Back to Journals » Drug Design, Development and Therapy » Volume 17
Clinical Trials in Hypertrophic Cardiomyopathy Therapy: A Comprehensive Analysis of Trials Registered in Global Clinical Databases
Authors Zhang H, Yu C, Cheng Y, Chen Z, Chen M, He W, Jin Z, Cai S , Yu L
Received 18 March 2023
Accepted for publication 2 June 2023
Published 21 June 2023 Volume 2023:17 Pages 1863—1877
DOI https://doi.org/10.2147/DDDT.S413136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Huan Zhang,1,2,* Cheng Yu,1,* Yuanling Cheng,1 Zhi Chen,2 Min Chen,2 Wangan He,1 Zhigang Jin,1 Shaoqian Cai,1 Lijuan Yu2
1Department of Cardiology, China Resources & Wisco General Hospital, Wuhan University of Science and Technology, Wuhan, People’s Republic of China; 2Wuhan University of Science and Technology Medical College, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaoqian Cai; Zhigang Jin, Department of Cardiology, China Resources & Wisco General Hospital, Wuhan University of Science and Technology, Wuhan, Hubei, 430080, People’s Republic of China, Email [email protected]; [email protected]
Background: With the disappointing results associated with the use of cardiac myosin inhibitors in the treatment of hypertrophic cardiomyopathy (HCM), the development of new therapies in clinical trials for HCM has rapidly increased. We assessed the characteristics of therapeutic intervention in HCM registered on ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP).
Methods: We conducted a cross-sectional, descriptive study of clinical trials for therapeutic intervention in HCM registered on ClinicalTrials.gov and ICTRP.
Results: This study analyzed 137 registered trials. Regarding study designs of these trials, 77.37% were purpose of treatment, 59.12% were randomized, 50.36% were parallel assignment, 45.26% were performed with masking, 48.18% recruited less than 50 participants, and 27.74% were Phase 2 trials. In total, 67 trials were new drug trials, of which 35 drugs were tested in these trials, and 13 trials involved treatment with mavacamten. Of these 67 clinical drug trials, 44.78% of trials involved the study of amines, and 16.42% involved 1-ring heterocyclic compounds. Regarding the NCI Thesaurus Tree, 23.81% of trials involved myosin inhibitors, 23.81% of trials involved drugs belonging to agents affecting the cardiovascular system, and 20.63% were involved in testing cation channel blockers. The drug-target network showed that myosin-7, potassium voltage-gated channel subfamily h member 2, beta-1 adrenergic receptor, carnitine o-palmitoyltransferase 1, and liver isoform were the most targeted pathways of the clinical trials analyzed in the drug-target network.
Conclusion: The number of clinical trials investigating therapeutic interventions for HCM has increased in recent years. Ultimately, recent HCM therapeutic clinical trials generally did not incorporate either randomized controlled trials or masking and were small studies recruiting fewer than 50 participants. Although recent research has focused on targeting myosin-7, the molecular signaling mechanisms involved in the pathogenesis of HCM have the potential to elucidate novel target pathways.
Keywords: clinical trials, obstructive hypertrophic cardiomyopathy, nonobstructive hypertrophic cardiomyopathy, myosin-7, amines, myosin inhibitors
Introduction
Hypertrophic cardiomyopathy (HCM) is a common inherited myocardial disorder that is caused by mutations in the sarcomere genes. HCM is a genetic disorder transmitted as an autosomal dominant trait, with incomplete penetrance and variable expressivity.1,2 Epidemiological surveys based on echocardiography indicate that the prevalence of HCM is estimated at 1/500 in the general population.3,4 Further, approximately 20 million people worldwide suffer from HCM, far exceeding initial predictions of disease burden.2
As of now, 29 genes have been linked to HCM, and over 1500 mutation sites have been closely associated with the development of the disease.5 MYH7 and MYBPC3, which encode beta-myosin heavy chain and myosin-binding protein C, are the two most common causative genes, collectively accounting for roughly 40% of all HCM cases.6–9 Additionally, existing research suggests that prevalent metabolic alterations prompted by mutations in the sarcomere genes in cases of HCM predominantly entail a rise in sensitivity to calcium ions (Ca2+) within the sarcomere and impairments in sarcomere energy metabolism, including suboptimal energy usage, and heightened energy requirements.10,11 Certain metabolic consequences brought on by these metabolic changes include disruptions in Ca2+ metabolic disorders,10,12 malfunctioning of mitochondria,13,14 and metabolic reconfiguration.15,16
The European Society of Cardiology (ESC) has classified HCM into two broad categories.17 Obstructive hypertrophic cardiomyopathy (oHCM; also known as HOCM), which represents ~70% of HCM, is characterized by left ventricular outflow tract (LVOT) obstruction (LVOTO) and defined as an instantaneous peak Doppler LV outflow tract pressure gradient ≥30 mm Hg at rest or during physiological provocation such as the Valsalva maneuver, standing, or exercise.18 In contrast, nonobstructive hypertrophic cardiomyopathy (nHCM) does not have significant LVOTO (<30 mm Hg) at rest or with provocation.19 Regardless of hemodynamic characteristics, sarcomeric gene mutations result in excessive cardiac actin-myosin cross-bridging20,21 that culminates in impaired myocardial relaxation, hyperdynamic contractile properties, and abnormal compliance that are hallmarks of this disease.1 Currently, the management of HCM focuses on the alleviation of symptoms, the prevention of sudden cardiac death, and family screening.17,22 Treatment of HCM symptoms includes: (1) the use of beta-blockers and non-dihydropyridine calcium-channel blockers to relieve obstruction,17 (2) alcohol septal ablation (ASA) is an effective therapy and the gold standard for patients with LVOTO and drug-refractory symptoms,23,24 and (3) treatment with a selective allosteric inhibitor of cardiac myosin ATPase to improve exercise capacity, LVOT obstruction, New York Heart Association (NYHA) functional class, and health status.25–27
Clinical trials are the most effective strategy for evaluating the efficacy of a drug for a specific disease28,29 and are a critical step in the successful development of novel effective drugs.30 Thus, one of the most important aspects of laying the foundation for future clinical practice is analyzing registered clinical trial data. Established in 2006, the WHO International Clinical Trials Registry Platform (ICTRP) is composed of partner regional or national clinical trial registers that upload information on the studies they hold at defined intervals.31 Currently, the ICTRP is a platform integrating information from primary registries in 17 countries/areas. This platform is free to access, allowing anyone the access to collect information on clinical trials all over the world.31–33 ClinicalTrials.gov is a public trials registry provided by the US National Library of Medicine and the US Food and Drug Administration, accounting for more than 80% of all studies in the ICTRP.34 Therefore, to better understand the current research progress in HCM treatment to provide the latest research hotspots to clinicians and researchers, we performed a cross-sectional study to investigate the characteristics of registered trials on ClinicalTrials.gov and ICTRP regarding HCM therapy.
Methods
Search Strategy and Selection Criteria
A cross-sectional, descriptive study of clinical trials for HCM registered on the ClinicalTrials.gov database (https://clinicaltrials.gov) and ICTRP (https://trialsearch.who.int/) was conducted. The trials were obtained from ClinicalTrials.gov and ICTRP using the advanced search function with the search terms “Hypertrophic Cardiomyopathy” for “condition or disease” and the term “Hypertrophic Cardiomyopathy” for “Health Condition or Problem studied” on November 10, 2022. Next, by using the Venny online software (version 2.1, http://bioinfogp.cnb.csic.es/tools/venny), we found the trials common to both ClinicalTrials.gov and ICTRP. All of the identified clinical trials were assessed to obtain records of all studies. Patient inclusion criteria for this study require a diagnosis of HCM consistent with current guidelines from the American College of Cardiology Foundation/American Heart Association and European Society of Cardiology. All intervention studies on control or compression, outcome measures, and time frames for evaluation of outcomes were collected without any limitations or restrictions. The following information and data were extracted: register number, title, study type, conditions, interventions, locations, start date, the status of the trial, study results, study samples, participant ages, primary sponsor, location, primary purpose, phases of each trial, allocation, intervention model, masking, and intervention. All trials were then further subclassified according to their study type. We used descriptive statistics to characterize trial categories, and frequencies and percentages were provided for categorical data. All analyses were performed using Microsoft Excel (Microsoft Office Excel 2010, Microsoft Corporation). Exclusion criteria included: 1) observation studies, 2) study subjects without HCM, and 3) non-human studies (Laboratory Analysis). All trials were then further subclassified according to their study type. We used descriptive statistics to characterize trial categories, and frequencies and percentages were provided for all categorical data.
Data Analysis
Descriptive analyses were used to analyze collected data. Values were entered into the cumulative calculation for that region if different sites were analyzed in the same region, and we defined locations spanning two or more continents as global. Categorical data are reported as frequencies and percentages. Correlations were analyzed using Spearman correlation. All of the analyses were executed using SPSS 20.0. P-values < 0.05 were considered to be statistically significant.
Results
Screening and Included Trials
The initial search identified 195 clinical trials and 105 clinical trials on HCM registered on the ClinicalTrials.gov database and the ICTRP database, respectively, from January 1, 1990, through November 10, 2022. After excluding duplicate trials, 242 trials remained (Figure 1A), which was reduced to 139 trials after excluding observation trials and relative factors research trials. After carefully reviewing all of the information, two trials not for HCM therapy were excluded. Finally, a total of 137 registered trials were ultimately evaluated (Figure 1B).
General Characteristics of Included Trials
Over 80% of trials (117/137; 85.40%) were from ClinicalTrials.gov, which is consistent with previous literature findings.34 More than half of the trials (72/137; 52.55%) were registered in databases since 2018.
We analyzed to determine whether the number of annual clinical trial registrations included in the study has changed over time. Our analysis, depicted in Figure 2, showed that annual clinical trial registrations increased significantly over time (r = 0.8329, t = 6.8969, p = 8.1494e−7 <0.05). A total of 43 trials (31.39%) were completed, followed by those recruiting (28.47%), of unknown status (16.79%), and those active but not recruiting (10.95%). The majority of trials (89.05%) had no results available, with only 15 trials (10.95%) having results reported on ClinicalTrials.gov and ICTRP. Nearly half of trials (49.56%) recruited adults and older adults as study subjects, with 11 trials (8.03%) selecting adult as study participants, and 16 trials (11.68%) enrolling subjects comprising children. In total, 59 trials (43.07%) were performed in Europe, followed by North America (27.01%), Asia (15.33%), and 12 trials (8.76%) performed in greater than or equal to two continents. The characteristics of included trials are shown in Table 1.
 |
Table 1 Characteristics of All Included Trials (n = 137) |
 |
Figure 2 Correlation between the number of trials and beginning year in all 137 included trials. |
Study Designs of Included Trials
The primary purpose of the majority of included trials (77.37%) was treatment, followed by diagnostic (7.30%), not applicable, basic science (4.38%), three trials (2.19%) for screening, three trials (2.19%) for supportive care, and two trials (1.46%) for device feasibility. More than half of allocations were randomized (59.12%), followed by not applicable (29.33%), and non-randomized (10.95%). More than half of the intervention models were parallel assignment (50.36%). A total of 63 trials (45.99%) were performed without masking, 12 (8.76) were with unknown masking, and 62 (45.26%) were performed with masking (12 single maskings, 22 double maskings, 11 triple maskings’s, and 17 quadruple maskings). Trial phases were as follows: Phase 1 (8.76%), phase 2 (27.74%), phase 2/Phase 3 (2.92%), phase 3 (8.76%), Phase 4 (8.03%), or not applicable (43.80%). Further, a total of 66 trials (48.18%) recruited less than 50 participants, 34 trials (24.82%) recruited 50–100 individuals, 23 trials (16.79%) recruited 201–500 individuals, 1 trial recruited greater than 500 individuals, and 1 trials did not indicate the number of participants (detailed data are depicted in Table 2).
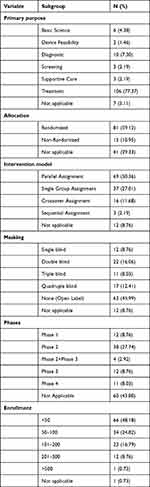 |
Table 2 Study Design Elements of Included Trials (n = 137) |
Overview of Investigated Drugs
A total of 67 trials were involved in clinical drug trials and investigated 35 drugs in 137 intervention trials. Of these 35 drugs, 13 trials involved the drug mavacamten (Figure 3A). We used two major classification systems to categorize these 35 compounds: the Medical Subject Headings Classification (MeSH) (https://meshb.nlm.nih.gov/) and the NCI Thesaurus Tree (https://ncit.nci.nih.gov). Of these 67 clinical drug trials, 44.78% of the trials involved amines and 16.42% involved heterocyclic 1-ring compounds (Figure 3B). Of the compounds categorized by the NCI Thesaurus Tree, 23.81% were myosin inhibitors (13 trials for mavacamten and two trials for MYK-224), 23.81% of trials involved agents affecting the cardiovascular system, 20.63% involved cation channel blockers, and 12.70% involved agents affecting nervous system. Of the 15 trials investigating agents affecting the cardiovascular system, 53.33% of trials assessed anti- hypertensive drugs, 20.00% of drugs were classified as not applicable, 13.33% analyzed cardiotonic agents, and 13.33% tested anti- arrhythmic agents (Figure 4).
 |
Figure 3 (A) Overview of investigated drugs and (B) their classification through Medical Subject Headings (MeSH). Notes: Data from MeSH Tree, https://meshb.nlm.nih.gov/; Mavacamten formerly known as MYK-461; Aficamten formerly known as CK-3773274; Binimetinib formerly known as MEK162; Ninerafaxstat formerly known as IMB-1018972; Vastarel formerly known as Trimetazidine dihydrochloride; TY-0305 formerly known as Cibenzoline. |
 |
Figure 4 Overview of NCI Thesaurus Tree for investigated drugs. (A) Proportion of each classification via NCI Thesaurus Tree; (B) Proportion of agents affecting the cardiovascular system; (C) Proportion of drugs used as myosin inhibitors. Note: Data from NCI Thesaurus Tree, https://ncit.nci.nih.gov. |
Overview of Investigated Targets
We further identified all 69 targets of the 35 investigated drugs using the DrugBank database (https://go.drugbank.com/). Next, a drug-target network was created and visualized using Cytoscape (Version 3.8.0). The results showed that myosin-7, potassium voltage-gated channel subfamily H member 2, beta-1 adrenergic receptor, carnitine o-palmitoyltransferase 1, and liver isoform were the most targeted proteins of included intervention trials (Figure 5A). The top 10 targets with the maximum number of trials were identified and shown in Figure 5B.
 |
Figure 5 Overview of Investigated Targets. (A) Diagram of the formulated drug-target network. (B) Graph showing the top seven agents tested in the clinical trials assessed. |
Randomized controlled, masked, and appropriate patient-population trials are critical components of high-quality clinical trials.35 In further analyzing these 67 clinical drug trials, we narrowed our selection to those registered after 2013, excluding trials without randomization, masking, and parallel assignment. This resulted in a selection of 18 trials for further analysis, which is presented in Table 3. Interestingly, of these 18 trials, only four trials were able to complete both recruitment and clinical testing to date. Among these four, three trials were Phase 1–3 clinical trials for mavacamten in the treatment of HCM (NCT02356289, NCT03442764, NCT03470545), and the remaining one was a Phase 2 clinical trial for valsartan in the treatment of early sarcomeric HCM (NCT01912534). The US FDA approved mavacamten on April 28th, 2022, and is now approved for treating symptomatic New York Heart Association (NYHA) class II–III obstructive HCM in adults to improve functional capacity and symptoms.36 Of these 18 trials, 5 clinical trials utilized peak oxygen consumption (pVO2) as their primary outcome measure. It has been recognized that exercise capacity is a prognostic indicator in HCM, and Peak Oxygen Consumption is an independent predictor of survival and outcomes in patients with HCM.37–39
 |
Table 3 Characteristics of High-Quality Clinical Trials in HCM Therapy |
Discussion
HCM is a myocardial disease characterized by primary left ventricular hypertrophy, LVOTO, hyperdynamic contractile properties, and diastolic abnormalities.1,2 Patients with oHCM are often symptomatic and can have atrial fibrillation, heart failure, and malignant ventricular arrhythmias.2,54 Previously, drug treatment for oHCM focused on symptomatic relief with β blockers, non-dihydropyridine calcium channel blockers, and disopyramide.17,55,56 However, selective allosteric inhibitors of cardiac myosin ATPase have been widely studied in recent years. Specifically, phase 3 clinical studies were recently completed for mavacamten,57 and are ongoing for aficamten (CK-3773274) (http://www.chinadrugtrials.org.cn/clinicaltrials.searchlistdetail.dhtml). We analyzed the correlation between the number of trials and the beginning year in all 137 included trials. The number of trials was significantly correlated with the year the trial started (r = 0.8329, P < 0.001).
Among all 35 drugs, the top 5 in terms of number of registrations included mavacamten (13 trials), perhexiline (5 trials), aficamten (3 trials), metoprolol (3 trials), and trientine (3 trials). Mavacamten is a first-in-class small molecule selective allosteric inhibitor of cardiac myosin ATPase. It was specifically developed to target the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation,57 thereby reducing contractility and improving myocardial energetic potential.27 Of the 67 trials drugs testing 35 drugs, the top 5 drug types (MeSH Tree) by number of registrations were amines (30 trials), heterocyclic 1-ring compounds (11 trials), heterocyclic 2-ring compounds (5 trials), hydrocarbons (4 trials), and lactones (3 trials). The drug-target network showed that myosin-7, potassium voltage-gated channel subfamily H member 2, beta-1 adrenergic receptor, carnitine o-palmitoyltransferase 1, and liver isoform were the most targeted proteins in the trials analyzed.
Clinical trials are critical to clinical practice and decision-making.58 Moreover, randomized controlled, masked, and appropriate patient-population trials are critical components of high-quality clinical trials.35 In our study, more than half of the primary trial purposes were treatment (77.37%), allocations were randomized trials (59.12%), and intervention models were parallel assignment (50.36%). Altogether, 54.75% of the trials were performed without masking, 43.80% of trials were without application of phases, and 48.18% of trials recruited less than 50 participants. There was a certain gap between the majority of clinical trials in this study and high-quality clinical trials. The main reason for this finding is that phase 3 or phase 4 trials only accounted for 16.79%. Further, before the approval of mavacamten, HCM was mainly treated by surgery, which was only performed in a small number of hospitals worldwide.
There are a number of limitations to this study. First, we could have missed some clinical trials whose protocols had not been registered on ClinicalTrials.gov and ICTRP and may instead have been registered in other countries clinical trial platforms, such as the Chinese Clinical Trial Registry Platform (http://www.chictr.org.cn/searchproj.aspx). In addition, we note that several new compounds are lacking MeSH, NCI Thesaurus Tree, and targets, thus limiting our analysis and potentially biasing our estimates by limiting the generalizability of our findings.
Currently, mavacamten and aficamten are the most promising drugs to treat HCM, having significant therapeutic effects. Although both are cardiac myosin inhibitors, they differ slightly in their pharmacological properties. For instance, mavacamten takes longer to reach steady-state blood drug concentrations (6 weeks) and has a sustained pharmacological effect even after discontinuation.25,59 On the other hand, aficamten reaches steady-state blood drug concentrations more quickly (2 weeks) and has reversible effects after discontinuation.41 As more clinical trial data accumulates, we will have a better understanding of the differences between these drugs and their optimal application in the treatment of HCM. As the clinical trials for mavacamten are ongoing globally, experimental outcomes related to it have been released consistently. Some studies have demonstrated mavacamten has significantly reduced the fraction of patients with oHCM who meet the guideline criteria for septal reduction therapy (SRT) after 16 weeks. Furthermore, LVOT obstruction has significantly decreased after treatment, along with a marked improvement in the quality of life.60 In addition, a long-term clinical trial (PIONEER-OLE) was conducted 6–18 months after completion of PIONEER-HCM and has found that mavacamten is still effective in reducing LVOT obstruction, improving symptoms, and maintaining normal LVEF levels in patients.61
The US FDA approved the use of mavacamten in April 2022. The “WARNINGS AND PRECAUTIONS” notice mentions that mavacamten may reduce systolic contraction and has the potential to cause heart failure or obstruct ventricular function. Patients who experience a serious intercurrent illness or arrhythmia are at a higher risk of developing systolic dysfunction and heart failure. Mavacamten is primarily metabolized by CYP2C19 and CYP3A4 enzymes. Therefore, concomitant use of mavacamten and drugs that interact with these enzymes may result in life-threatening drug interactions, such as heart failure or a loss of effectiveness.36
Conclusions
The number of clinical trials investigating therapeutic intervention for HCM has increased in recent years. The main characteristics of clinical trials for therapeutic intervention in HCM include lack of randomized control, lack of masking, and recruiting less than 50 participants. Amines, myosin inhibitors, and agents affecting the cardiovascular system have been the focus of most current HCM research. With increased research in targeting myosin-7, the molecular signaling mechanisms involved in the pathogenesis of HCM have the potential to elucidate novel pathways and drug targets.
Funding
This study was supported by the Hubei Province Health and Family Planning Scientific Research Project (WJ2018H0036), Wuhan Health and Family Planning Scientific Research Project (WX17C34).
Disclosure
We declare no competing interests.
References
1. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749–770. doi:10.1161/circresaha.117.311059
2. Maron BJ, Longo DL. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(7):655–668. doi:10.1056/NEJMra1710575
3. Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64(1):83–99. doi:10.1016/j.jacc.2014.05.003
4. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124(24):2761–2796. doi:10.1161/CIR.0b013e318223e230
5. Kraker J, Viswanathan SK, Knöll R, Sadayappan S. Recent advances in the molecular genetics of familial hypertrophic cardiomyopathy in South Asian descendants. Front Physiol. 2016;7:499. doi:10.3389/fphys.2016.00499
6. Alfares AA, Kelly MA, McDermott G, et al. Results of clinical genetic testing of 2912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet Med. 2015;17(11):880–888. doi:10.1038/gim.2014.205
7. Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60(8):705–715. doi:10.1016/j.jacc.2012.02.068
8. Biagini E, Olivotto I, Iascone M, et al. Significance of sarcomere gene mutations analysis in the end-stage phase of hypertrophic cardiomyopathy. Am J Cardiol. 2014;114(5):769–776. doi:10.1016/j.amjcard.2014.05.065
9. Marian AJ. Molecular genetic basis of hypertrophic cardiomyopathy. Circ Res. 2021;128(10):1533–1553. doi:10.1161/circresaha.121.318346
10. Ormerod JO, Frenneaux MP, Sherrid MV. Myocardial energy depletion and dynamic systolic dysfunction in hypertrophic cardiomyopathy. Nat Rev Cardiol. 2016;13(11):677–687. doi:10.1038/nrcardio.2016.98
11. van der Velden J, Tocchetti CG, Varricchi G, et al. Metabolic changes in hypertrophic cardiomyopathies: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc Res. 2018;114(10):1273–1280. doi:10.1093/cvr/cvy147
12. Ho CY, Lakdawala NK, Cirino AL, et al. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail. 2015;3(2):180–188. doi:10.1016/j.jchf.2014.08.003
13. Sacchetto C, Sequeira V, Bertero E, Dudek J, Maack C, Calore M. Metabolic alterations in inherited cardiomyopathies. J Clin Med. 2019;8(12):2195. doi:10.3390/jcm8122195
14. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi:10.1042/bj20081386
15. Aoyama R, Takano H, Kobayashi Y, et al. Evaluation of myocardial glucose metabolism in hypertrophic cardiomyopathy using 18F-fluorodeoxyglucose positron emission tomography. PLoS One. 2017;12(11):e0188479. doi:10.1371/journal.pone.0188479
16. Pereira RO, Wende AR, Olsen C, et al. Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc. 2013;2(5):e000301. doi:10.1161/jaha.113.000301
17. Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779. doi:10.1093/eurheartj/ehu284
18. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232–2239. doi:10.1161/circulationaha.106.644682
19. Ho CY, Mealiffe ME, Bach RG, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75(21):2649–2660. doi:10.1016/j.jacc.2020.03.064
20. McNamara JW, Li A, Dos Remedios CG, Cooke R. The role of super-relaxed myosin in skeletal and cardiac muscle. Biophys Rev. 2015;7(1):5–14. doi:10.1007/s12551-014-0151-5
21. McNamara JW, Li A, Smith NJ, et al. Ablation of cardiac myosin binding protein-C disrupts the super-relaxed state of myosin in murine cardiomyocytes. J Mol Cell Cardiol. 2016;94:65–71. doi:10.1016/j.yjmcc.2016.03.009
22. Lafreniere-Roula M, Bolkier Y, Zahavich L, et al. Family screening for hypertrophic cardiomyopathy: is it time to change practice guidelines? Eur Heart J. 2019;40(45):3672–3681. doi:10.1093/eurheartj/ehz396
23. Liebregts M, Vriesendorp PA, Mahmoodi BK, Schinkel AF, Michels M, ten Berg JM. A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Fail. 2015;3(11):896–905. doi:10.1016/j.jchf.2015.06.011
24. Wells S, Rowin EJ, Boll G, et al. Clinical profile of nonresponders to surgical myectomy with obstructive hypertrophic cardiomyopathy. Am J Med. 2018;131(6):e235–e239. doi:10.1016/j.amjmed.2017.12.031
25. Grillo MP, Erve JCL, Dick R, et al. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica. 2019;49(6):718–733. doi:10.1080/00498254.2018.1495856
26. Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292(40):16571–16577. doi:10.1074/jbc.M117.776815
27. Anderson RL, Trivedi DV, Sarkar SS, et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115(35):E8143–e8152. doi:10.1073/pnas.1809540115
28. Feizabadi M, Fahimnia F, Mosavi Jarrahi A, Naghshineh N, Tofighi S. Iranian clinical trials: an analysis of registered trials in International Clinical Trial Registry Platform (ICTRP). J Evid Based Med. 2017;10(2):91–96. doi:10.1111/jebm.12248
29. Chen L, Su Y, Quan L, Zhang Y, Du L. Clinical trials focusing on drug control and prevention of ventilator-associated pneumonia: a comprehensive analysis of trials registered on ClinicalTrials.gov. Original Research. Front Pharmacol. 2019;9. doi:10.3389/fphar.2018.01574
30. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516–2521. doi:10.1200/JCO.2011.39.5186
31. Bhaskar SB. Clinical trial registration: a practical perspective. Indian J Anaesth. 2018;62(1):10–15. doi:10.4103/ija.IJA_761_17
32. Adams M. Chinese research register joins WHO network, raising hopes for improved clinical trials. Bull World Health Organ. 2007;85(9):653–654. doi:10.2471/blt.07.010907
33. Krleza-Jerić K. Clinical trial registration: the differing views of industry, the WHO, and the Ottawa Group. PLoS Med. 2005;2(11):e378. doi:10.1371/journal.pmed.0020378
34. Zarin DA, Ide NC, Tse T, Harlan WR, West JC, Lindberg DAB. Issues in the registration of clinical trials. JAMA. 2007;297(19):2112–2120. doi:10.1001/jama.297.19.2112
35. Zwierzyna M, Davies M, Hingorani AD, Hunter J. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ. 2018;361:k2130. doi:10.1136/bmj.k2130
36. FDA US. FDA approves new drug to improve heart function in adults with rare heart condition. Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-drug-improve-heart-function-adults-rare-heart-condition.
37. Tompkins JDV, Day SM, Jacoby DL, et al. Abstract 14251: peak oxygen consumption is an independent predictor of survival and outcomes in obstructive and non-obstructive Hypertrophic Cardiomyopathy (HCM) patients: results from the International Sarcomeric Human Cardiomyopathies Registry (SHaRe). Circulation. 2018;138(Suppl_1):A14251–A14251. doi:10.1161/circ.138.suppl_1.14251
38. Contaldi C, Lombardi R, Giamundo A, Betocchi S. Abstract 19346: peak oxygen consumption predicts outcome in hypertrophic cardiomyopathy. Circulation. 2014;130(suppl_2):A19346–A19346. doi:10.1161/circ.130.suppl_2.19346
39. Saberi S, Day SM. Exercise and hypertrophic cardiomyopathy. Circulation. 2018;137(5):419–421. doi:10.1161/CIRCULATIONAHA.117.029989
40. Ramli FF, Hashim SAS, Raman B, Mahmod M, Kamisah Y. Role of trientine in hypertrophic cardiomyopathy: a review of mechanistic aspects. Pharmaceuticals. 2022;15(9):1145. doi:10.3390/ph15091145
41. Chuang C, Collibee S, Ashcraft L, et al. Discovery of aficamten (CK-274), a next-generation cardiac myosin inhibitor for the treatment of hypertrophic cardiomyopathy. J Med Chem. 2021;64(19):14142–14152. doi:10.1021/acs.jmedchem.1c01290
42. Maron Martin S, Masri A, Choudhury L, et al. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2023;81(1):34–45. doi:10.1016/j.jacc.2022.10.020
43. Wang J, Huang X, Liu H, et al. Empagliflozin ameliorates diabetic cardiomyopathy via attenuating oxidative stress and improving mitochondrial function. Oxid Med Cell Longev. 2022;9(1122494). doi:10.1155/2022/1122494
44. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi:10.1056/NEJMoa2022190
45. Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568–574. doi:10.1038/s41591-021-01659-1
46. Zakir RM, Folefack A, Saric M, Berkowitz RL. The use of midodrine in patients with advanced heart failure. Congestive Heart Failure. 2009;15(3):108–111. doi:10.1111/j.1751-7133.2008.00042.x
47. Lafitte S, Peyrou J, Reynaud A, et al. Midodrine hydrochloride and unexpected improvement in hypertrophic cardiomyopathy symptoms. Arch Cardiovasc Dis. 2016;109(3):223–225. doi:10.1016/j.acvd.2015.11.005
48. Tremaine L, Al-Fayoumi S, van de Wetering J, van Iersel T, Patel J, Chamberlin P. Abstract 10372: a clinical drug-drug interaction study of Imb-1018972, a novel investigational cardiac mitotrope in phase 2 development for the treatment of myocardial ischemia and hypertrophic cardiomyopathy. Circulation. 2021;144(Suppl_1):A10372–A10372. doi:10.1161/circ.144.suppl_1.10372
49. Hundertmark M, Siu AG, Matthews V, et al. A phase 2a trial investigating ninerafaxstat – a novel cardiac mitotrope for the treatment of diabetic cardiomyopathy (IMPROVE-DiCE). Eur Heart J. 2022;43(Supplement_2):
50. Horowitz JD, Chirkov YY. Perhexiline and hypertrophic cardiomyopathy. Circulation. 2010;122(16):1547–1549. doi:10.1161/circulationaha.110.981464
51. Napoli PD, Giovanni PD, Gaeta MA, Taccardi AA, Barsotti A. Trimetazidine and reduction in mortality and hospitalization in patients with ischemic dilated cardiomyopathy: a post hoc analysis of the Villa Pini D’Abruzzo trimetazidine trial. J Cardiovasc Pharmacol. 2007;50(5):585–589. doi:10.1097/FJC.0b013e31814fa9cb
52. Ho CY, Day SM, Axelsson A, et al. Valsartan in early-stage hypertrophic cardiomyopathy: a randomized phase 2 trial. Nat Med. 2021;27(10):1818–1824. doi:10.1038/s41591-021-01505-4
53. Kario K, Sun N, Chiang F-T, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in asian patients with hypertension. Hypertension. 2014;63(4):698–705. doi:10.1161/hypertensionaha.113.02002
54. Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018;138(14):1387–1398. doi:10.1161/circulationaha.117.033200
55. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(24):e783–831. doi:10.1161/CIR.0b013e318223e2bd
56. Kaltenbach M, Hopf R, Kober G, Bussmann WD, Keller M, Petersen Y. Treatment of hypertrophic obstructive cardiomyopathy with verapamil. Br Heart J. 1979;42(1):35–42. doi:10.1136/hrt.42.1.35
57. Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–769. doi:10.1016/s0140-6736(20)31792-x
58. Mastroianni A, Kahn J. Swinging on the pendulum. Shifting views of justice in human subjects research. Hastings Cent Rep. 2001;31(3):21–28. doi:10.2307/3527551
59. Heitner SB, Jacoby D, Lester SJ, et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy. Ann Intern Med. 2019;170(11):741–748. doi:10.7326/M18-3016
60. Desai MY, Owens A, Geske JB, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. journal article; clinical trial protocol. J Am Coll Cardiol. 2022;80(2):
61. Wang A, Heitner SB, Jacoby D, et al. Long-term safety and effectiveness of mavacamten in symptomatic obstructive hypertrophic cardiomyopathy (oHCM) patients (PTS): update from PIONEER open-label extension (PIONEER-OLE) study. Conference Abstract. Eur Heart J. 2019;40:65. doi:10.1093/eurheartj/ehz747.0063
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

