Back to Journals » International Journal of Nanomedicine » Volume 18
Clinical Trials for Oral, Inhaled and Intravenous Drug Delivery System for Lung Cancer and Emerging Nanomedicine-Based Approaches
Authors Aryal S, Park S, Park H , Park C, Kim WC, Thakur D, Won YJ, Key J
Received 29 August 2023
Accepted for publication 19 November 2023
Published 21 December 2023 Volume 2023:18 Pages 7865—7888
DOI https://doi.org/10.2147/IJN.S432839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Susmita Aryal,1 Sanghyo Park,1 Hyungkyu Park,1 Chaewon Park,1 Woo Cheol Kim,1 Deepika Thakur,1 Young-Joo Won,2 Jaehong Key1
1Department of Biomedical Engineering, Yonsei University, Wonju, Gangwon Province, 26493, Korea; 2Division of Health Administration, College of Software Digital Healthcare Convergence, Yonsei University, Wonju, Gangwon State, 26493, Korea
Correspondence: Jaehong Key, Department of Biomedical Engineering, Yonsei University, 1 Yonseidae-Gil, Wonju, Gangwon State, 26493, Korea, Tel +82-33-760-2857, Fax +82-33-760-2919, Email [email protected]
Abstract: Lung cancer is one of the most common malignant tumors worldwide and is characterized by high morbidity and mortality rates and a poor prognosis. It is the leading cause of cancer-related death in the United States and worldwide. Most patients with lung cancer are treated with chemotherapy, radiotherapy, or surgery; however, effective treatment options remain limited. In this review, we aim to provide an overview of clinical trials, ranging from Phase I to III, conducted on drug delivery systems for lung cancer treatment. The trials included oral, inhaled, and intravenous administration of therapeutics. Furthermore, the study also talks about the evolving paradigm of targeted therapy and immunotherapy providing promising directions for personalized treatment. In addition, we summarize the best results and limitations of these drug delivery systems and discuss the potential capacity of nanomedicine.
Keywords: lung cancer, clinical trials, nanomedicine, oral, intravenous, inhalation
Introduction
Lung cancer is the second most commonly diagnosed cancer worldwide overall, and it is the most commonly detected cancer in the male population.1 Moreover, lung cancer has the highest mortality rate among all cancer types, which corresponds to 18% of total cancer deaths.2 Thus, lung cancer is a major concern because of the rapid increase in its incidence and contribution to a substantial number of cancer cases.3 One of the main challenges in lung cancer is early diagnosis. The disease often lacks noticeable symptoms in its early stages and screening techniques are inadequate for widespread early detection purposes. Consequently, most patients with lung cancer are diagnosed at a later stage, leading to a lower survival rate.4–6 This emphasizes the need for innovative approaches to improve early detection and treatment outcomes.
There are different types of lung cancers. The two broad histological subtypes of lung cancer are small cell lung cancer (SCLC), which is the cause of 15% cases and non-small cell lung cancer (NSCLC), which accounts for 85% of cases. NSCLC is further subdivided into adenocarcinoma (ADC), squamous cell carcinoma (SCC), neuroendocrine tumors, large cell carcinoma (LCC).7–10 Small cell lung cancer, although less in common, is highly aggressive.
Lung cancer is also known to metastasize to various organs. In the metastatic process, cancer cells from the lungs can spread to distant sites including breasts, colon, or prostate.11 Addressing metastasis proves challenging owing to the complex nature, characterized by factors like tumor heterogeneity and the unique tumor microenvironment (TME). The challenges posed by metastasis and tumor heterogeneity are further compounded by the distinctive characteristics of the tumor microenvironment in comparison to healthy tissues. In the TME, angiogenesis, the formation of new blood vessels, is generally chaotic, immature and excessively branched with varying diameters and shunts. This leads to abnormal organization, function and structure of these vessels, resulting in heterogeneous and compromised blood flow.12,13 Moreover, the rapid growth of tumors outpaces the development of functional vessels, creating regions of hypoxia and increased acidity due to altered metabolic processes.14,15 The extracellular matrix (ECM) within the TME undergoes modifications, facilitating the invasion of cancer cells into surrounding tissues.16 Furthermore, the TME possess the capacity to suppress the immune response enabling cancer cells to evade detection. These unique features of the TME, including disorganized vasculature, acidic pH and hypoxic conditions, present a tremendous challenge in terms of evaluating and treating cancer. Addressing these challenges requires tailored efforts to develop effective therapies that account for these distinct characteristics.
Various treatment approaches are currently available for lung cancer, including surgery, ablation, radiation, chemotherapy, targeted therapy, and immunotherapy.17 The treatment regimen for lung cancer depends on the patient’s health and cancer stage. Surgery is considered the most effective approach for early-stage NSCLC, whereas chemotherapy is commonly used for advanced-stage lung cancer to prolong survival and improve the quality of life. Surgical resection is the primary option with consistently successful results, but it is only feasible for patients with an operable region who can receive the proposed surgical intervention.18
Both chemotherapy and chemoradiotherapy can be administered for all stages of small cell lung carcinoma (SCLC); however, surgical resection is the most frequent option for stage 1 NSCLC, and other treatment approaches such as chemotherapy, chemoradiotherapy, post-surgery, or neoadjuvant chemotherapy must be considered for patients with stage 2 and 3 NSCLC.19,20 Once diagnosed, patients with locally advanced or metastatic disease can be treated with chemotherapy. Generally, patients diagnosed with resected stage IIA/IIIA NSCLC undergo adjuvant chemotherapy.21 Chemotherapy can be administered alone or in combination with other anticancer agents, and significant progress has been made in improving its therapeutic efficacy in recent years.6 While platinum-based or non-platinum-based chemotherapeutic treatments show good efficacy in patients with stage IV disease, combining more than three cytotoxic drugs can cause a high incidence rate of anticancer toxicity without therapeutic improvement.
Nanomedicine is a rapidly growing field that harnesses the power of nanotechnology to prevent and treat diseases. Nanomedicine which utilizes nanoscale materials can potentially improve the delivery and effectiveness of anticancer drugs.22 Nanoparticles have unique properties that allow them to be suitable drug carriers. They enhance drug solubility, prolong circulation half-life, and facilitate targeted drug delivery to tumor tissues.23,24 For example, lipid-based liposomes have been used to encapsulate anticancer drugs, such as cisplatin; these nanoparticles specifically accumulate in lung tumor tissues, improving drug delivery and reducing toxicity in healthy cells. STEALTH® Liposomal Cisplatin, for instance, has shown potential for enhanced efficacy in patients with stage IIIB and IV NSCLC who have failed previous platinum-based treatments.25
Nanoparticles can be engineered for passive targeting based on enhanced permeability and retention effects, as well as active targeting through specific ligands. Active targeting involves ligands or molecules recognizing unique markers or receptors on cancer cells.26 Active and passive targeting are two distinct approaches; these strategies aim to deliver therapeutic agents selectively to cancer cells while minimizing damage to healthy tissues. Active targeting involves the use of specific ligands or molecules that can recognize and bind to unique markers or receptors present on the surface of cancer cells. A significant example of active lung cancer targeting is the selective targeting of Epidermal Growth Factor Receptor (EGFR), a protein that is typically overexpressed in Non-Small Cell Lung Cancer (NSCLC). This method employs the use of a specifically designed ligand such as the antibody Cetuximab, which binds specifically to EGFR.27,28 The selected targeting ligand is conjugated to a drug carrier. Upon systemic administration, the ligand on the drug carrier recognizes and binds to the overexpressed EGFR on the surface of lung cancer cells, facilitating internalization and subsequent drug release within the cancer cells. This approach enhances precision, delivering the therapeutic payload directly to cancer cells while minimizing the impact on normal tissues.29,30
On the other hand, Passive targeting relies on the distinctive characteristics of tumor tissues, such as leaky vasculature and defective lymphatic drainage, which allow drug carriers to accumulate selectively within the tumor.31 Nano sized drugs, such as nanoparticles or liposomes, which are not small enough to be excreted by the kidney or large enough to be rapidly recognized and trapped by the RES is tend to accumulate more in tumors due to the enhanced permeability and retention (EPR) effect.32 This effect allows these carriers to passively diffuse into the tumor tissue through leaky blood vessels and stay trapped there due to poor drainage.33 Passive targeting can be used by formulating drug carriers with the optimal size and surface charge, smarter mechanism for drug release and administration kinetics. The EPR effect entails the increased accumulation of nanomaterial accumulation in tumors due to features such as vascular fenestrations, inadequate lymphatic drainage and a thick extracellular matrix. This action permits nanoparticles to remain in the tumor for a longer period after entering it. Fibronectin and collagen are ECM components that serve as adhesive agents, potentially trapping nanoparticles. Tumor-associated macrophages can potentially aid in intratumoral transport. However, the EPR impact varies by person, and clinical trials demonstrate significant variation. Nanoparticle accumulation is influenced by tumor size, vascular density, and necrotic regions.34
In the battle against lung cancer, both active and passive targeting strategies can be employed in various ways: Researchers often combine active and passive targeting to maximize drug delivery to lung cancer cells while minimizing off-target effects. Ultimately, the choice between active and passive targeting, or a combination of both, depends on the specific characteristics of the patient’s lung cancer and the therapeutic agents being used. These targeting strategies represent important tools in the fight against lung cancer, contributing to more effective and less toxic treatments.
Targeted nanoparticle systems have also been developed to specifically deliver therapeutic agents to lung cancer cells, thereby increasing treatment effectiveness. One approach involves functionalizing nanoparticles with ligands that bind to overexpressed proteins in lung cancer cells, such as the epidermal growth factor receptor (EGFR).30 By incorporating ligands or antibodies onto the surface of nanoparticles, they can recognize and bind to unique biomarkers expressed on cancer cells within lung metastases. This targeted approach improves drug delivery, enhances therapeutic efficacy, and minimizes damage to healthy lung tissue.
The development of PEGylated triangular gold nanoplates functionalized with an anti-EGFR peptide for targeted delivery to lung tumors is a promising advancement in the field of nanomedicine.35 This approach leverages the overexpression of EGFR, a transmembrane protein, often upregulated in NSCLC,36 as a specific target for drug delivery. EGFR plays a key role in cell proliferation, survival, and differentiation.37 Targeting EGFR is exemplified using monoclonal antibodies like cetuximab and tyrosine kinase inhibitors such as erlotinib and gefitinib.38 Cetuximab effectively inhibits EGFR by blocking its ligand-induced activation,39 while erlotinib inhibits the intracellular tyrosine kinase domain of EGFR.40
Another intriguing target is the CXCR4, often overexpressed in various cancers including NSCLC and plays an important role in tumor growth and metastasis.41 Drugs like AMD3100 (plerixafor) inhibit the CXCR4 receptor and have been investigated for their potential in limiting cancer progression. Notably, AMD3100 has demonstrated efficacy in overcoming chemotherapeutic resistance in NSCLC cancer-initiating cells. Furthermore, it has also been observed to impede these cells from maintaining their stem-like properties.42
Furthermore, the Luteinizing Hormone-Releasing Hormone (LHRH) receptor is another potential target. LHRH receptors are expressed in various cancers and targeting them can be achieved by attaching LHRH analogs to drug carriers. This approach aims to leverage the overexpression of LHRH receptors in NSCLC cells for enhanced drug delivery. For eg - In the context of lung cancer treatment, the combination of targeted Deslorelin-docetaxel (D-D) chemotherapy and RGD-conjugated Flt3k anti-VEGF nanoparticles (RGD-Flt23k-NP) emerges as a promising strategy. D-D exhibits superior cytotoxicity by targeting LHRH receptors, while RGD-Flt23k-NP, a gene delivery system, enhances therapeutic efficacy. This combined approach demonstrates potent anti-tumor effects, surpassing individual therapies or docetaxel alone, suggesting a promising avenue for advanced lung cancer therapy.43
These examples showcase the diversity of receptors that can be targeted in NSCLC, each presenting unique opportunities for therapeutic intervention. Combining these targeted strategies with advanced drug delivery systems, such as nanoparticles, can further improve the precision and efficacy of lung cancer treatment.
In addition to their therapeutic applications, nanoparticles can serve as diagnostic tools. They can act as contrast agents in various imaging techniques including computed tomography, magnetic resonance imaging, and positron emission tomography. When functionalized with targeting ligands, they can specifically accumulate in lung tumors, aiding their detection and characterization.44
Furthermore, antibody-drug conjugates (ADCs) represent a promising treatment option for lung cancer-targeted therapy.45 ADCs combine the specificity of monoclonal antibodies with the cytotoxicity of small-molecule drugs;46 so, ADCs can spare healthy cells and improve treatment outcomes.47 Several ADCs targeting specific proteins that are overexpressed in cancer cells are currently undergoing clinical trials for lung cancer treatment. For example, rovalpituzumab tesirine (Rova-T), which specifically targets delta-like protein 3 (DLL3),48 has shown promising results in Phase 2 clinical trials for patients with SCLC who had previously received two lines of therapy.49
Immunotherapy, particularly with immune checkpoint inhibitors, has revolutionized the field of lung cancer treatment. These inhibitors block certain molecules that inhibit the immune response, allowing the immune system to effectively target and destroy cancer cells.50,51 Immune checkpoint inhibitors have shown promising results in NSCLC and have been approved for clinical use. In 2015, two immune checkpoint inhibitors targeting programmed cell death-1 (PD-1), namely nivolumab and pembrolizumab, gained approval for second-line therapy of NSCLC. Subsequently, in 2016, another checkpoint inhibitor targeting programmed death-ligand 1 (PD-L1), atezolizumab, was approved for the same indication. Furthermore, in 2016, pembrolizumab received approval for first-line NSCLC treatment in patients with high PD-L1 expressing tumors. These recent approvals firmly establish immunotherapeutic agents as the preferred second-line therapy for NSCLC, sparking increased interest in potential additional clinical applications for these agents.52 However, response rates to immunotherapy can vary, and biomarker screening is essential to identify patients suitable for this treatment approach.53
The route of administration also plays a significant role in optimizing therapeutic applications and controlling side effects associated with the type of anticancer drug. Choosing a specific route of administration, taking into consideration the site of action, type of drug, pH, volume, and solubility, is essential to avoid adverse effects and tackle obstacles.54
We summarized the various approaches for lung cancer treatment after searching all clinical trials registered in clinicaltrial.gov and conducted from early 1999 to late 2022. This review focuses on recent progress in clinical trials involving drug delivery systems for lung cancer treatment via oral, inhaled, and intravenous (IV) administration (Figure 1). This study aims to achieve two things: 1) evaluate the efficacy of monotherapy, combination therapy, and targeted therapy in lung cancer patients, and 2) highlight the challenges for implementing new drugs and the specific considerations for their clinical application and sustainable use in the future. In conclusion, these advancements in medicine and therapeutics have provided hope for patients with lung cancer and we hope that further research will continue to improve the lives of those affected by this devastating disease.
 |
Figure 1 Schematic representation showing current status of clinical trials of lung cancer through delivery pathway. Created with Biorender.com. |
Biological Barrier for DDS Depending on the Administration Routes
The respiratory system is a complex network of organs that work together to facilitate the exchange of gases between the environment and the body. It is divided into two main functional zones: conducting and respiratory. The conduction zones are responsible for transporting air to the respiratory zones, whereas the respiratory zones are responsible for the actual exchange of gases.55
The conducting zones consist of the trachea, bronchi, and bronchioles, whereas the respiratory zone comprises the airways and alveoli (Figure 2). There are approximately 5.6×108 human alveoli with an estimated total surface area of approximately 140 m2.56 The pseudostratified epithelium provides a barrier for absorption into the bloodstream and differs between distinct parts of the lungs. The airway epithelia constitute gradually thinning columnar epithelium, with the bronchial and bronchiolar epithelia having thicknesses of 3.5 mm and 0.5–1 mm, respectively. In contrast, the alveolar epithelium is one cell thick, and the distance between the alveolar lumen and the bloodstream is less than 400 nm. Owing to the large accessible surface area of the alveoli and the close air-blood contact in this region, this zone is ideal for gaseous exchange and absorption of inhaled aerosols, including drug delivery systems.57
The continuous development of medical technology in recent decades has led to an in-depth understanding of lung function and characteristics. The lung is an ideal organ for drug delivery owing to its large absorptive surface area, abundant capillaries, and minimal transport distance. Physiochemical properties such as size, shape, and surface characteristics are among the key parameters that should be considered for an efficient drug delivery system for a particular therapeutic agent via a specific administration route.24
Pulmonary administration has attracted considerable scientific attention for drug delivery; however, it has some limitations.58 The primary disadvantage is that most drugs need to be administered at least three to four times a day, owing to the short duration of the resultant clinical effects. Additionally, the rapid absorption of drugs such as bronchodilators and corticosteroids from the lung epithelium results in undesirable side effects.59
Understanding the transport and deposition of drugs is fundamentally important for drug administration. Herein, we report different administration therapies and their clinical effects, advantages, and limitations.
Oral Administration
Despite the significant advancements in drug delivery via other routes, oral drug delivery remains the most promising and popular approach. This is due to the convenience, therapeutic efficacy, controlled and targeted delivery, enhanced mucosal immune response, non-invasiveness, and long half-life of solid formulations. Additionally, higher patient preference is another factor contributing to its popularity.60,61 Furthermore, controlled drug release is mainly applicable to oral administration by designing the drug dosage form at various concentrations.62
Recently, several oral anticancer drugs have been approved and many other developments are underway, with new drugs for oral chemotherapy on the horizon. Table 1 summarizes the orally administered drugs that are currently being tested and developed in clinical trials for patients with lung cancer.
 |
Table 1 The Synopsis and Results of Clinical Studies on Drugs Administered Orally |
With the increasing number of oral agents on the market, we can expect a rapid increase in the use of oral chemotherapy in the coming years. Although over 60% of all anticancer drugs are available in oral dosage forms for clinical therapy,76 due to limited oral bioavailability, very few of them are used in clinical practice. However, oral agents such as vinorelbine, gemcitabine, paclitaxel, and docetaxel are useful and often as effective as their IV formulations,77 and are widely used in improving therapeutic index.
In this study, Bordonaro et al sought to determine whether oral chemotherapy has an advantage over IV chemotherapy. They compared oral vinorelbine with IV gemcitabine63 and found no significant difference in efficacy. However, patients treated with oral vinorelbine reported significantly improved quality of life, role, emotional, and social functioning, whereas cognitive functioning improved with IV gemcitabine. Additionally, oral vinorelbine was well tolerated and showed improvements in symptoms such as dyspnea, sore mouth, and chest pain, and the patient satisfaction rate was higher than with IV gemcitabine. Therefore, owing to its comparable efficacy and toxicity profiles, oral vinorelbine was more effective than IV gemcitabine.
The treatment option for NSCLC is based not only on the stage of cancer, but also on other factors such as the patient’s health status, lung function, and specific characteristics of the cancer itself. For example, metronomic chemotherapy, which can minimize the toxicity caused by traditional chemotherapeutic agents, involves the close and regular administration of chemotherapeutic agents at a less toxic dose over a longer period. Guetz et al reported a phase I dose-finding study in patients with advanced NSCLC treated with daily oral vinorelbine at a fixed dose concentration of 20–50 mg/d.64 The results showed that the recommended dose (30–40 mg/d) of oral vinorelbine (Navelbine®oral, NVBo) is feasible and safe, but high concentrations resulted in neutropenia and fever.
A combination of cytotoxic drugs has successfully improved efficacy and led to acceptable toxicity in first- and second-line treatments. Cisplatin has been used effectively and actively in combination therapy.65 Tan et al investigated the first-line treatment efficacy and compared IV and oral vinorelbine with cisplatin versus docetaxel in a cisplatin-based combination for NSCLC.66 This study demonstrated that doublet chemotherapy combinations of NVBiv (IV vinorelbine), NVBo (oral vinorelbine), cisplatin, and docetaxel (DCT) achieved similar results in terms of response, temporal parameters, and quality of life, with an acceptable tolerability profile. In this study, oral vinorelbine was found to be more effective than IV vinorelbine.
Similarly, Lerouge et al conducted a Phase II study to evaluate the efficacy and safety of fractionated oral vinorelbine with cisplatin as induction chemotherapy, followed by chemotherapy and radiotherapy. The results showed an overall response rate of 50%, disease control rate of 81.42%, and survival rates of 68.6% and 37% at one year and two years, respectively.78 Moreover, oral vinorelbine in combination with cisplatin was well tolerated, and had the highest response rate compared to IV vinorelbine-based chemoradiotherapy regimens.
Ding et al used apatinib, a tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor-2, in daily dose of 850 mg, 28 days per cycle to treat two patients with NSCLC after the failure of first- or third-line chemotherapy.79 The progression-free survival (PFS) of the first patient increased to 4.6 months after palliative therapy with apatinib compared to almost 6 months in the second patient. Although hypertension and hand-foot syndrome are the most common side effects of apatinib, its toxicity is controllable and tolerable. Therefore, apatinib may be a treatment option for advanced NSCLC after failure of chemotherapy or other targeted therapies.
Cancer cachexia is a weight loss syndrome caused by the loss of fat and muscle due to chronic diseases such as cancer. Typically, 50–80% of patients in the terminal stage of cancer show signs of cachexia. Xie et al investigated the clinical value of treating lung cancer cachexia using a combination of thalidomide and cinobufagin.67 They compared an experimental group that received thalidomide and cinobufagin with a control group that received only cinobufagin. The results showed a significant improvement in the nutritional status, quality of life, and survival of patients receiving thalidomide and cinobufagin compared to those receiving cinobufagin alone. Additionally, the combination of thalidomide and cinobufagin showed better tolerability and efficacy than cinobufagin alone. Therefore, the combination of thalidomide and cinobufagin demonstrates a promising combination regarding safety, efficacy, and good tolerance in clinical settings and could be used in lung cancer cachexia.
Epidermal growth factor receptor stimulates the growth and differentiation of cancer cells, and EGFR tyrosine kinase inhibitors block EGFR activity, further halting or slowing cancer cell growth. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (EGFR-TKIs) are an important class of targeted therapies in the treatment of non-small cell lung cancer.80 The first-generation EGFR-TKIs, include erlotinib and gefitinib. These drugs inhibit kinase activity by competitively interacting with the ATP-binding site of EGFR, preventing auto-phosphorylation and consequently inhibiting downstream signaling. This inhibition leads to apoptosis in cells dependent upon EGFR signaling, such as those with EGFR mutations. Resistance to first-generation EGFR TKIs has prompted the development of second-generation TKIs. These newer inhibitors are designed to circumvent resistance mechanisms and perhaps outperform earlier EGFR TKIs such as erlotinib or gefitinib. Second-generation TKIs frequently create irreversible covalent connections with the EGFR kinase domain, displaying increased action against other EGFR family members and structurally related receptors such as VEGF. Their covalent binding may improve efficacy against resistance-inducing mutations like T790M, which are less sensitive to first-generation TKIs. This is an encouraging step forward in the treatment of EGFR-mutated malignancies.81 Despite advancements, resistance mechanisms persist, leading to the emergence of third-generation EGFR-TKIs like osimertinib. Osimertinib is a very successful third-generation covalent inhibitor that targets both EGFR-sensitizing and T790M resistant mutations in advanced non-small cell lung cancer patients. When administered as a second-line treatment, clinical studies have shown outstanding overall response rates of more than 50%. Common adverse events with a moderate side effect profile.82 The evolution from first-to-third generation EGFR-TKIs highlights the dynamic landscape of precision medicine in lung cancer, demonstrating the continual efforts to overcome resistance and enhance treatment outcomes for patients with NSCLC. Therefore, the development of targeted therapies has progressive implications for the treatment of lung cancer. Tyrosine kinase inhibitors are a good example of successful cancer targeting to improve therapeutic efficacy and overcome resistance problems. Eg, Gefitinib is an EGFR-TKI and has recently been approved in several countries for use in advanced or metastatic NSCLC. The 63-patient trial focuses on individuals who got gefitinib after conventional chemotherapy failed or because they were unable to accept conventional treatment. The results show promising results, with a median time to progression of 161 days and an overall response rate of 11%, including full remission in 2% of patients and partial remission in 9%. Furthermore, 38% of patients had stable disease. Gefitinib indicated anti-tumor efficacy in both pretreated and previously untreated NSCLC patients during a median of 311 days of follow-up. Importantly, the medicine had a low toxicity profile and was well tolerated, indicating that gefitinib might be a viable treatment option for people with NSCLC and promoting its use in clinical trials.83
AZD9291 is an orally administered potent irreversible EGFR tyrosine kinase inhibitor. It is selective for EGFR tyrosine kinase inhibitor-sensitizing mutations and T790M resistance mutations. To determine the safety and efficacy of AZD9291, Janne et al administered AZD9291 at doses ranging from 20 to 240 mg. They found an overall tumor response rate of 61%. Thus, AZD9291 may be highly effective in patients with lung cancer harboring the EGFR T790M mutation.68 No limiting toxic effects were observed in patients receiving doses ranging from 20 mg to 40 mg. Moreover, only 10% of patients experienced side effects such as diarrhea, rash, and nausea, and the severity of these symptoms increased with increasing doses.
Rociletinib is an orally administered EGFR inhibitor that is effective against EGFR-mutated NSCLC with or without T790M. To investigate the side effects, pharmacokinetics, and anti-tumor activity in patients with advanced EGFR mutated NSCLC, Sequist et al used rociletinib at a dose ranging from 500 mg to 1000 mg and reported a response rate of 59% and disease control rate of 93% in T790M- positive tumors, and 29% response rate and 59% disease control rate in T790M-negative tumors.69 In this study, Rociletinib did not cause rash, stomatitis, or paronychia, but caused hyperglycemia. Although Rociletinib showed tumor response and sustained disease control in patients with EGFR-mutated NSCLC, it may not be effective owing to the toxic effects of hyperglycemia. Moreover, Sequist et al investigated the response to osimertinib in patients with EGFR mutation-positive lung cancer who had previously received rociletinib.84 They found that patients with rociletinib resistance benefited from the use of osimertinib.
Brigatinib is an oral tyrosine kinase inhibitor used to treat patients with anaplastic lymphoma Kinase (ALK)+NSCLC. Camidge et al found that patients with ALK+ NSCLC showed significant improvements in anti-tumor activity.70 However, several side effects have been observed in patients after treatment, including nausea, diarrhea, fatigue, cough, and headache.
Lin et al evaluated the safety and pharmacokinetics of trametinib in Kirsten rat sarcoma (KRAS)-mutated nonmetastatic NSCLC71 while its efficacy and safety still need further research, it has been reported that trametinib, when combined with dabrafenib, had durable clinical effects over a 5-year survival period in patients with BRAF V600E-mutant metastatic NSCLC.85
Yang et al reported improved overall survival rates with afatinib compared to patients who received cisplatin, pemetrexed, or gemcitabine.72 In their study, patients with EGFR mutation-positive stage IIIB or IV lung adenocarcinoma were treated with afatinib or cisplatin plus pemetrexed or gemcitabine. Accordingly, afatinib was approved for first-line treatment of metastatic EGFR mutations and non-resistant mutations.
A phase 3 clinical study showed significant improvement in the overall survival rate in patients who received dacomitinib and suggested that it could be the standard treatment for patients with advanced NSCLC and activating mutations in EGFR. Patients who took dacomitinib showed a lower death rate and improved overall survival rate, which was 34.1 months versus 26.8 months with gefitinib.73
In conclusion, the use of oral chemotherapy in the treatment of lung cancer shows promise in improving patient outcomes and quality of life. Oral vinorelbine is as effective as IV chemotherapy, with significantly improved quality of life and patient satisfaction rates. Metronomic chemotherapy and combination therapy have also been successful in improving efficacy and reducing toxicity. Targeted therapies such as EGFR tyrosine kinase inhibitors and ALK inhibitors have shown promising results in patients with specific genetic mutations.
For example, Shaw et al initiated a Phase III clinical study and evaluated lorlatinib, a third generation ALK inhibitor the authors introduced. In this study, patients with previously untreated advanced ALK-positive NSCLC who received lorlatinib had longer PFS and a higher frequency of intracranial response (78% (95% confidence interval [CI], 70–84) in the lorlatinib group and 39% (95% CI, 30–48) in the crizotinib group).74
Despite the advantages of oral administration, there are some limitations. The harsh environment of the gastrointestinal tract, including digestive enzymes, gastric acids, pH, and poor permeability of the intestinal epithelium, are major obstacles to minimizing the therapeutic effects of drugs through oral administration (Figure 3).86,87 Entrectinib is a ROS1 inhibitor that effectively penetrates the CNS75 however, due to its poor aqueous solubility, advanced self-nano emulsifying drug delivery systems (SNEDDS) have been suggested to improve solubility and emulsification. Reddy et al suggested an optimized design to overcome the poor solubility, moderate permeability, and pH-dependent solubility of entrectinib.88
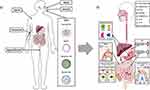 |
Figure 3 Schematic depiction of the different routes for nanoparticle drug delivery, particularly emphasizing oral administration and interactions within the intestinal barrier.89 Figure (a) depicts various routes for nano-drug delivery (b) highlights challenges in oral delivery such as enzymatic degradation (pepsin, lipase, peptidase, amylase), mucus entrapment, and pH variations in the gastrointestinal tract. The enterocyte transport mechanisms involve transcytosis, direct passage, and paracellular routes, while M cells in the gut associated lymphoid tissue (GALT) play a role in antigen detection for immune response. Notes: Reprinted with permission from Vitulo M, Gnodi E, Meneveri R, Barisani D. Interactions between nanoparticles and intestine. International Journal of Molecular Sciences. 2022 Apr 14;23(8):4339. Copyright © 2022.89 This image even after increasing resolution words are blurred because it’s the same in original article also. |
Nanomedicine-based drug delivery is an effective approach to overcoming existing challenges in drug administration.90 To improve oral administration, a wide range of drug carriers, including vesicles, micelles, solid particles, hollow structures, porous materials, tubes, mesoporous and hydrogels (Figure 4) have been intensively investigated.91 Each carrier type presents unique advantages and functionalities, contributing to potential application in oral drug delivery.
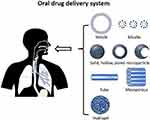 |
Figure 4 Illustration of diverse Nanodrug designs for oral administration: A Spectrum of Vesicle, Micelle, Solid, Hollow, Pored microparticles, Tube, Mesoporous and Hydrogel Architectures. Notes: Reprinted with permission from Homayun, Bahman, Xueting Lin, and Hyo-Jick Choi. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 11.3 (2019): 129. Copyright © 2019.60 |
Of all the particle designs, polymeric nanoparticles have been extensively studied and have shown advantages in improving barriers encountered during oral delivery, such as enzymatic degradation and poor membrane permeability.92,93 For example, Razak et al demonstrated versatile nanotechnological approaches for treating lung cancer.94 They summarized various nanocarrier systems, such as nanoparticles, solid lipid nanoparticles, liposomes, polymeric nanoparticles, polymeric micelles, lipid-polymer hybrid nanoparticles, carbon nanotubes, mesoporous silica nanoparticles, and gold nanoparticles. As a therapeutic dosage-release system, nanoparticle formulations can reduce drug toxicity and enhance drug delivery to tumor sites either passively or actively.
Multidrug resistance (MDR) is a major obstacle to the success of cancer therapy, with one of its primary contributors being the overproduction of P-glycoprotein (P-gp), which acts as an efflux pump for various anticancer drugs.95 This active efflux mechanism reduced intracellular drug concentrations and thereby impeded accessibility of drugs to their sites of action, ultimately leading to reduced susceptibility.96 The clinical implications of P-gp-mediated MDR are profound, impacting the efficacy of treatments across various cancer types. Researchers have endeavored to address this challenge through strategies such as P-gp inhibitors, nanoparticle-based drug delivery systems to enhance drug bioavailability97 and the exploration of alternative treatment modalities like immunotherapy.98 Overcoming P-gp-mediated MDR is a critical focus in cancer research, aiming to enhance the success of treatment regimens and improve patient outcomes in the face of this complex resistance mechanism.
Low bioavailability is another problem with orally administered drugs, especially anticancer drugs such as paclitaxel and docetaxel.99 The low systemic bioavailability of paclitaxel is due to its high affinity for the multidrug efflux pump, p-glycoprotein. It is particularly important to bypass p-glycoprotein, which can cause drug resistance. To solve this problem, Liqin Jiang et al developed thiolated chitosan-modified PLA-PCL-TPGS (poly (lactide-co ε-caprolactone)-d-α-tocopheryl polyethylene glycol 1000 succinate) nanoparticles to increase paclitaxel transport by opening tight junctions and bypassing the efflux pump of p-glycoprotein.100,101
Jian Hou et al induced a paclitaxel-loaded mixed micelle (PTX-TP-M) with vitamin E-TPGS (TPGS) and Plasdone®S-630 Copovidone (PVPS 630), with vitamin E-TPGS enhancing permeability for intestinal absorption and acting as a p-glycoprotein inhibitor.102 The mixed micelles contain paclitaxel inside, with TPGS having a high affinity for the membranes of lung cancer cells. Therefore, owing to the enhanced solubility of paclitaxel and absorption moieties such as TPGS and PVPS630, the permeability of PTX-TP-M is significantly increased, and efflux pumps are modulated through its p-glycoprotein inhibitor activity.
Therefore, nanocarriers such as polymeric nanoparticles and mixed micelles are a promising approach for the oral delivery of anticancer drugs. By using these nanocarriers, the bioavailability of drugs can be improved, leading to improved efficacy and reduced toxicity.103,104
Administration by Inhalation
Another route for the administration of anticancer drugs is through the respiratory tract, by delivering them directly to the lungs through the nose. Pulmonary inhalation has received significant attention because of its large absorptive surface area, low epithelial barrier thickness, and good blood supply to the lungs.105 This method offers a noninvasive route of drug delivery that facilitates the local delivery of anticancer drugs directly into tumor tissues while bypassing non-targeted organs.106 Moreover, chemotherapeutic drugs administered by inhalation help reduce pulmonary side effects and reduce the risk of distribution to healthy lung cells.107,108
Compared to oral and IV administration, pulmonary delivery improves drug bioavailability in the lungs owing to limited intracellular and extracellular drug-metabolizing enzyme activities in the lungs, unlike in the gastrointestinal tract and liver. Inhalation also delivers chemotherapeutic agents directly to tumor tissues, reducing the risk of damage to adjacent healthy cells.109,110 For instance, Koushik Os111 et al used nanostructured lipid carriers (NLC) and liposomes as model carriers to deliver drugs locally to the lungs. They found that after IV injection, 40–60% of both NLC and liposomes accumulated in the liver, whereas less than 25% of the injected dose was found in the lungs. In contrast, the same carriers delivered via inhalation accumulated approximately 80% in the lungs, showing better localization after inhaled administration.
Storti et al summarized preclinical studies and showed that the inhalation of aerosolized drugs is a promising option for treating lung cancer.112 For example, it could reduce the systemic distribution of drugs and patient discomfort, such as pain due to needle injection from IV administration. Jyoti et al synthesized cationic small unilamellar niosomes loaded with curcumin (Cur-C-SUNS), which exhibited a positive surface charge that enhanced the penetration rate between the nanovesicles and the mucosal surface to increase drug stability.113 They demonstrated that the accumulation rate in the lungs could be controlled by different surface charges. Assuming they have to bypass filtration in the primary and secondary bronchi and bronchioles, these nanovesicles are optimized to a diameter of 50— 200 nm, and the size of the nanobubbles varies according to the drug dose. Cur-C-SUNS accumulated more strongly in A549 cells than non-cationic small unilamellar liposomes (Cur-SUNS).
Table 2 summarizes the clinical studies conducted to evaluate the safety and efficacy of inhaled drugs. For example, Otterson et al used inhaled doxorubicin in combination with cisplatin and docetaxel to treat patients with NSCLC.114 Although this therapy was considered safe as expected, no therapeutic effect was achieved.
 |
Table 2 The Synopsis and Results of Clinical Studies on Drugs Administered Through Respiratory Inhalation |
Paul et al used inhaled carboplatin with a combination of IV infusion and aerosol to treat 60 patients aged 18–70 years old admitted with NSCLC. The primary endpoint was the evaluation of efficacy and safety.115 There was a significant improvement in patient survival, and this therapy was better than IV infusion alone. Etienne et al investigated the biodistribution, pharmacokinetics, safety profile, and feasibility of aerosolized gemcitabine in patients with lung carcinoma and found that aerosolized gemcitabine was safe with minimal toxicity.116
Researchers are actively addressing challenges associated with pulmonary drug delivery. In the context of pulmonary administration, a significant limitation arises from the potential bolus release of administered drugs, which can pose a risk of pulmonary toxicity.116 The bolus release refers to the rapid and concentrated delivery of a drug upon administration. This phenomenon can result in localized toxicity within the pulmonary tissues. The abrupt exposure of lung cells to high concentration of certain drugs may lead to adverse effects, inflammation, or damage to the delicate respiratory structures.117 To address this challenge, researchers are actively exploring controlled- release formulations and advanced drug delivery systems that can provide a more sustained and regulated release of therapeutic agents, aiming to enhance the safety and efficacy of pulmonary drug delivery for conditions such as lung cancer. This consideration underscores the importance of refining administration techniques to mitigate the risk of pulmonary toxicity associated with bolus drug release.
However, the effectiveness of inhaled drugs depends on overcoming various immune barriers, including mucociliary clearance, alveolar macrophage clearance, enzymatic degradation, rapid systemic absorption, nasal passages, pharynx, larynx, and bronchial wall,118–122 that can reduce the delivery efficiency of drugs (Figure 5).
 |
Figure 5 Barriers that impact the effectiveness for treating lung infection via pulmonary drug delivery is depicted. (a) Mucociliary clearance (b) biofilm (c) pulmonary surfactant and (d) alveolar macrophage clearance. Notes: Reprinted with permission from He S, Gui J, Xiong K, Chen M, Gao H, Fu Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. Journal of nanobiotechnology. 2022 Mar 3;20(1): 101. Copyright © 2022.123 |
To overcome these challenges and increase efficiency, particulate-based drug delivery systems have been introduced. Particulate-based drug delivery offers several advantages, including protecting the drug from enzymatic degradation, evading pulmonary clearance, slowing down drug absorption, delivering the drug directly to the target site in the lungs, providing controlled drug release, reducing dose frequency, maximizing therapeutic efficacy, and minimizing adverse side effects.
To achieve the therapeutic potential of inhaled drugs, several factors, such as the aerodynamic diameter, shape, and surface properties of the carriers, should be tailored and optimized. For example, particles smaller than 5–6 µm settle in the tracheal-bronchial area, while ultrafine particles with a size of 1–2 µm are deposited in the bronchioles. Nanoparticles less than 1 µm in size reach the lower respiratory system. Dendrimers, which are ultra-small nanoparticles measuring less than 20 nm, are effective in delivering drugs to the alveoli; however, their retention in the lungs is limited because they quickly enter the bloodstream.124,125
Despite these advancements, no clinical trials have confirmed the effectiveness of inhalation as a treatment method. The primary challenge in this area is the difficulty of replicating the complex structure and function of human lungs in their entirety, especially given the differences in organ size, vital capacity, and circulation between mice and humans. If these challenges are addressed, inhalation could become an efficient treatment option for lung cancer and other respiratory diseases.
Intravenous Administration
IV administration refers to the direct injection of drugs into the vein using a needle or tube. This method ensures high drug concentration and rapid injection into blood vessels, allowing the drug to enter the bloodstream quickly and directly without passing through the gastrointestinal tract (Figure 6). It also provides high bioavailability and the fastest capacity to overcome physiological barriers to drug absorption.
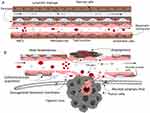 |
Figure 6 Nanoparticle – guided journey: The microenvironment of (A) healthy and (B) tumor tissues are compared. The chaotic components of the tumor microenvironment (hypoxic core, obstructed lymphatic drainage, insufficient pericyte population, disorganized basement membrane, and broad fenestration are used to enhance the EPR effect. Notes: Reprinted with permission from Ejigah V, Owoseni O, Bataille-Backer P, Ogundipe OD, Fisusi FA, Adesina SK. Approaches to improve macromolecule and nanoparticle accumulation in the tumor microenvironment by the enhanced permeability and retention effect. Polymers. 2022 Jun 27;14(13):2601. Copyright © 2022.122 |
Table 3 summarizes clinical studies on intravenous drug delivery to patients with lung cancer. Von Pawel et al reported that amrubicin, a third-generation anthracycline and topoisomerase inhibitor II that is administered Intravenously, significantly improved all secondary efficacy endpoints in patients with SCLC.126 Amrubicin demonstrated efficacy and a safety profile comparable to that of topotecan and showed greater response rates and a 2-week survival benefit in patients with refractory illness, a patient category for which there are currently no approved treatments.
 |
Table 3 The Synopsis and Results of Clinical Studies on Drugs Were Administrated by Vein |
When combined with chemotherapy, the effect of treatment can change depending on the sequence of drug administration. S-1 is an oral anticancer agent consisting of tegafur, 5-chloro-2, 4-dihydroxypyridine, and potassium oxonate. In a study by Ichinose et al, combination chemotherapy with oral administration of S-1 and IV administration of cisplatin showed promising efficacy with acceptable toxicity rates in patients with advanced NSCLC.127
Blumenschein et al studied the efficacy and safety of combination treatment with IV radiation. Cetuximab is a monoclonal antibody that targets EGFR-expressing non-small cell lung cancer and has demonstrated radiosensitive properties in preclinical models. Combining cetuximab with chemoradiotherapy (CRT) for unresectable stage III NSCLC resulted in a statistically significant improvement in median and overall survival compared to that previously reported by the Radiation Therapy Oncology Group.128
Fink et al investigated a new combination of chemotherapies and compared the safety and efficacy of topotecan-cisplatin (TP), topotecan-etopside (TE), and cisplatin-etopside (PE) in SCLC with extensive disease (ED-SCLC).128 The results showed that the combination of IV topotecan/cisplatin is an active regimen for ED-SCLC and is non-inferior to standard PE therapy. TP is associated with a higher percentage of hematological toxicities, resulting in higher transfusion rates, particularly for red blood cells. A higher rate of treatment-related deaths was observed; however, this difference was not statistically significant. Despite higher response rates and longer progress free survival (PFS), TP will not replace the standard PE therapy in the first-line treatment of ED-SCLC owing to its higher toxicity. However, topotecan remains the first-line treatment for relapses.
In contrast, researchers who have invented new drugs that can be administered intravenously are focusing on the use of viral vectors that express tumor suppressor genes and are limited to intratumoral administration or a single IV dose owing to systemic immune responses.137 Lu et al found that functional TSG (Tumor-Suppressor Genes) can be administered intravenously to human cancer cells, which can express mRNA and proteins in cancer cells and mediate anti-tumor activity.138 This was the first systemic gene therapy study in humans to administer the tumor suppressor gene TUSC2, which is known to mediate the induction of apoptosis and alter relevant pathways in cancer cells.130
Although metal-based anticancer drugs have side effects, ruthenium has been considered a candidate for new anticancer drugs owing to its unique properties. These include transferrin transport, activation by reduction, slow ligand-exchange kinetics, and DNA binding, which can be applied to ruthenium-derived anticancer drugs.139,140
Leijen et al studied the combination of gemcitabine and a ruthenium-derived anticancer drug, NAMI-A (Novel Anti-tumor Metastasis Inhibitor A), which was administered intravenously to patients with NSCLC after first-line treatment.141 However, the results showed that the combination of NAMI-A and gemcitabine was only moderately tolerated and less effective than gemcitabine alone in patients with NSCLC after first-line treatment. Although NAMI-A has demonstrated its potential as an effective agent for treating metastasis in preclinical studies, its toxicity profile and efficacy in these settings raise uncertainties about its future role as a treatment option.
Horinouchi et al administered simultaneous chemoradiotherapy with cisplatin and vinorelbine in patients with stage III NSCLC and approximately 15% of patients were cured with no severe toxicities observed in the following 10 months.131
Pemetrexed is approved as first-line and second-line therapy for patients with progressive relapsed NSCLC. However, its significant toxicity, including myelosuppression, skin rash, mucositis, and fatigue, makes it ineffective when used alone.142 Alex et al investigated the use of pemetrexed in combination with bevacizumab injected intravenously every 3 weeks in patients with NSCLC.131 Although this study did not reach its primary endpoint of 3-month PFS, it confirmed 4-month survival, which is promising.
In addition, there are many ongoing studies investigating new approaches for the anti-tumor activity and treatment of malignancies in various tumor types, including NSCLC. Among the recently approved immune checkpoint inhibitors, nivolumab binds to programmed cell death protein 1 (PD-1) receptors expressed in T cells and restores T cell effector function.
Gettinger et al investigated the safety, objective response rate, and PFS rate of nivolumab monotherapy133 and concluded that it was well tolerated as first-line therapy for patients with advanced NSCLC and showed a durable response.
In contrast, atezolizumab and PD-L1 antibodies can restore tumor-specific T cell immunity. In a Phase 1 study involving patients with relapsed or refractory small cell lung cancer, vedolizumab monotherapy had acceptable side effects and safety profiles.
Horn et al evaluated the efficacy and safety of co-administering atezolizumab or a placebo with carboplatin and etoposide, which is the first-line treatment for extensive-stage SCLC.134 The study found that the combination of chemotherapy with atezolizumab prolonged overall survival (OS) and PFS in patients with SCLC compared with chemotherapy alone. Therefore, this study suggests that the combination of checkpoint inhibition with cytotoxic chemotherapy may have a synergistic effect and improve efficacy.
Kluger et al evaluated the efficacy and safety of APX005M in combination with nivolumab in patients with NSCLC. In their clinical study, one of four patients who did not receive immunotherapy showed a clinical response (CR), and two patients had stable disease.135
Pembrolizumab, known as anti-PD1 (programmed death ligand), was approved for patients who have metastatic NSCLC with tumors expressing PD-L1 (TPS (Tumor Proportion Score) ≥1%) with progression, or after platinum-containing chemotherapy in 2015, and was allowed as the first-line treatment for patients with stage III NSCLC in 2019.136
IV administration is considered one of the most effective methods of drug delivery for the treatment of lung cancer because it allows drugs to enter the bloodstream quickly and directly, bypassing the gastrointestinal tract. When combined with other treatment regimens, such as oral administration and radiotherapy, IV administration has shown promising results in producing more effective outcomes than single treatment methods, offering the possibility of developing a more comprehensive and effective treatment plan for lung cancer patients.143
One of the key advantages of IV administration is its precise control over drug dosage, which can be particularly important in treating lung cancer. However, this method of administration carries some risks, such as direct exposure of drugs in the systemic circulation, requiring close monitoring by healthcare professionals to ensure proper administration and minimize potential complications, which are expensive and painful.144 However, these challenges can be overcome using nanomedicines which permit the development of novel platforms for the efficient transport and controlled release of drug molecules.145 Efficient nanostructured delivery systems allow for reduced side effects caused by non-optimized drug dosages and present a variety of synergistic properties.
One example is Abraxane (nab-paclitaxel). When comparing the conventional approach, which involves using Cremophor EL (CrEL) and ethanol as solvents, to nab-paclitaxel, notable differences were observed in the pharmacokinetic and biodistribution properties. Nab-paclitaxel has been shown to display improved anti-tumor efficacy and a better safety profile while reducing the risk of prolonged systemic exposure and slow tissue distribution.146
Conclusion and Future Perspective
Lung cancer is a complex and heterogeneous disease with various genetic mutations that necessitate personalized treatment plans based on individual circumstances.147 Various systemic treatment options are available, including different modes of drug delivery such as oral, IV, and inhalation. However, each mode has limitations and nanomedicines have emerged as promising solutions to overcome these challenges.148
Clinical trials using conventional drug delivery systems have shown varying degrees of success, depending on the mode of administration and the specific drug being delivered. Oral administration, while convenient, can result in poor bioavailability owing to drug degradation in the gastrointestinal tract. Alternatively, IV administration can overcome bioavailability issues but carries the risk of adverse reactions and requires skilled personnel. Inhalation offers a non-invasive route for targeted drug delivery to tumor tissues; however, obstacles such as mucociliary clearance and enzymatic degradation can reduce the delivery efficiency.149
Immunotherapy, especially immune checkpoint inhibitors, has shown remarkable success in directing the body’s immune system against lung cancer cells. This presents a promising alternative for advanced-stage patients, offering potential for prolonged survival and improved quality of life. Nanomedicines have demonstrated the potential to enhance drug delivery efficacy by improving the solubility, stability, absorption, and controlled release of drugs. Personalized nanomedicines tailored to an individual’s genetic makeup or disease state offer targeted therapies for specific cells or tissues. The nano drug delivery system, which is gaining attention as an emerging therapy, can be customized for each patient’s health condition, which enhances drug efficacy and controls side effects.150 For instance, targeted nanoparticles functionalized with specific ligands such as antibodies can selectively bind to cancer cells, enabling precise drug delivery to tumors. These nanoparticles can be engineered to respond to specific stimuli, thereby enhancing their specificity and effectiveness.
However, despite the potential benefits, developing personalized nanomedicine presents significant challenges in terms of safety, efficacy, regulatory approval, and complex technology. The interaction of nanomedicines with the human immune system is a critical challenge that needs more understanding and additional research. The inherent complexity of scaled-up synthesis, which is necessary for bringing nanomedicines from the laboratories to large-scale manufacturing, requires innovative engineering and rigorous techniques. The regulatory framework complicates matters further by imposing rigorous guidelines. While these criteria are important for safety, they also serve as restrictions. In order to fully exploit the transformative potential of personalized nanomedicine in healthcare, the journey from concept to clinical reality requires scientific knowledge, a strategic approach to address safety concerns, rationalization of regulatory processes, and overcome technological hurdles.151
To address these challenges, in-depth research into the interactions between nanomedicine and biological systems is crucial. Understanding the pharmacokinetics, biodistribution, and cellular uptake of nanocarriers provides insights into refining their design and optimizing their behavior within the body. Advancements in nanocarrier design, interdisciplinary collaboration, and continued research efforts will help overcome these challenges and harness the full potential of nanomedicine to improve therapeutic efficacy.
One innovative approach to overcome these challenges is the use of microfluidics-based organ on chip models. These models offer a powerful tool to access the efficacy and safety of nanomedicines in a more ethical, efficient and personalized manner. By mimicking human organ functions and structure, organ-on-a-chip models reduce the reliance on animal testing. Their ability to accurately predict drug responses in humans holds the potential to revolutionize precision medicine.
In conclusion, while lung cancer treatment options vary based on individual factors, nanomedicine offers a promising approach to drug delivery with the potential to enhance therapeutic outcomes and minimize their impact on healthy tissues. Addressing these challenges through research, interdisciplinary collaboration and innovative techniques like organ-on-a-chip models will bring us closer to realizing the full potential of nanomedicines and their impact on healthcare and potential outcomes.
Acknowledgment
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government [MSIT; grant nos. 2022RIS-005 and 2022R1F1A1069516].
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chhikara BS, Parang K. Global cancer statistics 2022: the trends projection analysis. Chem Biol Lett. 2023;10(1):451.
2. Sabarwal A, Kumar K, Singh RP. Hazardous effects of chemical pesticides on human health–cancer and other associated disorders. Environ Toxicol Pharmacol. 2018;63:103–114. doi:10.1016/j.etap.2018.08.018
3. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
4. Walter FM, Rubin G, Bankhead C, et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br J Cancer. 2015;112(1):S6–13. doi:10.1038/bjc.2015.30
5. Huang CY, Ju DT, Chang CF, Reddy PM, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine. 2017;7(4). doi:10.1051/bmdcn/2017070423
6. Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;Volume 11:943–953. doi:10.2147/CMAR.S187317
7. Inamura K. Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol. 2017;7:193. doi:10.3389/fonc.2017.00193
8. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non–small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058
9. Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40(4):558–566. doi:10.1016/j.ctrv.2013.10.001
10. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–72. doi:10.1016/S1470-2045(14)71180-5
11. Niveria K, Yadav M, Dangi K, Verma AK. Overcoming challenges to enable targeting of metastatic breast cancer tumour microenvironment with nano-therapeutics: current status and future perspectives. OpenNano. 2022;8:100083. doi:10.1016/j.onano.2022.100083
12. Rieger H, Welter M. Integrative models of vascular remodeling during tumor growth. Wiley Interdiscip Rev Syst Biol Med. 2015;7(3):113–129. doi:10.1002/wsbm.1295
13. Awad NS, Salkho NM, Abuwatfa WH, Paul V, AlSawaftah NM, Husseini GA. Tumor vasculature vs tumor cell targeting: understanding the latest trends in using functional nanoparticles for cancer treatment. OpenNano. 2023;11:100136. doi:10.1016/j.onano.2023.100136
14. Singh L, Nair L, Kumar D, et al. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Front Oncol. 2023;13:1034205. doi:10.3389/fonc.2023.1034205
15. Calvin JR, Sanderlin EJ, Yang LV. Molecular connections between cancer cell metabolism and the tumor microenvironment. Int J Mol Sci. 2015;16(5):11055–11086.
16. Brassart-Pasco S, Brézillon S, Brassart B, Ramont L, Oudart JB, Monboisse JC. Tumor microenvironment: extracellular matrix alterations influence tumor progression. Front Oncol. 2020;10:397. doi:10.3389/fonc.2020.00397
17. Saintigny P, Burger JA. Recent advances in non-small cell lung cancer biology and clinical management. Discov Med. 2012;13(71):287–297.
18. Little AG. No nodes is good nodes. the annals of thoracic surgery. Ann Thorac Surg. 2006;82(1):4–5. doi:10.1016/j.athoracsur.2006.03.051
19. Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94(8):1599–1622. doi:10.1016/j.mayocp.2019.01.034
20. Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856(2):189–210. doi:10.1016/j.bbcan.2015.08.002
21. Pfister DG, Johnson DH, Azzoli CG, et al. American society of clinical oncology treatment of unresectable non–small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22(2):330–353. doi:10.1200/JCO.2004.09.053
22. Hamid R, Manzoor I. Nanomedicines: nano based drug delivery systems challenges and opportunities. Altern Med. 2021;27:59.
23. Bhalani DV, Nutan B, Kumar A, Singh Chandel AK. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines. 2022;10(9):2055. doi:10.3390/biomedicines10092055
24. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
25. Kim ES, Lu C, Khuri FR, et al. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer. 2001;34(3):427–432. doi:10.1016/S0169-5002(01)00278-1
26. Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Delivery Rev. 2014;66:2–5. doi:10.1016/j.addr.2013.11.009
27. Landi L, Rossi A. Cetuximab in the treatment of advanced non-small cell lung cancer: fISHing for a miraculous catch. J Thorac Dis. 2018;10(Suppl 17):S1940. doi:10.21037/jtd.2018.04.126
28. Forde PM, Ettinger DS. Targeted therapy for non-small-cell lung cancer: past, present and future. Expert Rev Anticancer Ther. 2013;13(6):745–758. doi:10.1586/era.13.47
29. Master AM, Sen Gupta A. EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine. 2012;7(12):1895–1906. doi:10.2217/nnm.12.160
30. Bajracharya R, Song JG, Patil BR, et al. Functional ligands for improving anticancer drug therapy: current status and applications to drug delivery systems. Drug Deliv. 2022;29(1):1959–1970. doi:10.1080/10717544.2022.2089296
31. Subhan MA, Yalamarty SS, Filipczak N, Parveen F, Torchilin VP. Recent advances in tumor targeting via EPR effect for cancer treatment. J Pers Med. 2021;11(6):571. doi:10.3390/jpm11060571
32. Nakamura Y, Mochida A, Choyke PL, Kobayashi H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjugate Chem. 2016;27(10):2225–2238. doi:10.1021/acs.bioconjchem.6b00437
33. Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11(8):771. doi:10.3390/jpm11080771
34. Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98. doi:10.1016/j.nantod.2019.02.005
35. Zhao Y, Liu W, Tian Y, et al. Anti-EGFR peptide-conjugated triangular gold nanoplates for computed tomography/photoacoustic imaging-guided photothermal therapy of non-small cell lung cancer. ACS Appl Mater Interfaces. 2018;10(20):16992–17003. doi:10.1021/acsami.7b19013
36. Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2(1):48.
37. Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9(5):52. doi:10.3390/cancers9050052
38. Dassonville O, Bozec A, Fischel JL, Milano G. EGFR targeting therapies: monoclonal antibodies versus tyrosine kinase inhibitors: similarities and differences. Crit Rev Oncol Hematol. 2007;62(1):53–61. doi:10.1016/j.critrevonc.2006.12.008
39. Li X, Lu Y, Pan T, Fan Z. Roles of autophagy in cetuximab-mediated cancer therapy against EGFR. Autophagy. 2010;6(8):1066–1077. doi:10.4161/auto.6.8.13366
40. Park JH, Liu Y, Lemmon MA, Radhakrishnan R. Erlotinib binds both inactive and active conformations of the EGFR tyrosine kinase domain. Biochem J. 2012;448(Pt 3):417. doi:10.1042/BJ20121513
41. Cavallaro S. CXCR4/CXCL12 in non-small-cell lung cancer metastasis to the brain. Int J Mol Sci. 2013;14(1):1713–1727. doi:10.3390/ijms14011713
42. Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non-small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3(1):26. doi:10.7150/thno.4922
43. Sundaram S, Trivedi R, Durairaj C, Ramesh R, Ambati BK, Kompella UB. Targeted drug and gene delivery systems for lung cancer therapy. Clin Cancer Res. 2009;15(23):7299–7308. doi:10.1158/1078-0432.CCR-09-1745
44. Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomed. 2015;10:1727. doi:10.2147/IJN.S76501
45. Desai A, Abdayem P, Adjei AA, Planchard D. Antibody-drug conjugates: a promising novel therapeutic approach in lung cancer. Lung Cancer. 2022;163:96–106. doi:10.1016/j.lungcan.2021.12.002
46. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93. doi:10.1038/s41392-022-00947-7
47. Peters C, Brown S. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35(4). doi:10.1042/BSR20150089
48. Morgensztern D, Besse B, Greillier L, et al. Efficacy and safety of rovalpituzumab tesirine in third line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the Phase II TRINITY StudyPhase II Study results of Rova-T in DLL3-expressing SCLC. Clin Cancer Res. 2019;25(23):6958–6966. doi:10.1158/1078-0432.CCR-19-1133
49. Carbone DP, Morgensztern D, Le Moulec S, et al. Efficacy and safety of rovalpituzumab tesirine in patients with DLL3-expressing, ≥ 3rd line small cell lung cancer: Results from the phase 2 TRINITY study. 2018:8507–8507.
50. Su WP, Chang LC, Song WH, et al. Polyaniline-based random-condensation on Au nanoparticles enhances immunotherapy in lung cancer. ACS Appl Mater Interfaces. 2022;14(21):24144–24159. doi:10.1021/acsami.2c03839
51. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974. doi:10.1200/JCO.2014.59.4358
52. Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. 2017;6(2):196. doi:10.21037/tlcr.2017.03.01
53. Chen SJ, Wang SC, Chen YC. Immunotherapy for colorectal cancer, lung cancer and pancreatic cancer. Int J Mol Sci. 2021;22(23):12836. doi:10.3390/ijms222312836
54. Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50(5):600–613.
55. Patwa A, Shah A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J Anaesth. 2015;59(9):533–541. doi:10.4103/0019-5049.165849
56. Ochs M, Nyengaard JR, Jung A, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169(1):120–124. doi:10.1164/rccm.200308-1107OC
57. Dhand C, Prabhakaran MP, Beuerman RW, Lakshminarayanan R, Dwivedi N, Ramakrishna S. Role of size of drug delivery carriers for pulmonary and intravenous administration with emphasis on cancer therapeutics and lung-targeted drug delivery. RSC Adv. 2014;4(62):32673–32689. doi:10.1039/C4RA02861A
58. Patil JS, Sarasija S. Pulmonary drug delivery strategies: a concise, systematic review. Lung India. 2012;29(1):44–49. doi:10.4103/0970-2113.92361
59. Borghardt JM, Kloft C, Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can Respir J. 2018 ;2018:1–11. doi:10.1155/2018/2732017
60. Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11(3):129. doi:10.3390/pharmaceutics11030129
61. Eek D, Krohe M, Mazar I, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence. 2016;24:1609–1621.
62. Arafat M. Approaches to achieve an oral controlled release drug delivery system using polymers: a recent review. Int J Pharm Pharm Sci. 2015;7(7):16–21.
63. Bordonaro S, Vizzini L, Spinnato F, et al. Oral chemotherapy in elderly patients with advanced non-small cell lung carcinoma. WCRJ. 2014;1(2):E223.
64. Guetz S, Tufman A, von Pawel J, et al. Metronomic treatment of advanced non-small-cell lung cancer with daily oral vinorelbine–a Phase I trial. Onco Targets Ther. 2017;10:1081. doi:10.2147/OTT.S122106
65. Crinò L, Calandri C, Maestri A, Marrocolo F. Gemcitabine and cisplatin combination in early-stage non-small-cell lung cancer. Oncology . 2001;15(3 Suppl 6):40–42.
66. Tan EH, Rolski J, Grodzki T, et al. Global Lung Oncology Branch trial 3 (GLOB3): final results of a randomized multinational phase III study alternating oral and iv vinorelbine plus cisplatin versus docetaxel plus cisplatin as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2009;20(7):1249–1256. doi:10.1093/annonc/mdn774
67. Xie M, Chen X, Qin S, Bao Y, Bu K, Lu Y. Clinical study on thalidomide combined with cinobufagin to treat lung cancer cachexia. J Cancer Res Ther. 2018;14(1):226–232. doi:10.4103/0973-1482.188436
68. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor–resistant non–small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi:10.1056/NEJMoa1411817
69. Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2015;372(18):1700–1709. doi:10.1056/NEJMoa1413654
70. Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(15_suppl):8062. doi:10.1200/jco.2015.33.15_suppl.8062
71. Lin SH, Lin HY, Verma V, et al. Phase I trial of definitive concurrent chemoradiotherapy and trametinib for KRAS-mutated non-small cell lung cancer. Cancer Treat Res Commun. 2022;30:100514. doi:10.1016/j.ctarc.2022.100514
72. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomized, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. doi:10.1016/S1470-2045(14)71173-8
73. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244. doi:10.1200/JCO.2018.78.7994
74. Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. doi:10.1056/NEJMoa2027187
75. Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):261–270. doi:10.1016/S1470-2045(19)30690-4
76. Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances in oral drug delivery. Front Pharmacol. 2021;12:618411. doi:10.3389/fphar.2021.618411
77. Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003;95(5):362–372. doi:10.1093/jnci/95.5.362
78. Lerouge D, Rivière A, Dansin E, et al. A phase II study of cisplatin with intravenous and oral vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy with oral vinorelbine and cisplatin for locally advanced non-small cell lung cancer. BMC Cancer. 2014;14(1):1–9. doi:10.1186/1471-2407-14-231
79. Ding L, Li QJ, You KY, Jiang ZM, Yao HR. The use of apatinib in treating nonsmall-cell lung cancer: case report and review of literature. Medicine. 2016;95(20):e3598. doi:10.1097/MD.0000000000003598
80. Chang JW, Huang CY, Fang YF, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non‐small cell lung cancer harboring uncommon EGFR mutations: real‐world data from Taiwan. Thoracic Cancer. 2023;14(1):12–23. doi:10.1111/1759-7714.14537
81. Helena AY, Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in lung cancers. J Natl Compr Canc Netw. 2013;11(2):161–169. doi:10.6004/jnccn.2013.0024
82. Gao X, Le X, Costa DB. The safety and efficacy of osimertinib for the treatment of EGFR T790M mutation positive non-small-cell lung cancer. Expert Rev Anticancer Ther. 2016;16(4):383–390. doi:10.1586/14737140.2016.1162103
83. Mehta AA, Jose WM, Pavithran K, Triavadi GS. The role of gefitinib in patients with non-small-cell lung cancer in India. Indian J Palliat Care. 2013;19(1):48. doi:10.4103/0973-1075.110237
84. Sequist LV, Piotrowska Z, Niederst MJ, et al. Osimertinib responses after disease progression in patients who had been receiving rociletinib. JAMA oncol. 2016;2(4):541–543. doi:10.1001/jamaoncol.2015.5009
85. Planchard D, Besse B, Groen HJ, et al. Phase 2 study of dabrafenib plus trametinib in patients with BRAF V600E-mutant metastatic non-small cell lung cancer: updated 5-year survival rates and genomic analysis. J Thorac Oncol. 2021;2021:1.
86. Cao SJ, Xu S, Wang HM, et al. Nanoparticles: oral delivery for protein and peptide drugs. Aaps Pharmscitech. 2019;20:1. doi:10.1208/s12249-019-1325-z
87. Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. Journal of Food and Drug Analysis. 2017;25(2):219–234. doi:10.1016/j.jfda.2017.02.001
88. Reddy MR, Gubbiyappa KS. Formulation development, optimization, and characterization of entrectinib-loaded supersaturable self-nanoemulsifying drug delivery systems. BioNanoScience. 2023;13(2):521–540. doi:10.1007/s12668-023-01094-1
89. Vitulo M, Gnodi E, Meneveri R, Barisani D. Interactions between nanoparticles and intestine. Int J Mol Sci. 2022;23(8):4339.
90. Liu C, Kou Y, Zhang X, Cheng H, Chen X, Mao S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin Drug Deliv. 2018;15(3):223–233. doi:10.1080/17425247.2017.1395853
91. Ahadian S, Finbloom JA, Mofidfar M, et al. Micro and nanoscale technologies in oral drug delivery. Adv Drug Delivery Rev. 2020;157:37–62. doi:10.1016/j.addr.2020.07.012
92. Viswanathan P, Muralidaran Y, Ragavan G. Nanostructures for oral medicine; 2017.
93. Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C. 2016;60:569–578.
94. Razak A, Mohd Gazzali SA, Fisol A, et al. Advances in nanocarriers for effective delivery of docetaxel in the treatment of lung cancer: an overview. Cancers. 2021;13(3):400. doi:10.3390/cancers13030400
95. Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade P‐glycoprotein or to exploit its expression. Med Res Rev. 2012;32(6):1220–1262.
96. Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013;7:DTI–S12519. doi:10.4137/DTI.S12519
97. Famta P, Shah S, Chatterjee E, et al. Exploring new horizons in overcoming P-glycoprotein-mediated multidrug-resistant breast cancer via nanoscale drug delivery platforms. Curr Res Pharmacol Drug Discovery. 2021;2:100054. doi:10.1016/j.crphar.2021.100054
98. Robinson K, Tiriveedhi V. Perplexing role of P-glycoprotein in tumor microenvironment. Front Oncol. 2020;10:265. doi:10.3389/fonc.2020.00265
99. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: what has been done and the challenges remain ahead. Int J Pharm. 2017;526(1–2):474–495. doi:10.1016/j.ijpharm.2017.05.016
100. Jiang L, Li X, Liu L, Zhang Q. Thiolated chitosan-modified PLA-PCL-TPGS nanoparticles for oral chemotherapy of lung cancer. Nanoscale Res Lett. 2013;8(1):1. doi:10.1186/1556-276X-8-66
101. Bansal T, Jaggi M, Khar R, Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci. 2009;12(1):46–78. doi:10.18433/J3RC77
102. Hou J, Sun E, Zhang ZH, et al. Improved oral absorption and anti-lung cancer activity of paclitaxel-loaded mixed micelles. Drug Deliv. 2017;24(1):261–269. doi:10.1080/10717544.2016.1245370
103. Amin H, Amin MA, Osman SK, Mohammed AM, Zayed G. Chitosan nanoparticles as a smart nanocarrier for gefitinib for tackling lung cancer: design of experiment and in vitro cytotoxicity study. Int J Biol Macromol. 2023;246:125638. doi:10.1016/j.ijbiomac.2023.125638
104. Amin H, Osman SK, Mohammed AM, Zayed G. Gefitinib-loaded starch nanoparticles for battling lung cancer: optimization by full factorial design and in vitro cytotoxicity evaluation. Saudi Pharm J. 2023;31(1):29–54. doi:10.1016/j.jsps.2022.11.004
105. Ruge CA, Kirch J, Lehr C-M. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. Lancet Respir Med. 2013;1(5):402–413. doi:10.1016/S2213-2600(13)70072-9
106. H. LW, Loo C–Y, Traini D, Young PM. Inhalation of nanoparticle–based drug for lung cancer treatment: advantages and challenges. Asian J Pharm Sci. 2015;2015:481–489.
107. Abdelaziz HM, Gaber M, Abd-Elwakil MM. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 2018;269:374–392. doi:10.1016/j.jconrel.2017.11.036
108. Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42(6):697–705. doi:10.1046/j.1365-2125.1996.00493.x
109. Koushik O, Rao Y, Kumar P, Karthikeyan R. Nano drug delivery systems to overcome cancer drug resistance—a review. J Nanomed Nanotechnol. 2016;7(378):2.
110. Storti C, Le Noci V, Sommariva M, Tagliabue E, Balsari A, Sfondrini L. Aerosol delivery in the treatment of lung cancer. Curr Cancer Drug Targets. 2015;15(7):604–612. doi:10.2174/1568009615666150602143751
111. Jyoti K, Pandey RS, Madan J, Jain UK. Inhalable cationic niosomes of curcumin enhanced drug delivery and apoptosis in lung cancer cells. Indian J Pharm Educ Res. 2016;50:S23–31.
112. Otterson GA, Villalona-Calero MA, Hicks W, et al. Phase I/II Study of inhaled doxorubicin combined with platinum-based therapy for advanced non–small cell lung cancerinhaled doxorubicin with iv chemotherapy for NSCLC. Clin Cancer Res. 2010;16(8):2466–2473. doi:10.1158/1078-0432.CCR-09-3015
113. Zarogoulidis P, Eleftheriadou E, Sapardanis I, et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investigational New Drug. 2012;30:1628–1640. doi:10.1007/s10637-011-9714-5
114. Lemarie E, Vecellio L, Hureaux J, et al. Aerosolized gemcitabine in patients with carcinoma of the lung: feasibility and safety study. J Aerosol Med Pulm Drug Deliv. 2011;24(6):261–270. doi:10.1089/jamp.2010.0872
115. Larsen ST, Jackson P, Poulsen SS, et al. Airway irritation, inflammation, and toxicity in mice following inhalation of metal oxide nanoparticles. Nanotoxicology. 2016;10(9):1254–1262. doi:10.1080/17435390.2016.1202350
116. Driscoll KE, Costa DL, Hatch G, et al. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 2000;55(1):24–35. doi:10.1093/toxsci/55.1.24
117. Pettit DK, Gombotz WR. The development of site-specific drug-delivery systems for protein and peptide biopharmaceuticals. Trends in biotechnology. Trends Biotechnol. 1998;16(8):343–349. doi:10.1016/s0167-7799(98)01186-x
118. Patton JS, Brain JD, Davies LA, et al. The particle has landed—characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Delivery. 2010;23(S2):S71–87. doi:10.1089/jamp.2010.0836
119. El-Sherbiny IM, El-Baz NM, Yacoub MH. Inhaled nano-and microparticles for drug delivery. Glob Cardiol Sci Pract. 2015;2015:2. doi:10.5339/gcsp.2015.2
120. Wang X, Chen Q, Zhang X, et al. Matrix metalloproteinase 2/9-triggered-release micelles for inhaled drug delivery to treat lung cancer: preparation and in vitro/in vivo studies. Int J Nanomed. 2018;13:4641. doi:10.2147/IJN.S166584
121. Park JY, Park S, Lee TS, et al. Biodegradable micro-sized discoidal polymeric particles for lung-targeted delivery system. Biomaterials. 2019;218:119331. doi:10.1016/j.biomaterials.2019.119331
122. Ejigah V, Owoseni O, Bataille-Backer P, Ogundipe OD, Fisusi FA, Adesina SK. Approaches to improve macromolecule and nanoparticle accumulation in the tumor microenvironment by the enhanced permeability and retention effect. Polymers. 2022;14(13):2601. doi:10.3390/polym14132601
123. He S, Gui J, Xiong K, Chen M, Gao H, Fu Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J Nanobiotechnology. 2022;20(1):101. doi:10.1186/s12951-022-01307-x
124. Chenthamara D. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):1–29.
125. von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. doi:10.1200/JCO.2013.54.5392
126. Blumenschein GR
127. Fink TH, Huber RM, Heigener DF, et al. Topotecan/cisplatin compared with cisplatin/etoposide as first-line treatment for patients with extensive disease small-cell lung cancer: final results of a randomized phase III trial. J Thorac Oncol. 2012;7(9):1432–1439. doi:10.1097/JTO.0b013e318260de75
128. Huang TT, Parab S, Burnett R, et al. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther. 2015;26(2):82–93. doi:10.1089/hum.2014.100
129. Lu C, Stewart DJ, Lee JJ, et al. Phase I clinical trial of systemically administered TUSC2 (FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS One. 2012;7(4):e34833. doi:10.1371/journal.pone.0034833
130. Ho YP, Au‐Yeung SC, To KK. Platinum‐based anticancer agents: innovative design strategies and biological perspectives. Med Res Rev. 2003;23(5):633–655. doi:10.1002/med.10038
131. Adjei AA, Mandrekar SJ, Dy GK, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non–small-cell lung cancer: NCCTG and SWOG Study N0426. J Clin Oncol. 2010;28(4):614. doi:10.1200/JCO.2009.23.6406
132. Horn L, Mansfield AS, Szcze Sna A, et al. First-line ate-zolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
133. Kluger H, Weiss SA, Olszanski AJ, et al. Abstract CT089: phase Ib/II of CD40 agonistic antibody APX005M in combination with nivolumab (nivo) in subjects with metastatic melanoma (M) or non-small cell lung cancer (NSCLC). Cancer Res. 2019;79(13_Supplement):CT089. doi:10.1158/1538-7445.AM2019-CT089
134. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369. doi:10.1200/JCO.20.00793
135. Chen R, Manochakian R, James L, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13(1):1–23.
136. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi:10.18632/oncotarget.16723
137. Rimkus T, Sirkisoon S, Harrison A, Lo HW. Tumor suppressor candidate 2 (TUSC2; FUS-1) and human cancers. Discov Med. 2017;23(128):325.
138. Motswainyana WM, Ajibade PA. Anticancer activities of mononuclear ruthenium (II) coordination complexes. Adv Chem. 2015;2015:1–21. doi:10.1155/2015/859730
139. Leijen S, Burgers SA, Baas P, et al. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investigational New Drug. 2015;33:201–214. doi:10.1007/s10637-014-0179-1
140. Horinouchi H, Sekine I, Sumi M, et al. Long‐term results of concurrent chemoradiotherapy using cisplatin and vinorelbine for stage III non‐small‐cell lung cancer. Cancer Sci. 2013;104(1):93–97. doi:10.1111/cas.12028
141. Gandara DR, Piperdi B, Walsh WV. A randomized Phase II Study of Schedule-Modulated Concomitant Pemetrexed (Alimta®) and Erlotinib (Tarceva®) vs single agent pemetrexed (Alimta®) in patients with progressive or recurrent Non-Small Cell Lung Cancer (NSCLC).
142. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2980–2987. doi:10.1200/JCO.2016.66.9929
143. June CH, Warshauer JT, Bluestone JA. Is autoimmunity the achilles’ heel of cancer immunotherapy? Nat Med. 2017;23(5):540–547. doi:10.1038/nm.4321
144. Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019 ;2019:1–26. doi:10.1155/2019/3702518
145. Desai N. Nanoparticle albumin-bound paclitaxel (Abraxane®). Albumin in medicine: pathological and clinical applications. 2016.
146. Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12(1):1–8. doi:10.1186/s13045-019-0818-2
147. Sharma P, Mehta M, Dhanjal DS, et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem Biol Interact. 2019;309:108720. doi:10.1016/j.cbi.2019.06.033
148. Anderson CF, Grimmett ME, Domalewski CJ, Cui H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(1):e1586. doi:10.1002/wnan.1586
149. Hafeez MN, Celia C, Petrikaite V. Challenges towards targeted drug delivery in cancer nanomedicines. Processes. 2021;9(9):1527. doi:10.3390/pr9091527
150. Rasool M, Malik A, Waquar S, et al. New challenges in the use of nanomedicine in cancer therapy. Bioengineered. 2022;13(1):759–773. doi:10.1080/21655979.2021.2012907
151. Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–1074. doi:10.3762/bjnano.9.98
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.