Back to Journals » International Journal of General Medicine » Volume 16
Clinical Significance of MMP7 Levels in Colorectal Cancer Patients Receiving FOLFOX4 Chemotherapy Treatment
Authors Zhou Y, Wang L, Zhou F
Received 9 April 2023
Accepted for publication 14 June 2023
Published 26 June 2023 Volume 2023:16 Pages 2671—2678
DOI https://doi.org/10.2147/IJGM.S416363
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yeting Zhou, Leiming Wang, Fei Zhou
Xuzhou Medical University Jiangsu Key Laboratory of Tumor Biotherapy, Shuyang People’s Hospital, Suqian, 221000, People’s Republic of China
Correspondence: Leiming Wang; Fei Zhou, Xuzhou Medical University Jiangsu Key Laboratory of Tumor Biotherapy, Shuyang People’s Hospital, Shuyang, Suqian, 221000, People’s Republic of China, Email [email protected]; [email protected]
Objective: Various studies have shown an association between the anti-cancer drug 5-fluorouracil and matrix metalloproteinase 7 (MMP7). The expression of MMP7 in the serum of colorectal cancer patients, as well as their sensitivity to chemotherapy, were examined using the FOLFOX4 chemotherapy treatment.
Methods: Serum samples were taken from 216 colorectal cancer patients who had undergone four cycles of gemcitabine and cisplatin treatment. The sera of 216 healthy persons were used as controls. MMP7 levels in the serum were measured by ELISA. Demographic and survival data were collected.
Results: MMP7 levels were not associated with sex, age, peritoneal dissemination, liver metastasis, lymph node metastasis, lymphatic invasion, or venous invasion in CRC patients, but were associated with histological grade, tumor size, TNM stage, and depth of tumor invasion. Patients’ serum MMP7 expression reduced after treatment. MMP7 expression was significantly lower chemotherapy-sensitive patients compared with chemotherapy-resistant patients. Elevated MMP7 expression was associated with worse prognosis and chemotherapy-sensitive patients had markedly better overall survival compared with chemotherapy-resistant patients.
Conclusion: MMP7 expression was potentially associated with the development of colorectal cancer and elevated levels were associated with chemoresistance in CRC patients. Serum MMP7 levels can be used to screen for drug resistance during FOLFOX4 chemotherapy treatment.
Keywords: MMP7, colorectal cancer, FOLFOX4 chemotherapy, chemotherapy-resistant
Introduction
Colorectal cancer (CRC) has a high recurrence rate as well as the third-highest morbidity and mortality rate of all neoplasms.1–3 Many people with CRC can be successfully treated with surgery during the initial stages of the disease, but those with locally advanced cancer and distant metastases will require post-surgery chemotherapy. In patients with locally advanced CRC, 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) chemotherapy is currently one of the most often utilized treatments.4 Chemotherapy, however, has significant side effects in people with chemoresistant malignancies.5 A minimally invasive biomarker that can predict the treatment response as soon as possible is required. Imaging modalities such as CT,6 and tumor markers, for instance, CEA7 and CA19-98 can be used to track the therapeutic effects of chemotherapy in the clinic. However, CT examinations cannot be conducted frequently due to the inherent radiation risk and, as monitoring biomarkers, CEA and CA19-9 have low sensitivity. Unfortunately, there is no method for predicting chemotherapeutic response. Thus, there is a pressing need to identify effective biomarkers that can predict whether or not a patient will respond to chemotherapy.
Matrix metalloproteinases (MMPs) belong to the family of zinc-dependent proteolytic enzymes that hydrolyze various components of the extracellular matrix constituents and may thus play a role in the regulation of tumor invasion. MMP7 was discovered to be elevated in several malignancies and has been recognized as an oncogene, promoting the incidence and growth of tumors, including hepatocellular carcinoma,9 gastric cancer,10 and colorectal cancer.11 MMP7 expression has also been found to be markedly higher in CRC patients11–14 and recent studies suggest that it may have an essential function in the development of chemotherapy resistance in several cancers, for instance, cross-talk between MMP7 and the FAS/FASL system has been observed during the acquisition of chemoresistance to oxaliplatin.15 There is also an association between MMP7 and heat shock protein 90 (HSP90) in acquired drug resistance and tumor metastasis.16
The present study investigated the expression of MMP7 in CRC patients receiving FOLFOX4 chemotherapy as well as the association between MMP7 expression and survival and chemotherapy resistance in these patients.
Materials and Methods
Subjects and Specimen Collection
Blood samples were collected from patients with pathologically confirmed CRC who volunteered for the study. The study, including the collection of specimens, retrieval of data, and subsequent patient follow-up, was approved by the Ethics Committee of Shuyang People’s Hospital (No. sy20210601008). The research strategy was formulated in accordance with the Declaration of Helsinki and STROBE Statement. All patients (or their legal representatives) provided written informed consent. A total of 216 blood samples were collected from healthy study participants.
Inclusion and Exclusion Criteria
The entire medical case histories of the CRC patients were collected retrospectively. CRC was diagnosed histologically and/or cytologically in all the patients. All enrolled patients were over the age of 18. The patients received only FOLFOX4 (85mg/m2 oxaliplatin, 200mg/m2 leucovorin and 5-fluorouracil via a 400mg/m2 bolus and a 22 hours continuous infusion of 600 mg/m2 5-fluorouracil on days 1–2) chemotherapy (except for surgery resection) without additional treatments such as chemotherapy, radiation, targeted therapy, or traditional Chinese medicine. Non-primary CRC patients, patients without cytology or histology results, patients with poor follow-up compliance, and patients with missing data were also excluded.
Serum MMP7 Measurement
A sensitive sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems, Australia) was used for measuring MMP7 levels in the blood. Sera were diluted 1:5 in the diluents provided by the manufacturer. All samples were assayed in duplicate. In one and two samples, the MMP7 levels were over or below the detection limit, respectively. Minimum and maximum detectable values were used to estimate MMP7 levels in these samples.
Follow-Up
Follow-ups were conducted on the telephone to obtain information about the living situations of the patients. The patients’ survival times were measured from the date of diagnosis to the date of death or last follow-up.
Measures of success: Four weeks following treatment, CRC patients were separated into two groups, namely, the chemotherapy-resistant and chemotherapy-sensitive groups, in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST). Outcomes in the chemotherapy-sensitive group were defined as complete response (CR) or partial response (PR), while the outcomes in the chemotherapy-resistant group were classified as stable disease (SD) or progressive disease (PD), according to the following criteria: CR, disappearance of lesions over > 4 weeks; PR, a decrease of >30% in the maximum diameter of the tumor > 4 weeks; SD, a decrease of <30% in the maximum diameter of the tumor or an increase ≤ 20%; PD, the maximum diameter of the tumor increased > 20%, or new lesions were found.
Statistical Analysis
GraphPad Prism 7 and SPSS 19.0 statistical tools were used to create an analytical database. For comparisons between CRC patients and healthy volunteers, as well as between subgroups of patients prior to and following treatment, the Chi-squared test was used. The median survival time was calculated, and survival curves were compiled using the Kaplan-Meier method. The Log rank test was used to compare the survival rates of various parameters. A Cox proportional hazards model was used for survival prediction. Two-sided tests were used for all experiments. Statistical significance was defined as P < 0.05.
Results
Demographics of Study Participants
A total of 216 CRC patients with comprehensive medical histories were enlisted. Of these, 110 were men and 106 were women with 99 patients over 60 years old and 117 patients under 60 years old. There were no significant differences in terms of age, sex, or other demographic data between the patient and control groups (Table 1).
 |
Table 1 Demographic Data of CRC Patients and Healthy Controls |
Relative MMP7 Expression Before FOLFOX4 Chemotherapy
The analysis showed that MMP7 levels were not associated with sex, age, peritoneal dissemination, liver metastasis, lymph node metastasis, lymphatic invasion, or venous invasion in CRC patients, but were associated with histological grade, tumor size, TNM stage, and depth of tumor invasion (Table 2).
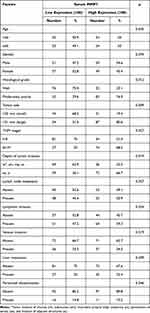 |
Table 2 Relationship Between Clinical Features and MMP7 Expression |
Survival of CRC Patients with High and Low MMP7 Expression
To assess the survival of the CRC patients in relation to MMP7 expression, the patients were allocated to two groups according to the median value of MMP7 expression, namely, a high MMP7-expression group and a low MMP7-expression group. Kaplan-Meier curves showed that patient survival in the high MMP7-expression group was significantly lower than that of patients in the low-expression group (Figure 1A). It was observed that MMP7 expression in CRC patients declined significantly after four cycles of FOLFOX4 treatment (Figure 1B). Patients were also divided into two subgroups according to their response to FOLFOX4 treatment based on the Response Evaluation Criteria in Solid Tumors (RECIST): chemotherapy-sensitive (CR+PR, n=102) and chemotherapy-resistant (SD+PD, n=114). Before FOLFOX4 chemotherapy, there was no statistical difference between the chemotherapy-resistant and chemotherapy-sensitive groups in terms of average MMP7 expression. After FOLFOX4 chemotherapy, MMP7 expression dropped considerably in each group in comparison with the levels before treatment, although patients in the chemotherapy-sensitive group showed a greater decline in MMP7 levels compared with those in the chemotherapy-resistant group. Patient survival was also observed to be higher in the chemotherapy-sensitive group compared with the chemotherapy-resistant group (Figure 1C). Additionally, to evaluate the diagnosability of MMP7 in the diagnosis of chemotherapy resistant CRC, we used Receiver operating characteristic (ROC) curve analysis. The results showed MMP7 Area was 0.9643 (95% confidence interval 0.9365 to 0.9921) among CRC patients, indicating that it may be a powerful potential indicator for the diagnosis of chemotherapy resistant CRC after FOLFOX4 treatment (Figure 2).
 |
Figure 2 Serum MMP7 levels acts as predictors of chemotherapy resistant CRC after FOLFOX4 treatment. |
Cox Proportional Hazards Analysis
The risk factors for CRC were then investigated in the patients. The relative expression of MMP7, tumor size, TNM stage, and depth of tumor invasion were all found to be risk factors for reduced survival in CRC patients (Table 3) while age, sex, and lymph node metastases were not found to be risk factors.
 |
Table 3 Logistic Regression Analysis of Factors Affecting the Overall Survival of CRC Patients |
Discussion
CRC is associated with high levels of mortality and a tendency to recurrence despite treatment. Aberrant expression of certain genes is linked to a variety of malignancies. For example, it is documented that many microRNAs are involved in the onset and progression of cancers, and their abnormal expression is associated with resistance to chemotherapy. Gremlin 1 from cancer-associated fibroblasts also increases breast cancer growth.17 The growth of human CRC-derived cells is enhanced through abnormal IL-6/JAK2/STAT3 signaling18 via an IL-33-TGF-associated signaling loop.19 Studies have also shown that certain genes are expressed in the peripheral blood of individuals with various malignancies.20,21 These features suggest that the expression of specific genes in the serum or plasma can be used as tumor indicators and many such genes have been identified as cancer biomarkers.
MMP7 expression is dysregulated in several cancer types. Previous studies have demonstrated aberrant expression of MMP7 in breast cancer,22 liver cancer,23 and gastric cancer,24 amongst others. Additionally, MMP7 acts as a potential biomarker of colon cancer and its prognostic value.25,26 The present study also showed that MMP levels were also significantly elevated in patients with CRC, where they were found to be linked to the clinical stage of the patients. MMP7 expression was also significantly associated with patient prognosis, suggesting that the gene plays a role in CRC development and progression.
Chemotherapy is a common treatment option for advanced CRC patients. The long-term success of chemotherapy is, however, limited by drug resistance. The most frequently used chemotherapy for CRC is FOLFOX4 which has been shown to have a positive therapeutic effect and is safe, has low toxicity, and is well tolerated. MMP7 has been reported to cause T-DM1 resistance and poor prognosis in gastric cancer via a DKK1-dependent mechanism.27 The HSP90 chaperone is also linked to acquired drug resistance and tumor metastasis in association with MMP7.16 The present results showed that MMP7 levels were higher in FOLFOX4 chemotherapy-resistant CRC patients than in FOLFOX4 chemotherapy-sensitive patients. This suggests that MMP7 may play a crucial role in the FOLFOX4 chemotherapy-resistance process; however, due to the small sample size, we were unable to investigate the precise part played by MMP7 in the development of resistance to FOLFOX4 in CRC patients. Further research into the function of MMP7 in FOLFOX4 resistance in CRC is needed. MMP7 expression in the serum of CRC patients may have clinical relevance in the diagnosis of the disease, although the use of biomarkers is generally insufficiently specific. The accuracy of diagnosis can be improved by using several biomarkers, such as miRNA levels and other types of tumor indicators, together with the construction of gene profiles. Detailed investigation into the use of serum proteins in cancer diagnosis and treatment is likely to yield productive results.
In conclusion, this MMP7 expression can be reduced by FOLFOX4 chemotherapy. The significant upregulation of MMP7 expression in chemotherapy-resistant patients may provide a reference and foundation for further investigation into chemotherapy resistance and the adjustment of FOLFOX4 chemotherapy during treatment.
Funding
This study was supported by Jiangsu Provincial Key Laboratory of Cancer Biotherapy (No. XZSYSKF2020007).
Disclosure
The authors declare that there are no potential conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi:10.3322/caac.21601
4. Suenaga M, Fujimoto Y, Matsusaka S, et al. Perioperative FOLFOX4 plus bevacizumab for initially unresectable advanced colorectal cancer (NAVIGATE-CRC-01). Onco Targets Ther. 2015;8:1111–1118. doi:10.2147/OTT.S83952
5. Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206:107447. doi:10.1016/j.pharmthera.2019.107447
6. Sali L, Falchini M, Taddei A, Mascalchi M. Role of preoperative CT colonography in patients with colorectal cancer. World J Gastroenterol. 2014;20(14):3795–3803. doi:10.3748/wjg.v20.i14.3795
7. Campos-da-Paz M, Dorea JG, Galdino AS, Lacava ZGM, de Fatima Menezes Almeida Santos M. Carcinoembryonic Antigen (CEA) and hepatic metastasis in colorectal cancer: update on biomarker for clinical and biotechnological approaches. Recent Pat Biotechnol. 2018;12(4):269–279. doi:10.2174/1872208312666180731104244
8. Hidaka E, Maeda C, Nakahara K, et al. High serum CA19-9 concentration predicts poor prognosis in elderly patients with stage IV colorectal cancer. Gastrointest Tumors. 2019;5(3–4):117–124. doi:10.1159/000493793
9. Rong W, Zhang Y, Yang L, et al. Post-surgical resection prognostic value of combined OPN, MMP7, and PSG9 plasma biomarkers in hepatocellular carcinoma. Front Med. 2019;13(2):250–258. doi:10.1007/s11684-018-0632-1
10. Fu CK, Chien YC, Chuang HY, et al. The association of MMP7 promoter polymorphisms with gastric cancer. Anticancer Res. 2020;40(2):695–702. doi:10.21873/anticanres.13999
11. Yu B, Liu X, Chang H. MicroRNA-143 inhibits colorectal cancer cell proliferation by targeting MMP7. Minerva Med. 2017;108(1):13–19. doi:10.23736/S0026-4806.16.04651-6
12. Gao Y, Nan X, Shi X, et al. SREBP1 promotes the invasion of colorectal cancer accompanied upregulation of MMP7 expression and NF-kappaB pathway activation. BMC Cancer. 2019;19(1):685. doi:10.1186/s12885-019-5904-x
13. Bufu T, Di X, Yilin Z, Gege L, Xi C, Ling W. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs. 2018;29(6):530–538. doi:10.1097/CAD.0000000000000621
14. Horndler C, Gallego R, Garcia-Albeniz X, et al. Co-expression of matrix metalloproteinase-7 (MMP-7) and phosphorylated insulin growth factor receptor I (pIGF-1R) correlates with poor prognosis in patients with wild-type KRAS treated with cetuximab or panitumumab: a GEMCAD study. Cancer Biol Ther. 2011;11(2):177–183. doi:10.4161/cbt.11.2.13839
15. Almendro V, Ametller E, Garcia-Recio S, et al. The role of MMP7 and its cross-talk with the FAS/FASL system during the acquisition of chemoresistance to oxaliplatin. PLoS One. 2009;4(3):e4728. doi:10.1371/journal.pone.0004728
16. Kumar P, Siripini S, Sreedhar AS. The matrix metalloproteinase 7 (MMP7) links Hsp90 chaperone with acquired drug resistance and tumor metastasis. Cancer Rep. 2020;5(12):e1261.
17. Ren J, Smid M, Iaria J, et al. Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Res. 2019;21(1):109. doi:10.1186/s13058-019-1194-0
18. Zhang X, Hu F, Li G, et al. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9(2):25. doi:10.1038/s41419-017-0176-3
19. Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, Oshimori N. Tumor-initiating cells establish an IL-33-TGF-beta niche signaling loop to promote cancer progression. Science. 2020;369(6501). doi:10.1126/science.aay1813
20. Thomas DS, Fourkala EO, Apostolidou S, et al. Evaluation of serum CEA, CYFRA21-1 and CA125 for the early detection of colorectal cancer using longitudinal preclinical samples. Br J Cancer. 2015;113(2):268–274. doi:10.1038/bjc.2015.202
21. Nikiteas NI, Tzanakis N, Gazouli M, et al. Serum IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients: prognostic implications. World J Gastroenterol. 2005;11(11):1639–1643. doi:10.3748/wjg.v11.i11.1639
22. Beeghly-Fadiel A, Shu XO, Long J, et al. Genetic polymorphisms in the MMP-7 gene and breast cancer survival. Int J Cancer. 2009;124(1):208–214. doi:10.1002/ijc.23859
23. Gao Q, Wang XY, Qiu SJ, et al. Tumor stroma reaction-related gene signature predicts clinical outcome in human hepatocellular carcinoma. Cancer Sci. 2011;102(8):1522–1531. doi:10.1111/j.1349-7006.2011.01981.x
24. Manuel V-A, Nuria T, Moisés B-C, Mar H-C, Luis -A-A. Serum levels of macrophage inhibitory cytokine-1 (MIC1) and matrix metallopeptidase-7 (MMP7) as diagnostic and prognostic markers in gastric cancer (GC) patients (pts). J Clin Oncol. 2012. doi:10.1200/jco.2012.30.15_suppl.e14595
25. Chen L, Ke X. MMP7 as a potential biomarker of colon cancer and its prognostic value by bioinformatics analysis. Medicine. 2021;100(9):e24953. doi:10.1097/MD.0000000000024953
26. Klupp F, Neumann L, Kahlert C, et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer. 2016;16(1):494. doi:10.1186/s12885-016-2515-7
27. Li H, Xu X, Liu Y, et al. MMP7 induces T-DM1 resistance and leads to the poor prognosis of gastric adenocarcinoma via a DKK1-dependent manner. Anticancer Agents Med Chem. 2018;18(14):2010–2016. doi:10.2174/1871520619666181203111329
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

