Back to Journals » OncoTargets and Therapy » Volume 14
Clinical Significance of the D-Loop Gene Mutation in Mitochondrial DNA in Laryngeal Cancer
Authors Wang L, Cheng HX, Zhou YH, Ma M
Received 1 February 2021
Accepted for publication 13 May 2021
Published 26 May 2021 Volume 2021:14 Pages 3461—3466
DOI https://doi.org/10.2147/OTT.S304836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Lei Wang,1 He-Xiang Cheng,2 Yan-Hui Zhou,1 Min Ma1
1Department of Otorhinolaryngology, Head and Neck Surgery, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, 471003, People’s Republic of China; 2Department of Otolaryngology, The Luoyang First People’s Hospital, Luoyang, 471000, People’s Republic of China
Correspondence: Min Ma
Department of Otorhinolaryngology, Head and Neck Surgery, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, No. 24 Jing Hua Road, Jianxi District, Luoyang, 471003, People’s Republic of China
Tel +8615290533346
Fax +86037969823266
Email [email protected]
Objective: The aim of the present study was to investigate the D-loop gene mutation and microsatellite instability in the mitochondrial DNA (mtDNA) and the correlation with the clinical and pathological parameters in laryngeal cancer.
Methods: The tumor tissues and paratumor tissues in 60 cases of laryngeal cancer were selected, and DNA was extracted from these tissues. The D-loop region in mtDNA was amplified by PCR with the gene sequence of the amplified product being detected. The gene sequence of the detected region was compared with the revised Cambridge Reference Sequence (rCRS) and the related database by using the Mitomaster software. The correlation between the D-loop gene mutation and the clinical and pathological parameters was investigated.
Results: A total of 174 mutations across 38 sites were detected in 51 (85%) of samples. Most of the mutations were concentrated in the high various (HV) I region, and the main types of mutations were the substitution of a single base or insertion and deletion of a single base. There was also microsatellite instability in the D310 region. The statistical results showed that there was no correlation between the age, gender, tumor diameter, and TNM stage, and the number of the D-loop mutations in mtDNA (P > 0.05).
Conclusion: There existed high-frequency mutation of the D-loop gene in mtDNA in laryngeal cancer, which might play an important role in the pathogenesis of laryngeal cancer.
Keywords: laryngeal cancer, mitochondrial DNA, D-loop, mutation
Introduction
Laryngeal cancer is a relatively common malignant tumor in the head and neck, accounting for 1–5% of malignant tumors in the body. Squamous cell carcinomas account for 96–98% of laryngeal cancers. Incidences of laryngeal cancer are increasing yearly as the smoking population expands, and the environment deteriorates. In 2015, there were 26,400 new cases of laryngeal cancer in China. Among these, 14,500 people died. The male to female ratio was 9:1.1 The incidence of laryngeal cancer is high in middle-aged and older males. In recent years, more incidences of laryngeal cancer are being found in the younger population, with significant differences among different races and regions.2 In China, the incidence of laryngeal cancer is highest in the Northeast and the North. In the United States, over the past 40 years, the percentage of patients with laryngeal cancer who survive for five years or more has decreased from 66% to 63%,3 suggesting the need for innovation in detection and treatment. Mitochondria are widely involved in cellular activities, including the regulation of cell cycle, oxidative stress, and apoptosis.4 Mitochondrial DNA (mtDNA) injury is closely correlated with tumors, aging, and neurodegenerative diseases.5,6 The D-loop region is the non-coding region in mtDNA. Most of the regulatory sequences related to the mtDNA replication, transcription, and translation are found in this region, which is prone to mutation. Although there have been achievements in the field concerning the relationship between tumorigenesis and mitochondrial mutation, and some related research at home and abroad have clarified some related mechanisms, the research on the relationship between tumorigenesis and mtDNA remains unclear. Moreover, no direct relationship between the abnormal changes of mtDNA and tumorigenesis has been found so far. Although many studies have reported the relationship between mitochondrial gene mutations and various tumors, the study on the mechanism of laryngeal cancer has not been reported. The purpose of the present study was to investigate the relationship between the D-loop gene mutation in mtDNA and the clinical and pathological parameters of laryngeal cancer by gene sequencing.
Subjects and Methods
Case Selection
From June 2013 to June 2019, 60 patients with squamous cell laryngeal carcinoma were selected from the First Affiliated Hospital of Henan University of Science and Technology. No patients received radiotherapy or chemotherapy before the operation. The age ranged between 40–80, with an average age of 59.12 ± 10.35. There were 55 males and five females. There were 15 cases in stage I, 26 in stage II, ten in stage III, and nine in stage IV (according to the 2017 UICC staging criteria). The specimens from all cases were diagnosed and screened by the pathological experts of the First Affiliated Hospital of Henan University of Science and Technology. This study was approved by the medical ethics committee of the Henan University of Science and Technology. Written informed consent was obtained from all participants. This study complied with the Declaration of Helsinki.
Main Reagents and Instruments
Reagents
DNA Marker DL2000 (CB15727772 TAKARA, Japan); 10Xbuffer (Mg2+plus) (DR001AM TAKARA, Japan); dNTPs (DR001AM TAKARA, Japan); Taq DNA polymerase (DR001AM TAKARA, Japan); agarose (D1200 Beijing Solabo Technology Co., Ltd); Primers (JN0060-02 Shanghai Bioengineering Technology Co., Ltd).
Instruments
Thermo high-speed centrifuge (USA); PCR instrument (ABI, USA); Gel imaging system (USA); −80°C refrigerator (Japan).
DNA Extraction
The specimens resected during laryngeal cancer surgery were removed, and the central part of the specimen with cancer focus was taken as the cancer tissue. Approximately 20 mg of the cancer tissue was cut and placed in a centrifuge tube. The tissue was broken into cell suspension by a high-speed tissue homogenizer. The following procedures were operated according to the kit instructions. After a series of extraction and washing, the final purified DNA solution was placed in a −20°C refrigerator for storage and further detection.
PCR Reaction
The sequence in the D-loop region in mtDNA was the target sequence with a full length of 1122 bp. The primers and probes were synthesized by the Shanghai Bioengineering Technology Service Co., Ltd. The sequences of primers were as follows: the upper primer 5′-CCATTAGCACCCAAAGCTAAG-3′, the lower primer 5′-TGCTTTGAGGTAAGCTACA-3′. The reaction system was 50 μL, with 5 μL of the 10 × buffer (Mg2+ plus) solution, 4 μL of the mixture of dNTP, 0.25 μL of the DNA polymerase, and 1 μL of the upper primer and 1 μL of the lower primer, 1 μL of the template DNA, and 37.75 μL of distilled water. The reaction conditions were as follows: 94°C 30s, 55°C 30s, 72°C 90s, with a total of 30 cycles. The electrophoresis of the PCR products was under the condition of 120V for 30 minutes. After the electrophoresis, the gel imaging system was used to take photos to confirm that the amplified fragment was the desired target fragment.
Gene Sequencing
The purification of the PCR products and the determination of gene sequences were completed by the Beijing Sequencing Department of Shanghai Yingjun Biotechnology Co., Ltd. The quality of the peak map was evaluated by Chromas 2.31 software, and the samples with low quality were re-sequenced. After landing on the website (https://www.Ncbi.Nlm.Nih.gov), the “Align two (or more) sequences using BLAST” software was used to compare the gene sequence of laryngeal cancer tissue with the Cambridge standard sequence. Once the mutation site is identified, the Chromas software should be adopted to observe the sequencing peak and compare it with the database’s reported mutation site. If there is no report, it is considered a novel mutation. At the same time, these mutation sites were compared with the sub-database of the polymorphic sites of the mtDB database. If the mutation frequency was more than 1% in all populations tested in the database, it was regarded as the single nucleotide polymorphism (SNP). The gene sequencing data was provided in the Supplementary Document.
Statistical Analysis
The Prism GraphPad 5.0 software was used for statistical analysis of the results. The Pearson’s or the Spearman correlation test was adopted to test the correlation between the two variables, and χ2 test was used to compare the number of mutations between genders. P < 0.05 was considered statistically significant.
Results
Results of the mtDNA PCR Product
The length of the target gene was 1122bp after the PCR amplification of the full length of the D-loop region (Figure 1). A single bright band was demonstrated by the gel imaging instrument, and the molecular weight was determined by the standard of DL2000DNAMark.
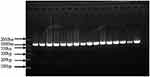 |
Figure 1 Results of the mtDNA PCR product. |
Sequencing Results and Analysis
Among the 60 samples, 38 mutation sites had been detected in 51 samples (85%), with a total of 174 mutations. In three HV in the D-loop, 160 mutations had been detected, accounting for 91.9% of the total mutations (Table 1). Microsatellite instability existed in the D310 regions in 15 cases (25%) (Figure 2), mainly manifested as the increased insertion of poly-C. Among them, nine cases showed insertion of one base C, six cases showed insertion of two bases, and the base pair exchanges within a microsatellite were found in two cases (Figure 3).
 |
Table 1 Sequencing Results and Analysis |
 |
Figure 2 Microsatellite instability in D310 region (insertion of base pair C). |
 |
Figure 3 Microsatellite instability in D310 region (T, C exchange). |
The Correlation Between the D-Loop Region Mutation in mtDNA and the Clinical and Pathological Parameters in Laryngeal Cancer
As shown in Table 2, there was no correlation between the age, gender, tumor diameter, TNM stage, and the number of the D-loop mutations in mtDNA in the 51 specimens with mutations (P > 0.05).
 |
Table 2 The Correlation Between the D-Loop Region Mutations in mtDNA and the Clinical and Pathological Parameters in Laryngeal Cancer (Number of Cases) |
Discussion
Mitochondria are the only organelles with DNA outside the nucleus in the eukaryotes, which are the main sites for oxidative phosphorylation to produce active oxygen and provide necessary energy and oxygen radicals for cell activities. When mtDNA mutation occurs, the cellular energy supply is dysfunctional, and a large number of ROS are produced. This leads to changes in cell function and even cell necrosis, thus showing a variety of clinical symptoms. Mitochondrial dysfunction may play an important role in tumor development, early diagnosis, drug resistance, prevention of recurrence, and prognosis.7–9 MtDNA is a closed-loop double-stranded DNA molecule composed of 16,569 base pairs. As the main non-coding region, the D-loop region of npl6024-np576 is responsible for regulating mtDNA replication and transcription.10 Sanchez-Cespedes et al11 found that 41% of head and neck squamous cell carcinoma had mutations in the D-loop region, including the deletion, insertion, and point mutation. Ha et al12 revealed that patients with head and neck tumors had the change of poly-C in the D-loop region of mtDNA. Our experiment confirmed this. In recent years, the D-loop region is agreed to be the HV of mtDNA mutations. In the present study, 91.9% of the mutation sites were concentrated in the HV region, consistent with the above view. Microsatellites are the short tandem repeats in the human genome. Microsatellite instability refers to the changes of microsatellite in length and the emergence of new microsatellite alleles in tumors due to the insertion or deletion of repeat units compared with normal tissues. Habano et al13 first proposed the mitochondrial microsatellite instability in a study on rectal cancer. In the present study, microsatellite instabilities were found in 15 cases of laryngeal cancer, mainly concentrated in the D310 region and manifested as the increase of poly-C insertion. Among these cases, nine showed insertion of one base C, six showed insertion of two bases, and base exchanges within a microsatellite were found in two cases. This was consistent with the study by Sanchez-Cespedes et al that 41% of head and neck squamous cell carcinoma had mutations in the D-loop region area.11 The HV of the D310 region may be related to its location in the intron, coder, and promoter of the gene and the repeated repair caused by slip error during replication.14 Therefore, the analysis of mtDNA mutations, especially the detection of changes in the D310 region, may play an important role in the cytological diagnosis, especially for cases with no obvious morphological changes or rare tumor cells. In conclusion, PCR amplification and direct sequencing were used in the present study to detect the D-loop gene changes in mtDNA in samples of laryngeal cancer tissue. It was found that there were a large number of mutations and microsatellite instability in the D-loop region in mtDNA of patients with laryngeal cancer, indicating that the D-loop gene mutations in mtDNA may play an important role in the development of laryngeal cancer.
Acknowledgments
We would like to acknowledge the hard and dedicated work of Dr. Kai Xi and Dr. Hao Chang that helped and contributed to us in terms of technology and software of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Shin JY, Truong MT. Racial disparities in laryngeal cancer treatment and outcome: a population-based analysis of 24,069 patients. Laryngoscope. 2015;125(7):1667–1674. doi:10.1002/lary.25212
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
4. Correia-Melo C, Passos JF. Mitochondria: are they causal players in cellular senescence? Biochim Biophys Acta. 2015;1847(11):1373–1379. doi:10.1016/j.bbabio.2015.05.017
5. Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):216–231. doi:10.1111/jnc.13731
6. Lee J, Chang JY, Kang YE, et al. Mitochondrial energy metabolism and thyroid cancers. Endocrinol Metab. 2015;30(2):117–123. doi:10.3803/EnM.2015.30.2.117
7. Chen N, Wen S, Sun X, et al. Elevated mitochondrial DNA copy number in peripheral blood and tissue predict the opposite outcome of cancer: a meta-analysis. Sci Rep. 2016;18(6):37404. doi:10.1038/srep37404
8. Huang X, Zhou X, Hu Q, et al. Advances in esophageal cancer: a new perspective on pathogenesis associated with long non-coding RNAs. Cancer Lett. 2018;28(413):94–101. doi:10.1016/j.canlet.2017.10.046
9. St John JC. Mitochondrial DNA copy number and replication in reprogramming and differentiation. Semin Cell Dev Biol. 2016;52:93–101. doi:10.1016/j.semcdb.2016.01.028
10. Morandi L, Asioli S, Cavazza A, Pession A, Damiani S. Genetic relationship among atypical adenomatous hyperplasia, bronchioloalveolar carcinoma and adenocarcinoma of the lung. Lung Cancer. 2007;56(1):35–42. doi:10.1016/j.lungcan.2006.11.022
11. Sanchez-Cespedes M, Parrella P, Nomoto S, et al. Identification of a mononucleotide repeat as a major target for mitochondrial DNA alterations in human tumors. Cancer Res. 2001;61(19):7015–7019.
12. Ha PK, Tong BC, Westra WH, et al. Mitochondrial C-tract alteration in premalignant lesions of the head and neck: a marker for progression and clonal proliferation. Clin Cancer Res. 2002;8(7):2260–2265.
13. Habano W, Sugai T, Nakamura SI, Uesugi N, Yoshida T, Sasou S. Microsatellite instability and mutation of mitochondrial and nuclear DNA in gastric carcinoma. Gastroenterology. 2000;118(5):835–841. doi:10.1016/S0016-5085(00)70169-7
14. Lin CS, Lee HT, Lee MH, et al. Role of mitochondrial DNA copy number alteration in human renal cell carcinoma. Int J Mol Sci. 2016;17(6):814. doi:10.3390/ijms17060814
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
