mann–Whitney test (between two groups) or the Kruskal–Wallis test (among three or four groups), which was followed by the Dunn’s multiple comparisons test to determine the differences of each group with every other group. The discriminating performance of FCP, CRP, and NBR in indicated scenarios was determined by receiver operator curves (ROC) analysis. The area under the ROC curve (AUC) between 0.5 and 0.6 suggests the bad accuracy of a diagnostic test. AUC between 0.6 and 0.7 suggests sufficient accuracy, between 0.7 and 0.8 good accuracy, between 0.8 and 0.9 very good accuracy, whereas AUC higher than 0.9 suggests the excellent accuracy of a diagnostic test.16 To determine the difference of NBR in the same patients but in different disease stage, the Wilcoxon signed rank test was performed. p value <0.05 was set as statistically significant.
Results
Basic Characteristics and Clinical Parameters of the Participants
In the present study, a cohort of 188 patients with UC and 145 non-IBD controls were enrolled. Demographic and clinical characteristics of both groups are depicted in Table 1. The female proportion of patients and control group was 51.1% (96 of 188) and 46.2% (67 of 145), respectively (p = 0.47). The median age of patients with UC was 38.0 years old (yr) (interquartile range: 29.3 to 46.6 yr). Control subjects (median age: 36.0, interquartile range: 31.0 to 44.5 yr) were age-matched with patients. Regarding UC extent, based on the Montreal classification system, 22.3% had proctitis (E1), 35.1% had left-sided colitis (E2), and 42.6% had extensive colitis (E3). Neutrophil percentages were examined by complete blood cell tests, and total bilirubin levels were detected by serum biochemistry tests. NBR was calculated as the ratio of neutrophil percentages over serum total bilirubin levels. NBR was 2-fold higher in patients with UC than that in controls (12.10, IQR: 9.85–16.69 versus 5.09, IQR: 3.94–6.55) (Table 1 and Figure 1A).
 |
Table 1 Demographics and Clinical Parameters of UC Patients and Controls |
NBR Reflects Clinical Disease Activity in UC
Since the treatment of UC is complex and the monitoring of disease activity is required in order to evaluate efficacy in achieving disease remission, we moved forward to explore whether NBR could be used to assess UC activity. According to p-Mayo scores, patients with UC were divided into 4 groups: 1 remission group and 3 active groups (Mild, Moderate, and Severe). The values of NBR were compared among all groups, showing that NBR in patients with active UC was markedly higher than that in patients in clinical remission (Figure 1B). Since previous research has demonstrated a role of bilirubin alone to distinguish active IBD,17,18 we compared the ability of NBR and bilirubin alone in this scenario. As shown in Supplementary Figure 1A, NBR (AUC = 0.8333) showed a better performance to differentiate active UC from the remission than bilirubin (AUC = 0.7439) or neutrophil alone (AUC = 0.7613).
Furthermore, patients with active UC were further categorized into three groups according to p-Mayo score (Figure 1C). Patients with severe UC (a p-Mayo score of ≥7) had the highest levels of NBR, followed by those with moderate UC (a p-Mayo score of 5 or 6). Patients with mild UC (a p-Mayo score of 3 or 4) showed lower NBR levels than the moderate or severe group. We also evaluated NBR levels in a subgroup of patients with UC whose normal CRP levels (≤10 mg/L, n = 38) and found that NBR levels were markedly elevated in patients with clinically active UC compared to those in remission (Figure 1D). These data demonstrate that NBR indicates the clinical disease severity of UC, even in patients with normal CRP levels.
Correlations of NBR with Endoscopic Disease Activity in UC
The disease activity of UC can be evaluated on several aspects: clinical, biochemical, radiographic, endoscopic, and histological. However, a patient with UC does not necessarily achieve endoscopic remission in the condition of clinical remission, and vice versa, a patient might complain gastrointestinal symptoms, probably due to concomitant functional disease of the gut, when there are no visible endoscopic inflammatory changes. Therefore, endoscopic remission remains the gold standard in the management of IBD.19–22 Next, we sought to investigate whether NBR was correlated with endoscopic disease activity in patients with UC. As shown in Figure 2A, endoscopic activity is significantly correlated with increased NBR. Elevated serum CRP levels were also significantly correlated with endoscopic activity (Figure 2B); however, unlike NBR, patients with a MES score of 0 and 2 showed no significant differences in CRP and neither did patients with a MES score of 1 and 2 (Figure 2B), suggesting that NBR might be more practical for discriminating endoscopic activity than CRP.
In the current study, endoscopic MH was defined as MES ≤ 1 (either completely normal or quiescent mucosa). We found that NBR was significantly lower in patients achieving MH (MES 0/1) than in those without MH (MES 2/3) (Figure 2C). As shown in Supplementary Figure 1B, NBR (AUC = 0.8152) showed a better performance to differentiate endoscopic MH (+) from MH (-) than bilirubin (AUC = 0.7217) or neutrophil alone (AUC = 0.7263). Additionally, a recent comprehensive analysis indicated that achieving complete MH (CMH, MES = 0) was more critical to avoid disease recurrence.23 Therefore, we assessed whether NBR could detect CMH in patients with UC and found that NBR in patients who achieved CMH was significantly lower than that in those without CMH (MES=1/2/3) (Figure 2D).
Efficacy of NBR as a Serological Marker for Monitoring MH in Patients with UC
Since we revealed a potential role of NBR as a biomarker in UC, we next evaluated its diagnostic accuracy in monitoring clinical activity and MH by ROC curve and AUC analysis. As shown in Figure 3A, NBR exhibited higher AUC than CRP for the determination of clinical remission by p-Mayo score. Moreover, the AUC for NBR was higher than that for CRP for the prediction of endoscopic MH (Figure 3B) and CMH (Figure 3C). We further detected fecal calprotectin (FCP) levels in a subgroup of patients (n = 94). In regard to the prediction of clinical activity and endoscopic MH, the AUC for NBR and FCP was comparable, although FCP showed a better performance in the determination of endoscopic CMH. Collectively, these findings strengthen our evidence that NBR is an effective serological biomarker for predicting MH in UC.
NBR as a Biomarker to Monitor MH During the Clinical Course of Patients with UC
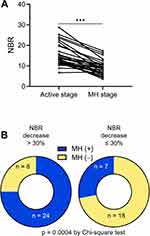
Next, we further determined the application values of NBR for the assessment of MH in UC by monitoring changes in NBR levels during the course of disease. We retrospectively included a subset of patients (n = 57) with active mucosal inflammation (a MES score of 2 or 3). During a median follow-up period of 16 months (interquartile range: 8 to 25 months), 31/57 patients achieved endoscopic MH (a MES score of 0 or 1). Serial NBR evaluation was performed at both the beginning and the end of follow-up. We analyzed changes of NBR levels in patients achieved endoscopic MH and found that NBR levels were significantly higher at the time of endoscopically active disease than at the time of MH (Figure 4A). Moreover, in these longitudinally followed patients, analysis of the association between changes of NBR levels and achievement of MH showed that 75% (24/32) patients with a decrease >30% in NBR established endoscopic MH, while only 28% (7/25) patients with a decrease ≤30% did (Figure 4B). These data indicate that NBR might be effective to reflect mucosal status during the disease course of patients with UC.
Discussion
To our best knowledge, this is the first study to investigate the clinical utility of NBR to help assess clinical and endoscopic disease activity of UC. The principal findings of our study were that NBR was significantly higher in patients with UC than that in controls and showed significantly positive associations with the clinical and endoscopic activity.
In the management of inflammatory bowel disease (IBD), biomarkers are increasingly utilized for diagnosis, monitoring, and individualized therapies. However, no biomarkers have been found that can replace endoscopy for these purposes. While some inflammatory biomarkers such as CRP and FCP have been validated in large populations, most biomarkers require further validation. The discovery of appropriate biomarkers could reduce the need for expensive and invasive endoscopy, which would decrease the healthcare burden on patients and clinicians. While blood routine examination markers such as white blood cell (WBC), neutrophil, and lymphocyte are commonly used as inflammatory indicators in clinical practice, their low sensitivity and specificity make it difficult to predict disease activity or progression using a single biomarker.24 In addition to such basic parameters, recent studies have focused on the combination of two parameters and have highlighted the potential value of the neutrophil-to-lymphocyte ratio (NLR),25 platelet-to-lymphocyte ratio (PLR),26 and lymphocyte-to-monocyte ratio (LMR)27 for assessing disease activity and predicting therapeutic effects. For example, NLR and PLR are elevated in active UC patients compared to inactive patients and healthy controls, indicating that an index that combines two blood parameters might be more useful than each single parameter.
Neutrophilic accumulation in inflamed mucosa tissues in the alimentary tract of IBD patients is a hallmark of the disease and intimately associated with disease activity.28 Moreover, disappearance of mucosal neutrophils is correlated with an improvement of clinical outcome. Therefore, the necessity of neutrophilic infiltration evaluation has been highlighted by ECCO guidelines for the histological diagnosis of this disease and its activity.29 On the other hand, bilirubin is generated during the breakdown of heme by the sequential action of heme oxygenase and biliverdin reductase. Bilirubin has been documented to play anti-oxidative and anti-inflammatory roles in many disorders, including colitis.30–34 Although high levels of serum bilirubin have known to be cytotoxic, multiple clinical reports have shown an inverse association between the risk of IBD and bilirubin levels. For, example, patients with the Gilbert’s polymorphism (who have a buildup of unconjugated bilirubin in their blood) have a decreased risk of CD,35,36 and patients with IBD show significantly lower levels of bilirubin than controls. Decreased bilirubin levels indicate higher CRP, ESR, fecal calprotectin and disease activity scores.18 Therefore, in the current study, we “augmented” the usefulness of neutrophil and bilirubin by adjusting to each other (the ratio of neutrophil-to-bilirubin) and investigated whether NBR might be more useful in monitoring clinical activity and MH in patients with UC than neutrophil or bilirubin alone.
Recent studies suggest that clinical remission may not be the optimal initial focus of UC therapy. As it is known that symptoms and the presence or activity of mucosal inflammation do not always correlate, therapeutic strategies based solely on symptoms may not provide the best management for UC.37 Patients may experience symptom relief while still having active mucosal inflammation, leading to under-treatment and a higher risk of recurrence. Conversely, some patients may continue to have symptoms despite there being no significant inflammation in the gastrointestinal mucosa, which may lead to the abandonment of current therapy or unnecessary replacement or escalation. Accumulating evidences have emphasized the importance of achieving MH in the management of UC. Mucosal healing is thought to be associated with a reduced risk of disease relapse and complications and an important predictor of long-term outcomes in UC patients. Achieving MH was associated with a lower risk of adverse outcomes such as hospitalization, surgery, and cancer.38–40 Furthermore, mucosal healing has been shown to improve quality of life and reduce the need for medication. Therefore, achieving endoscopic remission or MH is now considered the primary therapeutic goal.19 Despite the growing recognition of the importance of MH, achieving this goal remains a challenge in clinical practice. Patients with UC may require more aggressive therapy to achieve MH, such as early use of biologic agents or combination therapy. Close monitoring of disease activity and treatment response is also crucial to ensure that patients are on the right treatment path to achieve MH.
Although endoscopy is currently the most effective method for detecting mucosal inflammation in patients with UC, its clinical application is often limited due to its invasiveness, high cost, and the need for experienced endoscopists and expensive equipment. Furthermore, patients often experience discomfort, embarrassment, and inconvenience due to colonoscopy and bowel preparation.41,42 As a result, researchers have been actively searching for non-invasive biomarkers that are inexpensive, easily accessible, and accurate for evaluating endoscopic activity. To date, a variety of serological and fecal biomarkers have been investigated for their potential in assessing endoscopic activity.5,8 In this context, we have demonstrated that NBR may be a useful biomarker for monitoring endoscopic activity in patients with UC, particularly during long-term follow-up. Unlike other biomarkers, NBR combines two easily measurable parameters and has the potential for widespread clinical use. In addition, the use of NBR for monitoring endoscopic activity in UC patients may have significant clinical implications. It could potentially reduce the need for frequent endoscopic examinations, which are burdensome for patients, expensive, and require experienced endoscopists. Furthermore, NBR could facilitate earlier detection of disease activity and prompt adjustments to treatment strategies, potentially leading to better long-term outcomes for UC patients.
In spite of these findings, some limitations in our study need mentioning: 1) FCP is the current gold standard for non-invasive monitoring disease activity. Although we compared NBR and FCP in the current study, the sample size was small. The comparison needs to be further evaluated in greater depth in terms of disease activity monitoring in the following studies; 2) the sample size of this study is relatively small, so single-center or multi-center studies involving a much larger sample size are required to validate the clinical significance of NBR.
In conclusion, our findings suggest that NBR could be a rapid, non-invasive, and cost-effective tool for assessing disease activity in UC patients, particularly in resource-limited areas. Further studies are needed to validate the utility of NBR in larger patient cohorts and to evaluate its potential as a surrogate marker for MH in UC.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board for Clinical Research of Sichuan Provincial People’s Hospital (No.201685, 2020204). All subjects were well informed about the study and potential risk and signed an informed consent before participation.
Funding
This work was granted by the National Natural Science Foundation of China (82070985) and Sichuan Science and Technology Program grants (2021JDJQ0044).
Disclosure
The authors declare no competing interests.
References
1. Alatab S, Sepanlou SG, Ikuta K; Collaborators, G.B.D.I.B.D. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi:10.1016/S2468-1253(19)30333-4
2. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi:10.1038/nrgastro.2015.150
3. Park KT, Ehrlich OG, Allen JI, et al. The cost of inflammatory bowel disease: an initiative from the crohn’s & colitis foundation. Inflamm Bowel Dis. 2020;26:1–10. doi:10.1093/ibd/izz104
4. Rajbhandari R, Blakemore S, Gupta N, et al. Crohn’s disease in low and lower-middle income countries: a scoping review. World J Gastroenterol. 2020;26:6891–6908. doi:10.3748/wjg.v26.i43.6891
5. Soubieres AA, Poullis A. Emerging biomarkers for the diagnosis and monitoring of inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:2016–2022. doi:10.1097/MIB.0000000000000836
6. Sunakawa H, Yoda Y, Takahashi M, et al. A prospective study on the risk of transmission due to droplets and aerosols during an endoscopic procedure and the usefulness of extraoral suction devices. Dig Endosc. 2022;2022:1.
7. Chebli JMF, Queiroz NSF, Damião AOMC, et al. How to manage inflammatory bowel disease during the COVID-19 pandemic: a guide for the practicing clinician. World J Gastroenterol. 2021;27:1022–1042. doi:10.3748/wjg.v27.i11.1022
8. Caire MT, Strauss AT, Kumar A, Brady P. Emerging biomarkers for the diagnosis and monitoring of inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:E46. doi:10.1097/MIB.0000000000000973
9. Caviglia GP, Ribaldone DG, Rosso C, et al. Fecal calprotectin: beyond intestinal organic diseases. Panminerva Med. 2018;60:29–34. doi:10.23736/S0031-0808.18.03405-5
10. Zhou Z, Zhang Y, Yang X, et al. Clinical significance of novel neutrophil-based biomarkers in the diagnosis and prediction of response to infliximab therapy in Crohn’s disease. Front Immunol. 2022;13:865968. doi:10.3389/fimmu.2022.865968
11. Zhou Z, Zhang Y, Pan Y, et al. A novel neutrophil-based biomarker to monitor disease activity and predict response to infliximab therapy in patients with ulcerative colitis. Front Med. 2022;9:872831. doi:10.3389/fmed.2022.872831
12. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi:10.1136/gut.2005.082909
13. Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. doi:10.1002/ibd.20520
14. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi:10.1053/j.gastro.2006.12.038
15. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi:10.1056/NEJM198712243172603
16. Simundic AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203–211.
17. Su Q, Li X, Mo W, Yang Z. Low serum bilirubin, albumin, and uric acid levels in patients with Crohn’s disease. Medicine. 2019;98:e15664. doi:10.1097/MD.0000000000015664
18. Zhao X, Li L, Li X, et al. The relationship between serum bilirubin and inflammatory bowel disease. Mediators Inflamm. 2019;2019:5256460. doi:10.1155/2019/5256460
19. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi:10.1136/gutjnl-2012-302830
20. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi:10.1093/ecco-jcc/jjy113
21. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi:10.1053/j.gastro.2003.11.010
22. Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–413. doi:10.1053/j.gastro.2003.11.014
23. Naganuma M, Aoyama N, Suzuki Y, et al. Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohns Colitis. 2016;10:828–836. doi:10.1093/ecco-jcc/jjv208
24. Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149:1275–1285 e1272. doi:10.1053/j.gastro.2015.07.003
25. Torun S, Tunc BD, Suvak B, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36:491–497. doi:10.1016/j.clinre.2012.06.004
26. Akpinar MY, Ozin YO, Kaplan M, et al. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis. J Med Biochem. 2018;37:155–162. doi:10.1515/jomb-2017-0050
27. Cherfane CE, Gessel L, Cirillo D, Zimmerman MB, Polyak S. Monocytosis and a low lymphocyte to monocyte ratio are effective biomarkers of ulcerative colitis disease activity. Inflamm Bowel Dis. 2015;21:1769–1775. doi:10.1097/MIB.0000000000000427
28. Zhou GX, Liu ZJ. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J Dig Dis. 2017;18:495–503. doi:10.1111/1751-2980.12540
29. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–851. doi:10.1016/j.crohns.2013.06.001
30. He J, Jiang G, Li X, et al. Bilirubin represents a negative regulator of ILC2 in allergic airway inflammation. Mucosal Immunol. 2022;15:314–326. doi:10.1038/s41385-021-00460-0
31. Liu Y, Li P, Lu J, et al. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2008;181:1887–1897. doi:10.4049/jimmunol.181.3.1887
32. Zucker SD, Vogel ME, Kindel TL, et al. Bilirubin prevents acute DSS-induced colitis by inhibiting leukocyte infiltration and suppressing upregulation of inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2015;309:G841–854. doi:10.1152/ajpgi.00149.2014
33. Zhao C, Huang H, Pan Q, et al. Unconjugated Bilirubin attenuates DSS-Induced colitis potentially via enhancement of bilirubin reabsorption. Front Pharmacol. 2021;12:654808. doi:10.3389/fphar.2021.654808
34. Longhi MS, Vuerich M, Kalbasi A, et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight. 2017;2. doi:10.1172/jci.insight.92791
35. de Vries HS, Te Morsche RH, Jenniskens K, Peters WH, de Jong DJ. A functional polymorphism in UGT1A1 related to hyperbilirubinemia is associated with a decreased risk for Crohn’s disease. J Crohns Colitis. 2012;6:597–602. doi:10.1016/j.crohns.2011.11.010
36. Lenicek M, Ďuricová D, Hradsky O, et al. The relationship between serum bilirubin and Crohn’s disease. Inflamm Bowel Dis. 2014;20:481–487. doi:10.1097/01.MIB.0000440817.84251.98
37. Rogler G, Vavricka S, Schoepfer A, Lakatos PL. Mucosal healing and deep remission: what does it mean? World J Gastroenterol. 2013;19:7552–7560. doi:10.3748/wjg.v19.i43.7552
38. Froslie KF, Jahnsen J, Moum BA, Vatn MH, Group I. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi:10.1053/j.gastro.2007.05.051
39. Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483–489 e483. doi:10.1016/j.cgh.2010.12.028
40. Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–1816. doi:10.1136/gut.2003.038505
41. Navaneethan U, Parasa S, Venkatesh PG, Trikudanathan G, Shen B. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011;5:189–195. doi:10.1016/j.crohns.2010.12.005
42. Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1425–1433. doi:10.1097/MIB.0000000000001140
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.