Back to Journals » Journal of Inflammation Research » Volume 16
Clinical Significance of Mean Platelet Volume Combined with Neutrophil–Lymphocyte Ratio in Predicting the Therapeutic Effect of Splanchnic Neurolysis
Authors Dai J, Han Y, Fang T , Shao H, Teng L, Zou H
Received 3 July 2023
Accepted for publication 23 October 2023
Published 31 October 2023 Volume 2023:16 Pages 5027—5037
DOI https://doi.org/10.2147/JIR.S428641
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Junzhu Dai,1,* Yuxiang Han,1,* Tianyi Fang,2 Hongxue Shao,1 Lei Teng,1 Huichao Zou1
1Department of Pain Medicine, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Gastroenterological Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huichao Zou, Department of Pain Medicine, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, 150081, People’s Republic of China, Email [email protected]
Introduction: In most cases of pain related to abdominal tumors, increasing the dosage of analgesics still makes the pain difficult to alleviate. Splanchnic neurolysis is a new treatment option. However, not all patients receiving splanchnic neurolysis treatment will achieve satisfactory results. The aim of this study is to retrospectively analyze the predictive value of preoperative serum immune indicators (white blood cells, neutrophils, lymphocytes, and platelets) for the efficacy of splanchnic neurolysis.
Methods: The abdominal cancer patients (pancreatic cancer, liver cancer, gastric cancer, colorectal cancer, cholangiocarcinoma, and renal cancer) admitted to the Department of Pain Medicine, Harbin Medical University Cancer Hospital from January 2017 to October 2020 were collected. We evaluate the efficacy of splanchnic neurolysis by assessing the dosage of opioids and Numerical Rating Scale (NRS) scores of patients 24 to 48 hr before and after splanchnic neurolysis. The predictive value of preoperative serum immune indicators on the efficacy of splanchnic neurolysis was analyzed using Receiver Operating Characteristic (ROC). Contract the Nomogram prediction model by R software.
Results: We found that Mean Platelet Volume (MPV) has statistical significance for predicting splanchnic neurolysis efficacy in digestive system tumors. MPV and Neutrophil–Lymphocyte Ratio (NLR) are independent predictors and have statistical significance in predicting splanchnic neurolysis efficacy in pancreatic cancer. The combination of MPV and NLR had satisfactory predictive value in pancreatic cancer (AUC = 0.715) and the nomogram model was constructed. Furthermore, there was a negative correlation between lymphocyte count and NRS score, and a positive correlation between Platelet–Lymphocyte Ratio (PLR) and NRS score.
Discussion: The combined detection of MPV and NLR has important clinical predictive value for the postoperative efficacy of splanchnic neurolysis in pancreatic cancer.
Kewwords: neutrophil–lymphocyte ratio, pancreatic cancer, splanchnic neurolysis, efficacy, nomogram
A Letter to the Editor has been published for this article.
Introduction
Abdominal cancer is an important public health problem cause of high mortality and heavy economic and social burden. Although the overall incidence rate of colorectal cancer has declined, the trend is younger. Incidence continued to increase by about 1.6%~1.7% annually in liver cancer and by 1% per year for cancers of the kidney and pancreas.1 According to statistics, the incidence of pain in patients with cancer can reach about 50%, and 70% in patients with advanced cancer.2 In particular, the incidence of pain in patients with pancreatic cancer can reach 44% in the early stage.3 Abdominal pain caused by upper abdominal malignancies can affect the quality of life of patients seriously, especially pancreatic cancer pain. Pain caused by pancreatic cancer is commonly confined to the upper abdomen and sometimes radiates to the back. Most pancreatic cancers present as advanced disease, cannot be surgically removed, have poor sensitivity to radiotherapy and chemotherapy, and have poor prognosis at the time of initial diagnosis.4 For these reasons, cancer pain is often the most concerning and most urgent problem for pancreatic cancer patients.5 Cancer pain can cause negative symptoms such as fatigue, loss of appetite, insomnia, anxiety, depression, and so on, which seriously affect the quality of life of patients.6 Therefore, effective control of pain is the key to improving the quality of life of patients with advanced pancreatic cancer.
According to the World Health Organization (WHO) three-step analgesia ladder, systemic analgesic drug therapy is the most important and commonly used analgesic method.7 Rational application of analgesics can effectively relieve abdominal pain in most patients with pancreatic cancer. Despite this, some patients still suffer from insufficient analgesia after high-dose opioids, or cannot manage pain with adequate opioids due to intolerable side effects such as nausea, vomiting, constipation, and drowsiness.8,9 In addition, non-opioid treatments, including chemotherapy, radiotherapy, surgical resection, and rehabilitation, have specific limitations.10 In 1914, Kappis first proposed celiac plexus and splanchnic nerve block and it became an alternative strategy for pain treatment.11 Splanchnic neurolysis has the advantages of fewer traumas, higher efficiency, and fewer side effects, which can effectively help patients relieve pain, improve function, and reduce opioid tolerance and dependence.10 However, in clinical application, neurolysis does not achieve satisfactory analgesic effect in all cases. There are individual differences in the effect of postoperative pain relief. Previous studies reported pain relief in 40–90% of patients after neurolysis.12 In addition, in practical clinical application, this procedure is less widely promoted than traditional drug therapy among cancer pain patients due to its invasive operation, relatively high cost, and difference in the effectiveness of interventional therapy. Many patients show hesitation and delay about whether to perform this interventional analgesia therapy. Therefore, we urgently need to find a marker to judge the efficacy of the interventional surgery more effectively, in order to guide doctors and patients to choose a more appropriate analgesia method in clinical practice.
In recent years, more and more studies have shown that inflammation plays an important role in the development of cancer.13 Chronic pain caused by cancer, radiotherapy, or chemotherapy is associated with chronic neuroinflammation. Studies14,15 have found that non-neural cells such as immune cells and cancer cells play an active role in the occurrence and development of pain. The severity of pain in patients with cancer is closely related to an adverse tumor microenvironment, and the increased nerve damage occurs in a more toxic tumor environment.12 Inflammatory responses can be assessed by changes in white blood cells (WBC), neutrophils (NE), lymphocytes (LY), platelets (PLT), PLR, MPV, NLR, PLR, SII, SIRI, LMR, and so on. The level of inflammatory response is readily assessed with standard assays widely used in clinical practice.
This study firstly evaluated the relationship between clinical common serum immune indexes and pain degree and evaluated the relationship between serum immune indexes and the efficacy of splanchnic neurolysis in digestive system tumors. We further screened serum immunological indicators suitable for preoperative evaluation of the efficacy of splanchnic neurolysis in pancreatic cancer and constructed a clinical prediction model. This study will help screen patients with advanced pancreatic cancer who are more suitable for splanchnic neurolysis.
Materials and Methods
Patient Characteristics
A retrospective analysis was performed on patients with abdominal cancer admitted to the Department of Pain Medicine, Harbin Medical University Cancer Hospital from January 2017 to October 2020. Demographic and clinicopathological variables, such as age, gender, and tumor type, were extracted.
Inclusion criteria: Patients diagnosed of unresectable primary or secondary abdominal cancer and with epigastric pain; use of high-dose, combination pain medication regimen (opioids and NSAID) for the past 2 weeks, or contraindication to dose increase due to adverse drug reactions; the mean NRS>3 points in the past 1 week; no cognitive impairment and signed informed consent voluntarily; no other inflammatory and autoimmune diseases; on stable antitumor therapy; the patient’s blood routine examination and other data are complete.
Exclusion criteria: Coagulation disorders or oral anticoagulation; general contraindications to neurotherapy; patients with severe cachexia or severe cardiopulmonary insufficiency.
Splanchnic Neurolysis
Preoperative blood cell analysis was performed, and an operation agreement was signed. The patients were fasted for 6 hr before surgery and deprived of water for 4 hr. Analgesics were stopped on the day of operation, and intravenous access was opened. Keep communication with the patient throughout the procedure. Continuously monitor the patient’s heart rate, blood pressure, respiration, pulse, and blood oxygen.
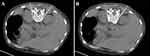
The patient entered the CT interventional operating room and kept the prone position with a pad placed under the abdomen to facilitate the curvature of the spine. CT scan was performed at a slice thickness interval of 5mm from T12 to L1. The anterior edge of bilateral T12 to L1 vertebral body was selected as the target points. They then drew up the puncture point, needle insertion route, and needle insertion direction and depth, and marked the body surface according to the CT positioning line. Routine disinfection and draping were performed, and local infiltration anesthesia with 0.5% lidocaine was applied. A 22G, 150 mm puncture needle was used to slide along the outer edge of the vertebral body to the prevertebral space and CT scan confirmed the correct position of the needle tip. No aspirates were seen coming through the inserted needles. A mixture of 2.5 mL 1% lidocaine and 0.5 mL iodohexol was injected on both sides, respectively. CT scan was performed again to observe the diffusion of contrast agent in the T12 to L1 prevertebral space and the posterolateral aspect of abdominal aorta. The upper abdominal pain of the patient was relieved after observation for 10 min. About 10 mL anhydrous ethanol was slowly injected into both sides, respectively. Patients should lie prone for 2–3 hr after operation. Vital signs were monitored, hypotension and ethanol poisoning were noted, and symptomatic and supportive treatment was provided if necessary. Patients’ activity can be guided out of bed by stable blood pressure on the second day. The intervention is depicted in Figure 1.
Evaluation of Postoperative Efficacy of Splanchnic Neurolysis
The opioid consumption of patients before and 24–48 hr after operation was collected to exclude the effect of postoperative pain after splanchnic neurolysis, and the equivalent oral morphine dose was calculated. Patients were assessed for pain before and 24–48 hr after the operation using a numerical scoring method, and adverse reactions were recorded. According to the NCCN guidelines, the analgesic target is pain numerical score (NRS)≤3 points, and the oral morphine measurement conversion standard: oral morphine 60 mg ≈ oral oxycodone 30 mg ≈ intravenous morphine 20 mg ≈ fentanyl transdermal patch 25 μg/h.
The patients were divided into two groups. (1) Group 1: significant effect meant the analgesic target could be achieved (NRS≤3 points) after the original analgesic drugs were reduced by more than 50% or completely discontinued. (2) Group 2: the general effect meant that the original analgesic drugs are reduced by less than 50% when the analgesic target is achieved, and the ineffectiveness means that the current dose or even the amount of analgesic drugs are still needed to achieve the analgesic target.
Hematology Analysis
The results of routine blood tests were collected 24–48 hr before surgery, and the values of WBC, MPV, PLR, LMR, NLR, SII, and SIRI were counted. The formula was calculated as platelet–lymphocyte ratio (PLR) = P/L, lymphocyte–monocyte ratio (LMR) = L/M, neutrophil–lymphocyte ratio (NLR) = N/L, systemic immune inflammation score (SII) = N×P/L, and systemic inflammation response index (SIRI) = N×M/L (N=Neutrophil count, L=Lymphocyte count, M=Monocyte count, and P=Platelets).
Statistical Analysis
Chi-squared test is used to analyze the relationship between preoperative serum immune indicators and NRS. We use Kruskal Wallis Test for data that do not correspond to Normal distribution. Odds ratio (OR) and 95% confidence intervals (CI) were estimated using Logistics regression models. We analyze the predictive value of preoperative serum immune indicators on the efficacy of splanchnic neurolysis using receiver operating characteristic curve (ROC). All bioinformatics analyses were performed using R Studio software (v4.0.2) and ‘rms’ and “resourceSelection” packages were used. We use the Hosmer Lemeshow Goodness-of-Fit to evaluate the calibration of the nomogram prediction model. The P value < 0.05 indicates that there is no significant difference between the predicted and observed values, indicating a satisfactory model calibration. We use Akaike Information Criterion (AIC) values to compare the goodness-of-fit of different prediction models. AIC is negatively correlated with model fits. For all statistical analyses, SPSS 24.0 (SPSS, USA) was used. Two-tailed P values < 0.05 were considered significant.
Results
Patient Characteristics
A total of 171 patients were treated with splanchnic neurolysis, and 143 patients were included in this study, including 94 cases of pancreatic cancer, 17 cases of liver cancer, 16 cases of gastric cancer, 5 cases of colorectal cancer, 4 cases of cholangiocarcinoma, and 7 cases of renal cancer with intraperitoneal metastasis. There were 89 males and 54 females with an average age of 61±8.99 years. The curative effect was excellent in 87 cases (60.84%) and average in 56 cases (39.16%). There were 53 males and 41 females with an average age of 62±8.41 years. The efficacy was excellent in 54 cases (57.45%) and average in 40 cases (42.55%).
Complications: 8 patients felt severe pain and needed to inject analgesic; 16 patients had diarrhea of different degrees after operation, and the symptoms were relieved within 3 days after symptomatic treatment; Hypotension occurred in 32 patients after intraoperative injection, and the blood pressure recovered after intravenous infusion of sufficient liquid and vasoactive drugs; 2 patients were complicated with symptoms of alcoholism after operation, which were relieved after naloxone rescue and fluid infusion. There were no severe complications such as obstinate hypotension, shock, arrhythmia, kidney injury, and pneumothorax.
Inflammatory Markers for Predicting Surgical Effectiveness in Digestive System Tumors
Among all 143 patients, WBC, PLR, MPV, NLR, PLR, SII, SIRI, and LMR were calculated to predict surgical efficacy. The ROC values were 0.473 (95% CI: 0.372–0.574, P=0.585), 0.439 (95% CI: 0.343–0.535, P=0.219), 0.635 (95% CI: 0.542–0.727, P<0.05), 0.596 (95% CI: 0.501–0.690, P=0.054), 0.541 (95% CI: 0.444–0.638, P=0.408), 0.537 (95% CI: 0.438–0.637, P=0.453) 0.530 (95% CI: 0.430–0.629, P=0.551), 0.443 (95% CI: 0.346–0.540, P=0.254) (Figure 2A). MPV has statistical significance for predicting efficacy, and the optimal cutoff value is 10.85 (Sensitivity=42.5%, Specificity=78.6%).
 |
Figure 2 Prediction of the effectiveness of immune markers for splanchnic neurolysis. (A) In digestive system tumors; (B) In pancreatic cancer; (C) Combined MPV and NLR in pancreatic cancer. |
Inflammatory Markers for Predicting Surgical Effectiveness in Pancreatic Cancer
Among 94 patients with pancreatic pain, the predictive values of WBC, PLR, MPV, NLR, PLR, SII, SIRI, and LMR for surgical efficacy were calculated, with ROC values of 0.529 (95% CI: 0.409–0.649, P=0.635), 0.409 (95% CI: 0.292–0.526, P=0.133), 0.665 (95% CI: 0.554–0.776, P<0.05), 0.654 (95% CI: 0.541–0.766, P<0.05), 0.513 (95% CI: 0.394–0.633, P=0.824), 0.578 (95% CI: 0.459–0.697, P=0.196), respectively, 0.575 (95% CI: 0.456–0.693, P=0.218), 0.412 (95% CI: 0.295–0.529, P=0.146) (Figure 2B). MPV and NLR have statistical significance in predicting efficacy, with the optimal Cutoff values of 10.15 and 3.65, respectively (Sensitivity=72.2%, Specificity=55.0%; Sensitivity=64.8%, Specificity=72.5%).
The Combination of MPV and NLR in Predicting the Efficacy of Splanchnic Neurolysis in Pancreatic Pain
In 94 patients with pancreatic pain, the predictive value of combined MPV and NLR for the efficacy of splanchnic neurolysis in pancreatic pain was calculated, with an AUC of 0.715 (95% CI: 0.611–0.818, P<0.001). The combination of MPV and NLR has a satisfactory predictive value (Sensitivity=77.5%, Specificity=59.3%, Figure 2C).
A Predictive Model Based on Preoperative Serum Inflammatory Indicators
In order to determine the independent factors that predict the efficacy of splanchnic neurolysis in patients with pancreatic cancer, a logistic risk regression model was used for univariate and multivariate analysis. We found that MPV and NLR were independent predictors of surgical efficacy in univariate analysis (P=0.004, P=0.025) and multivariate analysis (P=0.007, P=0.038) (Table 1).
 |
Table 1 Univariate Analysis and Multivariate Analysis of Preoperative Serum Inflammatory Indicators |
A nomogram model predicting the efficacy of splanchnic neurolysis was constructed for pancreatic cancer (Figure 3A). The AUC of the nomogram for predicting efficacy is 0.714 [95% CI: 0.609–0.819]. The sensitivity and specificity were 59.3% and 77.5%, respectively (Figure 3B).
Then, we verified that the prognostic model had satisfactory predictive value by calibration curve analysis, and the calibration analysis showed that the C-index was 0.714 (0.669–0.759). The P value of Hosmer-Lemeshow Goodness-of-Fit was 0.9537, which showed a satisfactory model calibration (Figure 3C). Decision curve analysis showed that the combination of MPV and NLR (AIC=119.71) was better than single use of NLR (AIC=125.89) or MPV (AIC=122.92) (Figure 3D).
The Relationship Between Preoperative Serum Inflammatory Markers and NRS Score
We retrospectively analyzed the relationship between preoperative NRS score and serum immunological indicators. We divided the NRS score into three groups based on 1–3 points (18 patients), 4–6 points (108 patients), and 7–10 points (17 patients). The serum immune indicators of three groups of patients were counted separately to observe whether inflammatory reactions would affect the patient’s NRS score. We found statistically significant differences in lymphocyte count, PLR, and NRS scores. There is a negative correlation between lymphocyte count and NRS score (P<0.05), and a positive correlation between PLR and NRS score (P<0.05). Although platelet count, NLR, and SII were not statistically significant, there was a statistical trend, which may be due to the small sample size of this study (Figure 4).
 |
Figure 4 There are statistically significant differences in lymphocyte count, PLR and NRS scores. |
Discussion
Primary abdominal malignancies or metastatic cancer may cause severe pain, especially pancreatic cancer.12 Pancreatic cancer is a fatal disease, with a 5-year survival rate of less than 2%. Clinical manifestations of pancreatic cancer include upper abdominal biting and blunt pain. Pain relief treatment is crucial in the disease management process of these cancer patients. Patients with pancreatic cancer pain often need to use strong opioids, but some still cannot achieve satisfactory analgesic effect.16 Severe nausea and vomiting, addiction to opioid drugs, respiratory depression, constipation, and other adverse reactions are the main limitations of its clinical application. In addition, this type of pain typically has a resistance to traditional opioid analgesics and standard treatment, resulting in significant negative impacts on patients’ emotional, physiological functions, and quality of life.
Compared with systemic medication, many studies have confirmed that celiac plexus block can alleviate upper abdominal pain more effectively, reduce opioid dosage and adverse reactions.17–19 The visceral nerve, along with T5-T12 preganglionic fibers and ventral nerve roots, constitutes the main sympathetic nerve of the celiac plexus. These fibers supply abdominal viscera including stomach, small intestine, pancreas, spleen, adrenal glands, and liver. The blockade of these nerves is termed as splanchnic nerve block.20 For patients with abdominal pain caused by malignant tumors, splanchnic neurolysis has been used to deal with pain mainly located in epigastrium, periumbilical, and other areas. However, it has not been widely applied in the field of cancer pain treatment in many developing countries. Due to the insufficient promotion of splanchnic neurolysis and its relatively high price, patients may hesitate to choose splanchnic neurolysis for pain relief without a sufficient understanding of its efficacy. Despite the shortcomings of poor efficacy and frequent side effects in the current use of analgesics, most patients still prefer to use analgesics to achieve their analgesic goals and refuse to intervene in analgesic treatment. Therefore, we urgently need to find a biomarker to predict the efficacy of splanchnic neurolysis, in order to provide more valuable reference for clinical application and patients to choose more suitable analgesic methods.
Current clinical studies have shown that inflammatory reactions play an important role in cancer. At the same time, cancer pain, especially neuropathic pain, is closely related to systemic inflammation.21 It is reported that TNF, CCL2, CCL5, IL-6, TRPA1, and other inflammatory factors can cause neuropathic pain.22–24 The increased infiltration of inflammatory cells and immune cells is accompanied by the progression of pancreatic cancer, and more and more studies show that these cells contribute to various pain states. Macrophages and other inflammatory or immune cells may play an important role in inducing and maintaining pancreatic cancer pain. Compared with other epigastrium tumors, pancreatic cancer has a more prominent ability to infiltrate neural structures due to its neurophilic growth characteristics.25 Pancreatic cancer cells can accumulate neurointima, axons, and Schwann cells. Therefore, nerve plexus infiltration around the pancreas is the most common way for pancreatic cancer to spread, and the pain caused by pancreatic cancer is often more severe.26 The main mechanism of pain in pancreatic cancer is pancreatic neuropathy. Neurogenic inflammation may be related to the generation, maintenance, and promotion of pain in pancreatic cancer. Histopathology changes and molecular-level changes can be observed in pancreatic cancer specimens, such as increased nerve density, infiltration of cancer cells in and around the nerve, infiltration of immune cells in the tumor microenvironment, and release of neurotrophic growth factors, which cannot be detected in normal pancreatic tissue.27 Some studies have also found that immune cell clusters including mast cells, T lymphocytes, and macrophages can be seen around the local pancreatic nerve. The pancreatic tissue structure is distorted due to the infiltration of cancer cells, which can promote the activation of local immune cells and lead to further damage caused by inflammation.28 Immune cell infiltration, peripheral nerve cell invasion, and the release of neurotrophic cytokines by tumors and immune cells lead to mitosis of pancreatic neurons, increasing their number and size.29 In animal models of pancreatic cancer, with the progression of the tumor, the density of macrophages increased significantly in pancreatic tissue, and a strong tumor growth factor NGF immunoreactivity could be observed. It was found that both mRNA and protein levels of NGF were up-regulated in pancreatic cancer. At the same time, the level of NGF seemed to be related to the degree of tumor peripheral nerve invasion and pain. In animal models, it has been found that IL-6 is elevated in some pain states. IL-8, IL-1b, TNF-α, and other cytokines involved in systemic inflammation may also be associated with pain. TRPV1 is a transient receptor potential cation channel. Local inflammation can activate the expression of TRPV1 in nerve endings in the pancreas. Quantitative and localization of TRPV1 in the pancreas by PCR revealed a positive correlation between TRPV1 mRNA levels and pain intensity.30,31 Another study found that compared with pancreatic cancer patients with mild pain or without pain, severe pain had a threefold increase in nerve hypertrophy. Patients with severe pain have almost twice the invasion score of nerve cancer cells compared to those without pain and those with mild pain.
Our study found for the first time that the immune response of peripheral blood is more effective in predicting the efficacy of splanchnic neurolysis in pancreatic cancer than other digestive system tumors. One of the most prominent characteristics of pancreatic cancer immune microenvironment is the presence of a large number of compact matrix components, including tumor cells, tumor-related fibroblasts, and various types of immune cells.32 Due to the influence of the immunosuppressive microenvironment of pancreatic cancer, the function of effector T cells is greatly inhibited, and helper T cells tend to differentiate into Th2 cells and secrete Th2 cytokines. In advanced pancreatic cancer, the accumulation of CD8+T cells in the stroma is significantly reduced while that of Treg cells is significantly increased.33 At the same time, pancreatic cancer cells promote the activation of surrounding stromal cells and the recruitment of immunosuppressive cells by secreting a variety of cytokines and chemokines. Activated stromal cells interfere with the infiltration of effector T cells and NK cells around tumor cells. These immunosuppressive cells lead to an imbalance between the number and function of normal immune effector cells by secreting immunosuppressive factors, forming a unique immunosuppressive tumor microenvironment for pancreatic cancer.24,34 Compared with other digestive system tumors, this special immune microenvironment may make immune cells in the peripheral blood more sensitive, which is more suitable for reflecting the immune status of pancreatic cancer tissue.
This study retrospectively analyzed the predictive effects of WBC, PLR, MPV, NLR, PLR, SII, SIRI, and LMR in peripheral blood on the efficacy of splanchnic neurolysis. We found that MPV has satisfactory value for the prediction of surgical efficacy in patients with digestive system tumors, with a cutoff value of 10.85. In pancreatic cancer, MPV and NLR are statistically significant for efficacy prediction, and the cutoff values are 10.15 and 3.65, respectively. When combining MPV and NLR to predict the efficacy of splanchnic neurolysis in pancreatic pain, the AUC was 0.715 (95% CI: 0.611–0.818, P<0.001), which has satisfactory predictive value. We find that MPV and NLR are independent predictors of surgical efficacy in univariate analysis (P=0.004, P=0.025) and multivariate analysis (P=0.007, P=0.038), and further construct a predictive model in pancreatic cancer. In addition, we analyzed the relationship between NRS score and preoperative serum immunological indicators and found that lymphocyte count and PLR had statistically significant effects on patient pain levels.
Nevertheless, our research also has some limitations. Firstly, our study is a single-center research and requires expanding the sample size, collecting data from more hospitals, and conducting internal and external validations separately. Changes in preoperative levels of inflammatory factors may indicate a non-specific inflammatory response to pancreatic cancer, leading to false-positive results when screening asymptomatic subjects.
Conclusion
The results of this study suggest that inflammation may be closely related to tumor progression and cancer pain. The combined detection of MPV and NLR has important clinical predictive value for the postoperative efficacy of splanchnic neurolysis in pancreatic cancer. Its detection has the advantages of simplicity and convenience, and it is worth expanding the sample size to further validate the results of this study, in order to be applied early in clinical practice and provide more references for patients to choose more suitable analgesic methods.
Data Sharing Statement
The datasets used in this study are available from the corresponding author on reasonable request.
Ethics Approval Statement
This study was approved by Harbin Medical University Cancer Hospital Ethics Committee. All the experiments were performed in accordance with established ethical guidelines, and informed consent was obtained from the participants. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Junzhu Dai and Yuxiang Han contributed equally to this work; Junzhu Dai, Yuxiang Han, Tianyi Fang, Hongxue Shao, and Lei Teng took part in drafting the article or revising it critically for important intellectual content; Junzhu Dai and Huichao Zou agreed to submit to the current journal; Junzhu Dai, Tianyi Fang, and Huichao Zou gave final approval of the version to be published; and Huichao Zou agrees to be accountable for all aspects of the work.
Funding
This work was supported by the Haiyan Research Fund of Harbin Medical University Cancer Hospital from Huichao Zou (JJZD2023-12).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
2. Portenoy RK. Treatment of cancer pain. Lancet. 2011;377(9784):2236–2247. doi:10.1016/S0140-6736(11)60236-5
3. Burton AW, Fanciullo GJ, Beasley RD, Fisch MJ. Chronic pain in the cancer survivor: a new frontier. Pain Med. 2007;8(2):189–198. doi:10.1111/j.1526-4637.2006.00220.x
4. Zhang ZK, Yang YM. Current research status and progress in comprehensive diagnosis and treatment of pancreatic cancer in the era of targeted therapy. Zhonghua Wai Ke Za Zhi. 2020;58(1):22–26. doi:10.3760/cma.j.issn.0529-5815.2020.01.006
5. Dobosz L, Kaczor M, Stefaniak TJ. Pain in pancreatic cancer: review of medical and surgical remedies. ANZ J Surg. 2016;86(10):756–761. doi:10.1111/ans.13609
6. Carter GT, Duong V, Ho S, Ngo KC, Greer CL, Weeks DL. Side effects of commonly prescribed analgesic medications. Phys Med Rehabil Clin N Am. 2014;25(2):457–470. doi:10.1016/j.pmr.2014.01.007
7. Anekar AA, Hendrix JM, Cascella M. WHO Analgesic Ladder. StatPearls. 2023.
8. Zhang H. Cancer Pain Management-New Therapies. Curr Oncol Rep. 2022;24(2):223–226. doi:10.1007/s11912-021-01166-z
9. Koller G, Schwarzer A, Halfter K, Soyka M. Pain management in opioid maintenance treatment. Expert Opin Pharmacother. 2019;20(16):1993–2005. doi:10.1080/14656566.2019.1652270
10. Patti JW, Neeman Z, Wood BJ. Radiofrequency ablation for cancer-associated pain. J Pain. 2002;3(6):471–473. doi:10.1054/jpai.2002.126785
11. Paul A, Borkar A. Fluoroscopy-Guided Splanchnic Nerve Block for Cancer-Associated Pain. Cureus. 2022;14(10):e30944. doi:10.7759/cureus.30944
12. Lohse I, Brothers SP. Pathogenesis and Treatment of Pancreatic Cancer Related Pain. Anticancer Res. 2020;40(4):1789–1796. doi:10.21873/anticanres.14133
13. Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol. 2020;119:199–245. doi:10.1016/bs.apcsb.2019.09.004
14. Ma H, Pan Z, Lai B, Li M, Wang J. Contribution of immune cells to cancer-related neuropathic pain: an updated review. Mol Pain. 2023;19:17448069231182235. doi:10.1177/17448069231182235
15. Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi:10.1126/science.aaf8924
16. Carvajal G. Pancreatic Cancer Related Pain: review of Pathophysiology and Intrathecal Drug Delivery Systems for Pain Management. Pain Physician. 2021;24(5):E583–E594.
17. Sachdev AH, Gress FG. Celiac Plexus Block and Neurolysis: a Review. Gastrointest Endosc Clin N Am. 2018;28(4):579–586. doi:10.1016/j.giec.2018.06.004
18. Dumitrescu A, Aggarwal A, Chye R. A retrospective case series of patients who have undergone coeliac plexus blocks for the purpose of alleviating pain due to intra-abdominal malignancy. Cancer Rep. 2020;3(5):e1265. doi:10.1002/cnr2.1265
19. Vig S, Bhan S, Bhatnagar S. Celiac Plexus Block - An Old Technique with New Developments. Pain Physician. 2021;24(5):379–398.
20. Paul A, Borkar A. Fluoroscopy-Guided Splanchnic Nerve Block for Cancer-Associated Pain. Cureus. 2022;14(10):e30944. doi:10.7759/cureus.30944
21. Sandy-Hindmarch O, Bennett DL, Wiberg A, Furniss D, Baskozos G, Schmid AB. Systemic inflammatory markers in neuropathic pain, nerve injury, and recovery. Pain. 2022;163(3):526–537. doi:10.1097/j.pain.0000000000002386
22. Duan YW, Chen SX, Li QY, Zang Y. Neuroimmune Mechanisms Underlying Neuropathic Pain: the Potential Role of TNF-alpha-Necroptosis Pathway. Int J Mol Sci. 2022;23(13). doi:10.3390/ijms23137191
23. Du S, Wu S, Feng X, et al. A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. J Clin Invest. 2022;132(13). doi:10.1172/JCI153563
24. Boakye PA, Tang SJ, Smith PA. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-alpha, Wnt Ligands, and Interleukin 1beta. Front Pain Res. 2021;2:698157. doi:10.3389/fpain.2021.698157
25. Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136(1):177–186 e1. doi:10.1053/j.gastro.2008.09.029
26. Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi:10.1093/jnci/djp456
27. Demir IE, Schorn S, Schremmer-Danninger E, et al. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One. 2013;8(3):e60529. doi:10.1371/journal.pone.0060529
28. Wang J, Chen Y, Li X, Zou X. Perineural Invasion and Associated Pain Transmission in Pancreatic Cancer. Cancers. 2021;13(18). doi:10.3390/cancers13184594
29. Li J, Kang R, Tang D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun. 2021;41(8):642–660. doi:10.1002/cac2.12188
30. Li F, Wang F. TRPV1 in Pain and Itch. Adv Exp Med Biol. 2021;1349:249–273. doi:10.1007/978-981-16-4254-8_12
31. Iftinca M, Defaye M, Altier C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs. 2021;81(1):7–27. doi:10.1007/s40265-020-01429-2
32. Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. doi:10.1186/s12943-018-0858-1
33. Goulart MR, Stasinos K, Fincham REA, Delvecchio FR, Kocher HM. T cells in pancreatic cancer stroma. World J Gastroenterol. 2021;27(46):7956–7968. doi:10.3748/wjg.v27.i46.7956
34. Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17(9):527–540. doi:10.1038/s41571-020-0363-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.