Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Utility of Contezolid-Containing Regimens in 25 Cases of Linezolid-Intolerable Tuberculosis Patients
Authors Wang J, Nie W, Ma L , Li Q, Geng R, Shi W, Chu N
Received 6 July 2023
Accepted for publication 9 September 2023
Published 19 September 2023 Volume 2023:16 Pages 6237—6245
DOI https://doi.org/10.2147/IDR.S425743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Jun Wang,1 Wenjuan Nie,1 Liping Ma,1 Qiang Li,1 Ruixue Geng,2 Wenhui Shi,1 Naihui Chu1
1Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, People’s Republic of China; 2Tuberculosis Department, The Second Hospital of Hohhot, Hohhot, People’s Republic of China
Correspondence: Naihui Chu; Jun Wang, Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis & Thoracic Tumor Research Institute, No. 9, Beiguan Street, Tongzhou District, Beijing, 101149, People’s Republic of China, Tel/Fax +86 108 950 9301, Email [email protected]; [email protected]
Objective: Linezolid is increasingly used in the treatment of multidrug-resistant (MDR) M. tuberculosis (TB) with good efficacy; however, its clinical use is limited by intolerable adverse events (AEs). This usually results in dose adjustment or even discontinuation. Contezolid is a new oxazolidinone antibiotic with in vitro antibacterial activity against MDR TB equivalent to linezolid, but its safety and efficacy in MDB TB treatment has not been established.
Methods: We conducted a retrospective study on 25 TB patients who received both linezolid and contezolid in Beijing Chest Hospital from January 1, 2022, to January 31, 2023. All patients received linezolid-containing anti-TB regimen first and then switched to contezolid-containing regimens due to the intolerable linezolid-related AEs.
Results: Most (68%, 17/25) of the patients were diagnosed with RR-TB or MDR-TB. A total of 30 AEs were reported in these patients. About 26.7% (8/30) of the AEs were Grade 3 (severe) in severity. After switching to contezolid-containing anti-TB regimens for at least 1 month, the linezolid-related AEs were resolved or improved in 90% of the cases. Clinical improvement was observed in all patients after treatment with contezolid-containing regimen, with negative results of sputum culture and/or smear for M. tuberculosis in 84% of the patients.
Conclusion: Contezolid can be the first choice instead of linezolid to combine with other anti-TB drugs if necessary. Well-designed clinical trials are required to further confirm the safety and efficacy of contezolid in the treatment of TB patients.
Keywords: contezolid, safety, outcome, tuberculosis, linezolid
Introduction
Drug-resistant tuberculosis (TB) still poses serious threats to public health globally.1,2 The widespread dissemination of drug-resistant TB will interfere with our efforts to cure patients and meet the ambitious targets of the End TB Strategy. It is estimated that in 2019, 361,000 new cases of multidrug-resistant (MDR) TB emerged globally, of which about 14% in China.1 In China, the prevalence of rifampicin resistant TB (RR-TB)/MDR-TB was 7.1% in the new cases and 23% in the previously treated cases in 2019. Drug-resistant TB is associated with heavier medical burden, higher morbidity, and poorer outcome. It is reported that the typical treatment duration of MDR-TB ranged from 18 months to more than 2 years. Moreover, some patients have to receive up to seven anti-TB medications. These multi-drug regimens were associated with high incidence of side effects, namely, moderate-to-severe adverse events were reported in 45% of the patients.3 Few treatment options are available for XDR-TB. Therefore, it is urgently needed to develop novel more effective and safer drugs to improve the treatment outcomes of MDR/RR-TB.
Linezolid (Lzd) is an oxazolidinone antibiotic with activity against MDR Mycobacterium tuberculosis. In 2018, the World Health Organization (WHO) endorsed the use of linezolid as a preferred agent for all patients with MDR-TB based on clinical trial results.4 However, a high percentage of patients had adverse events (AEs) related to linezolid during a study. Peripheral neuropathy was reported in 81% of the patients receiving linezolid-containing anti-TB regimen, and nearly half of the patients had evidence of hematologic toxic effects such as thrombocytopenia.3 These adverse effects have led to the intolerability to linezolid and discontinuation of treatment, resulting in unsatisfactory outcomes. A recent study, conducted within programmatic conditions, focused on a WHO-endorsed bedaquiline containing all oral longer regimens for treating MDR-TB. Notably, linezolid, serving as a crucial constituent of this regimen, necessitated withdrawal due to the emergence of adverse effects in 42.22% of people with MDR-TB.5 Prolonged linezolid treatments (ie, more than 10 days) are at risk of developing drug-related hematological toxicity, often resulting in temporary or permanent treatment discontinuation. Aging, renal dysfunction, low baseline platelet count, and linezolid plasma trough concentrations >8 mg/L are risk factors for the development of linezolid-related myelosuppression.6
Contezolid (Czd) is a next-generation oxazolidinone antibacterial drug with similar activity against M. tuberculosis, and better safety profile than linezolid.7 Czd works by inhibiting the formation of a functional 70S initiation complex that is necessary for bacterial multiplication. It is purposely designed to address the issues of myelosuppression and MAO inhibition observed in Lzd treatment. Studies have shown that Czd was well tolerated and safe in healthy volunteers at doses up to 800 mg every 12 hours for 28 days. Czd showed the same in vitro activity against M. tuberculosis strains as Lzd. Both drugs showed MIC50 of 0.5 mg/L and MIC90 of 1 mg/L.8 In a mouse M. tuberculosis infection model, Lzd (100 mg/kg once daily) and Czd (100 mg/kg once daily, 50 mg/kg twice daily, or 25 mg/kg twice daily) were used as treatment for 4 weeks (5 days/week). Czd 100 mg/kg group showed efficacy equivalent to linezolid in terms of the bacterial load in mouse lungs.7,8 Therefore, it is reasonable to include Czd in the anti-TB regimen, especially considering the frequent Lzd intolerance in the current treatment regimen.
Based on the literature and our observations in clinical practice, it has been observed that certain patients experienced intolerance and subsequently discontinued treatment with Lzd due to various known and/or unknown AEs as the treatment lasts longer time.5 For these patients, we used Czd to replace Lzd for the purpose of improving the safety and outcome of such anti-TB treatment. Our experience of Czd-containing regimens in Lzd-intolerable TB patients may inform the clinical utility of Czd in managing complicated and/or drug-resistant TB patients.
Materials and Methods
Study Participants
A retrospective study was conducted by searching the database of hospital information system to identify the TB patients who received both Lzd and Czd in the Clinical Center on TB, Beijing Chest Hospital, Capital Medical University from January 1, 2022, to January 31, 2023. The clinical data were reviewed by independent reviewers to collect the demographic data, anti-TB treatment regimen, adverse events, laboratory test results, and treatment outcomes. The Institutional Review Board (IRB) for Human Investigation at Beijing Chest Hospital of Capital Medical University, China, reviewed and approved the protocol and informed consent form (approval no. YJS-2021-022). The IRB considered that the protocol complied with relevant laws, regulations, and guideline, and study procedures and data processing would not affect the safety and rights of patients.
The inclusion criteria included laboratory confirmed diagnosis of TB; intolerance to or treatment failure of Lzd-containing regimen; and switch to treatment with Czd-containing regimen for at least 1 month. The patients were excluded if they received Czd for less than 1 month, or used Czd before Lzd.
The study was implemented in accordance with the guidelines outlined in the Declaration of Helsinki. Consent was obtained from the study participants before data collection. All the data of patients were kept anonymous during data analysis.
Treatment Regimens
Clinicians prescribed individualized background regimens based on the past anti-TB history and/or drug susceptibility testing (DST) results of the patient. Lzd or Czd was used in combination with other background anti-TB drugs. Lzd was administered at a dose of 600 mg once or twice daily, and Czd was used at doses of 400 mg or 800 mg twice daily. The dosage of linezolid and contezolid was determined according to the body weight and disease status. Both shorter and longer regimens were used as clinically indicated.
The background regimens included the anti-TB drugs such as isoniazid (H), rifampin (R), rifapentine (P), bedaquiline (Bdq), clofazimine (Cfz), amikacin (Am), prothionamide (Pto), delamanid (Dlm), pyrazinamide (Z), ethambutol (E), cycloserine (Cs), moxifloxacin (Mfx), levofloxacin (Lfx), sitafloxacin (Sfx), pasiniazid (Pzd), and para-aminosalicylic acid (PAS).
The treatment outcomes were evaluated in terms of clinical signs and symptoms, AEs, and detection of M. tuberculosis by sputum culture/smear. The BACTEC Mycobacteria Growth Indicator Tube (MGIT) 960 system was used to detect M. tuberculosis.
Safety Evaluation
The demographic information including age, sex, clinical history, medication history, background regimens, laboratory test results, and AEs were collected from the hospital information system. The AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA 25.0) and then assigned with severity (Grade 1, Grade 2, Grade 3, Grade 4, and Grade 5) according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). Grade 1 and 2 AEs belonged to mild or moderate in severity, while severe AEs included Grade 3–5 AEs (Grade 3: serious; Grade 4: life threatening; Grade 5: death). The outcome of AEs was evaluated in terms of severity after switching from Lzd to Czd regimens.
Specifically, hepatotoxicity was based on serum alanine and aspartate aminotransferases greater than 3-fold the upper limit of normal (ULN) with symptoms; or serum bilirubin greater than 2-fold ULN with symptoms; or serum transaminases or serum bilirubin greater than 5-fold ULN with or without symptoms. Nephrotoxicity was defined as the presence of at least one serum creatinine value greater than 133 μmol/L. Peripheral neuropathy was diagnosed by physician according to numbness, weakness, tingling, burning/pain in the extremities. Central nervous system disorders included headache, dizziness, and seizure activity as reported by patient or witness. Psychiatric disorders were reported if the presence of depression, anxiety, psychosis, suicide, nightmares, and convulsions was documented in the patient chart.
Dermatologic disorders included skin rash, itch, bronzing, black pigmentation, and photosensitivity reaction. Arthralgia was defined as pain, swelling, or stiffness in the joints as reported by patients. Gastrointestinal disorders included nausea, vomiting, anorexia, abdominal pain, diarrhea, epigastric discomfort, and hematemesis. Hypokalemia was defined as the presence of at least one serum potassium value <3.5 mmol/L. Cardiac disorders included symptoms of palpitations, fainting, fatigability, and prolonged QT (>500 ms) on electrocardiograph.9
Data Analysis
A descriptive analysis was carried out for the data of patients using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Non-normal distribution continuous variables were expressed as median [interquartile range (IQR)]. Categorical variables were summarized using numbers and percentages. Kaplan-Meier analysis was performed to present the time to the occurrence of adverse effects related to linezolid.
Results
A total of 25 eligible patients were identified from the hospital database, including 11 males and 14 females. These patients had a median age of 26 years (range from 19 to 76 years). All these patients first received Lzd-containing anti-TB regimen and then switched to Czd-containing regimens due to intolerable Lzd-related AEs. Most (68%, 17/25) of the patients were diagnosed with RR-TB or MDR-TB. The most frequently used background anti-TB drugs were cycloserine, bedaquiline, clofazimine, levofloxacin, moxifloxacin, isoniazid, ethambutol, and pyrazinamide. Clinical improvement was observed in all of these patients after switching to Czd-containing anti-TB regimens (Table 1).
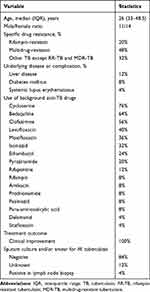 |
Table 1 Demographic and Clinical Data of the 25 Patients Who Received Contezolid-Containing Anti-TB Regimens Due to Intolerance to Linezolid Treatment |
Safety Outcomes After Switching from Lzd to Czd
All of the 25 patients received Lzd-containing anti-TB regimens. The mean time from initiation of Lzd treatment to the occurrence of Lzd-related AE was 130 days (4.3 months). The patients developed AE within 3 months of treatment in 32% of the cases, within 3–6 months in 48% of the cases, and in longer time (>6 months) in 20% of the cases (Table 2, Figure 1).
 |
Table 2 The Incidence of Linezolid-Related Adverse Events and AE Outcomes After Switching to Contezolid-Containing Anti-TB Regimens |
 |
Figure 1 Kaplan Meier analysis to present the time to the occurrence of linezolid-related adverse events in 25 tuberculosis patients. Abbreviation: AE, adverse event related to linezolid. |
Most of the Lzd-related AEs were peripheral neuropathy (numbness and pain of feet and/or lower limbs) and myelosuppression (anemia and pancytopenia). A total of 30 AEs were reported in the 25 patients. Most (73.3%) of these AEs were mild or moderate in severity (Grade 1 or 2). About 26.7% of the 30 AEs were evaluated as Grade 3 (Table 2). No SAEs were reported.
After switching to Czd-containing anti-TB regimens for at least 1 month, the Lzd-related AEs were resolved or improved in 90% of the cases. Only 3 of the 30 AEs were still ongoing (Table 2), including anemia and feet numbness (peripheral neuropathy) (Table 3). The demographic and clinical details of the 25 patients who received Czd-containing anti-TB regimens due to intolerance to Lzd are listed in Table 3.
 |
Table 3 Demographic and Clinical Details of 25 Patients Receiving Contezolid-Containing Anti-TB Regimens Due to Intolerance to Linezolid Treatment |
Discussion
The widespread dissemination of drug-resistant TB during the past 20 years, especially during the 3-year COVID-19 pandemic, has damped our efforts to reach the ambitious targets of the End TB Strategy. MDR-TB is caused by M. tuberculosis strains that are resistant to at least isoniazid and rifampicin. The MDR M. tuberculosis strains are also resistant to one or more other anti-TB drugs.2 More novel anti-TB drugs and/or regimens are being developed and introduced into clinical practice to address the emerging MDR/RR-TB. However, most MDR-TB regimens were designed to last at least 9–12 months, or even longer than 20 months. Long-term and multi-drug regimens are always associated with safety concerns.
WHO has listed Lzd as a useful anti-TB drug (Group A) for combination with other background anti-TB drugs.10 It is increasingly used in China to treat the refractory TB cases. Clinical trials indicated that the combination of Lzd, Bdq, and pretomanid showed favorable outcomes in highly drug-resistant TB cases.11 The accumulating body of clinical evidence for the benefits of Lzd treatment supports the role of Lzd in anti-TB regimen.4 However, Lzd causes potentially severe toxicities including myelosuppression, lactic acidosis, and neuropathies, which are ascribed to impairment of mitochondrial protein synthesis and consecutive mitochondrial dysfunction.12
In the present study, we found that the patients usually developed intolerable AEs such as peripheral neuropathy, anemia, and pancytopenia due to myelosuppression when Lzd treatment lasted longer time (>3 months). These findings are consistent with the safety profile of Lzd. Safety is one of the top priorities when prescribing anti-TB drugs because the anti-TB treatment usually lasts a long time, at least half or 1 year. The approval of Czd in China has provided the possibility for clinicians to try Czd instead of Lzd to avoid the Lzd-related AEs while keeping the same anti-TB activity.13–15
Czd was considered as an alternative to Lzd to combine with other anti-TB drugs to address the complicated RR-TB/MDR-TB cases, especially when the patients are intolerable to other treatments. This is based on the following facts. First, both Czd and Lzd had the same in vitro activities against the clinical isolates of M. tuberculosis in China in terms of MIC50 and MIC90. Second, Czd demonstrated better intracellular anti-TB effects than Lzd in terms of reducing the colony forming units (CFU), although both drugs inhibited the intracellular growth of M. tuberculosis in the macrophages in a dose-dependent manner.14 Third, the chemical structure of Czd enables the drug to largely avoid the AEs caused by myelosuppression and MAO inhibition, which were confirmed in the patients receiving long-term Lzd treatment in this report. Lastly, the in vitro antimicrobial susceptibility testing of the M. tuberculosis strains isolated in China, including the isolates in this report, indicated that Czd was active in vitro against most of the isolates. The results of the current study on compassionate use of Czd in complicated or refractory case of TB patients also supports the utility of Czd in managing TB patients.16,17
Some limitations are observed in this study. No control group was available. The symptoms and signs of the illness and/or underlying diseases (tuberculosis and diabetes mellitus) might affect the evaluation of Lzd-related AEs, although the reviewers had made every effort to provide a reasonable assessment. It was unable to correlate the drug exposure with the incidence of AEs due to fewer cases. Longer time follow-up after switching to Czd-containing regimens may be more convincing for our conclusion. Patients always took several background drugs for treatment of MDR/XDR-TB. For example, bedaquiline-containing regimens resulted in better treatment outcomes and similar safety relative to bedaquiline-free regimens for patients with refractory pulmonary RR/MDR/XDR-TB.18 This may also interfere with the clear causality assessment of AEs.
Conclusion
The preliminary experience of Czd-containing anti-TB regimens in the patients who cannot tolerate linezolid treatment due to AEs informs the clinicians of the potential role of Czd in such cases. If a patient has significant underlying diseases or other vulnerable conditions, Czd can be the first choice instead of Lzd to combine with other anti-TB drugs when necessary. However, well-designed clinical trials are still needed to further confirm the safety and efficacy of Czd in the treatment of TB patients.
Data Sharing Statement
The datasets generated or analyzed during this study are available from the corresponding author Dr. Naihui Chu on reasonable request.
Ethics Statement
The protocol and informed consent form were reviewed and approved by the Institutional Review Board for Human Investigation at Beijing Chest Hospital of Capital Medical University, China (approval no. YJS-2021-022).
Consent for Publication
All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China [grant No. 8210002].
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report. Geneva: World Health Organization; 2020. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
2. Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57(6):2003300. doi:10.1183/13993003.03300-2020
3. Conradie F, Diacon AH, Ngubane N, et al.; Nix-TB Trial Team. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. doi:10.1056/NEJMoa1901814
4. Du J, Gao J, Yu Y, et al. Low rate of acquired linezolid resistance in multidrug-resistant tuberculosis treated with bedaquiline-linezolid combination. Front Microbiol. 2021;12:655653. doi:10.3389/fmicb.2021.655653
5. Mishra G, Alffenaar J-W, Munje R, Khateeb S. Adverse drug reactions due to linezolid in the programmatic management of drug-resistant tuberculosis in India: a retrospective multicenter study. Indian J Tuberc. 2023. Available from: https://linkinghub.elsevier.com/retrieve/pii/S001957072300063X.
6. Cattaneo D, Marriott DJ, Gervasoni C. Hematological toxicities associated with linezolid therapy in adults: key findings and clinical considerations. Expert Rev Clin Pharmacol. 2023;16(3):219–230. doi:10.1080/17512433.2023.2181160
7. Yang M, Zhan S, Fu L, Wang Y, Zhang P, Deng G. Prospects of contezolid (MRX-I) against multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Drug Discov Ther. 2022;16(2):99–101. doi:10.5582/ddt.2022.01025
8. Shoen C, DeStefano M, Hafkin B, Cynamon M. In vitro and in vivo activities of contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018;62(8):e00493–e00518. doi:10.1128/AAC.00493-18
9. Nasasira M, Kalyango JN, Mupere E, Baluku JB. Incidence and predictors of adverse drug events among people receiving drug resistant tuberculosis treatment in Uganda: 8-year Retrospective Cohort Study. Ther Clin Risk Manag. 2022;18:1117–1127. doi:10.2147/TCRM.S381800
10. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Online annexes. Available from: https://apps.who.int/iris/bitstream/handle/10665/332678/9789240007062-eng.pdf.
11. Mishra G. Nix-TB and ZeNix trials: paving the way for shorter regimens for drug-resistant tuberculosis. Asian Pac J Trop Med. 2021;14(10):431–432. doi:10.4103/1995-7645.329004
12. Milosevic TV, Payen VL, Sonveaux P, Muccioli GG, Tulkens PM, Van Bambeke F. Mitochondrial alterations (Inhibition of mitochondrial protein expression, oxidative metabolism, and ultrastructure) induced by linezolid and tedizolid at clinically relevant concentrations in cultured human HL-60 promyelocytes and THP-1 monocytes. Antimicrob Agents Chemother. 2018;62(3):e01599–e01617. doi:10.1128/AAC.01599-17
13. Yuan H, Wu H, Zhang Y, et al. Clinical pharmacology and utility of contezolid in Chinese patients with complicated skin and soft-tissue infections. Antimicrob Agents Chemother. 2022;66(6):e0243021. doi:10.1128/aac.02430-21
14. Wang C, Wang G, Huo F, et al. Novel oxazolidinones harbor potent in vitro activity against the clinical isolates of multidrug-resistant Mycobacterium tuberculosis in China. Front Med. 2022;9:1067516. doi:10.3389/fmed.2022.1067516
15. Gao JT, Du J, Wu GH, et al. Bedaquiline-containing regimens in patients with pulmonary multidrug-resistant tuberculosis in China: focus on the safety. Infect Dis Poverty. 2021;10(1):32. doi:10.1186/s40249-021-00819-2
16. Kang Y, Ge C, Zhang H, Liu S, Guo H, Cui J. Compassionate use of contezolid for the treatment of tuberculous pleurisy in a patient with a leadless pacemaker. Infect Drug Resist. 2022;15:4467–4470. doi:10.2147/IDR.S373082
17. Guo W, Hu M, Xu N, et al. Concentration of contezolid in cerebrospinal fluid and serum in a patient with tuberculous meningoencephalitis: a case report. Int J Antimicrob Agents. 2023;62(2):106875. doi:10.1016/j.ijantimicag.2023.106875
18. Zhang SJ, Yang Y, Sun WW, et al. Effectiveness and safety of bedaquiline-containing regimens for treatment on patients with refractory RR/MDR/XDR-tuberculosis: a retrospective cohort study in East China. BMC Infect Dis. 2022;22(1):715. doi:10.1186/s12879-022-07693-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
