Back to Journals » Patient Preference and Adherence » Volume 16
Clinical Practice Management of Primary Open-Angle Glaucoma in the United States: An Analysis of Real-World Evidence
Authors Imperato JS , Zou KH , Li JZ , Hassan TA
Received 22 March 2022
Accepted for publication 5 August 2022
Published 18 August 2022 Volume 2022:16 Pages 2213—2227
DOI https://doi.org/10.2147/PPA.S367443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Joseph S Imperato,1 Kelly H Zou,1 Jim Z Li,1 Tarek A Hassan2
1Global Medical Analytics and Real-World Evidence, Viatris Inc., Canonsburg, PA, USA; 2Global Medical Affairs, Ophthalmology, Viatris Inc, Canonsburg, PA, USA
Correspondence: Tarek A Hassan, Global Medical Affairs, Ophthalmology, Viatris Inc, 1000 Mylan Boulevard, Canonsburg, PA, 15317, USA, Tel +1 347 443 2850, Email [email protected]
Purpose: To investigate clinical management of primary open-angle glaucoma (POAG) in the United States using real-world evidence and to examine healthcare resource utilization (HCRU), medication adherence/persistence, and procedure use.
Design: A cross-sectional, retrospective analysis of Optum’s de-identified Market Clarity Dataset (July 1, 2013–December 31, 2019).
Patients and Methods: Patients ≥ 18 years with POAG diagnosis and continuous enrollment for 1-year pre- and post-index were eligible and categorized into four mutually exclusive cohorts: CH1, treated with antiglaucoma medication(s) only; CH2, underwent glaucoma procedure(s) only; CH3, treated with antiglaucoma medication(s) and underwent procedure(s); and CH4, received no treatment for POAG. Adherence and persistence with antiglaucoma medications, and disease-specific HCRU were analyzed. Pairwise two-sample comparisons and multivariate regressions were conducted.
Results: Examined 232,572 eligible patients (CH1=60,895; CH2=4330; CH3=6027; CH4=161,320). Prostaglandin analogs were most prescribed antiglaucoma medications (CH1: 69.7%; CH3: 62.7%), of which latanoprost was most common (CH1: 51.3%; CH3: 46.1%). Disease-specific office visits occurred in 26.3%, 78.2%, 75.0%, 23.8%, and surgical services visits occurred in 3.8%, 36.3%, 42.5%, 3.3%, in CH1-CH4, respectively. Adherence was higher (medication possession ratio: 47.1% vs 39.4%; P< 0.0001), and more patients remained persistent across 1-year post-index period in CH1 vs CH3 (25.4% vs 16.1%; P< 0.0001). Positive predictors of medication persistence included being female, ≥ 55 years, and history of dyslipidemia or thyroid disease (all P≤ 0.0003).
Conclusion: Overall, 70% POAG patients might not have received antiglaucoma treatment. Since POAG is a slowly progressive blinding disease, the lack of antiglaucoma treatment and suboptimal adherence/persistence with medications are of major concerns. Targeted screening and educational approaches are needed to improve POAG management.
Keywords: adherence, antiglaucoma, health care resource utilization, persistence, real-world evidence
Plain Language Summary
Primary open-angle glaucoma (POAG) remains asymptomatic until late stage. Progression of POAG is slowed by lowering intraocular pressure. This study examines the clinical practice management of POAG in 232,572 subjects from the US. Seven of 10 patients might not have received POAG treatment. Less than 25% persisted with antiglaucoma medication. Thus, lack of patient treatment and medication compliance for POAG is of major concern.
Introduction
Primary open-angle glaucoma (POAG) is the most common form of glaucoma,1,2 and glaucoma is the second most common cause of irreversible blindness worldwide.3 Unlike cataracts that are surgically treatable with >90% success,4 blindness relating to POAG or other forms of glaucoma cannot be rectified.5 Glaucoma affects more than 70 million people worldwide,1 and furthermore, the worldwide prevalence is predicted to increase to 111.8 million in 2040, disproportionally affecting people residing in Asia and Africa.1 In the United States (US) prevalence of POAG alone was predicted to reach at least 3.36 million by 2020,6 and approximately 6.3 million by 2050.7 Common risk factors for POAG include elevated intraocular pressure (IOP), older age, African ethnic origin, family history of glaucoma, and myopia.8,9 Certain other comorbidities have been associated with a risk for developing glaucoma or POAG, including cardiovascular (CV) risk factors (dyslipidemia, hypertension, diabetes mellitus, and high body mass index [BMI]/obesity).10–13 However, progression of POAG and associated nerve damage can be halted by lowering IOP.14 This makes control of IOP paramount for preventing the major etiology of glaucoma and POAG blindness worldwide.3,15
Management of POAG is usually initiated with ocular hypotensive drops, but laser trabeculoplasty and/or surgery may also be used to slow disease progression.14,16,17 Although there are new surgical interventions, glaucoma remains a slowly progressive disease requiring long-term IOP lowering and frequently relies on the ongoing use of pharmacologic eye drops. Five main classes of topical drops are available: prostaglandin derivatives, beta-blockers, carbonic anhydrase inhibitors, sympathomimetics, and miotics.18 The primary aim is to reduce IOP by at least 20–30%, followed by ongoing monitoring of IOP with adjustment as indicated by disease course and severity.9 Clinical data have demonstrated that lowering IOP not only reduces the risk of developing POAG but slows progression of disease and delays visual field loss.19–22 Even with a known relationship between IOP and POAG, there is significant interindividual variation in the susceptibility of the optic nerve to IOP-related damage,23–26 and therefore ongoing monitoring is an important aspect of POAG management following diagnosis.
Despite availability of efficacious antiglaucoma medications with few side effects, as well as evidence for the importance of IOP reduction in POAG,19–22 many patients continue to go untreated or undertreated, leaving them at risk for future visual field loss.27,28 Indeed, as POAG is asymptomatic until late stage, a diagnosis is frequently delayed.29 Even among patients with diagnosed POAG, many were historically reported not to receive treatment.27 With a need for attentive management of IOP, there is a need to examine real-world evidence (RWE) to determine how patients are being managed in a clinical practice setting, and moreover, whether patients are sufficiently adherent and persistent with their pharmacologic management. Using claims data and electronic health records (EHR), this study sought to investigate clinical practice in the management of patients with POAG in the US. Specifically, the study compared the adherence and persistence patterns and disease-specific healthcare resource utilization (HCRU) across treatment approaches (both pharmacologic and surgical), alongside an analysis of key comorbidities that may influence treatment persistence.
Methods
Study Database
This study uses Optum’s de-identified Market Clarity Data (2007–2020), which combines robust transactional pharmacy and medical claims data with best-of-breed EHR data.30 The data are a fully Health Insurance Portability and Accountability Act (HIPAA)31 compliant, statistician-certified, de-identified precision data set. The Market Clarity Dataset links EHR data with historical, linked administrative claim data, pharmacy claims, physician claims, facility claims (with clinical information) and is inclusive of medications prescribed and administered. Clinically rich and specific data elements sourced from the EHR include lab results, vital signs and measurements, diagnoses, procedures, and information derived from unstructured clinical notes using natural language processing.
Study Design
A cross-sectional, retrospective study of Optum’s de-identified Market Clarity Data during the study period July 1, 2013 to December 31, 2019. Overall, the database contained 37,762,270 unique patients during the study period. As only de-identified data were used, no informed consent process was needed, and no Institutional Review Board approval was sought.
Study Population
Eligible patients were aged ≥18 years, with an International Classification of Diseases, Clinical Modification (ICD-CM), in both ICD-9-CM and ICD-10-CM versions, diagnosis code for POAG, ocular hypertension, or risk for POAG, and at least 24 months continuous enrollment in the database, to allow at least 1-year continuous assessment, pre- and post-index. Patient demographic and clinical characteristics were captured pre-index, including key comorbidities of interest, based on diagnosis codes. Disease-specific HCRUs, as well as antiglaucoma medication adherence and persistence, were captured in the post-index period. Medications of interest were identified based on national drug codes for commercial or generic therapeutic agents and included all common classes of topical medications used for POAG. Procedures of interest were identified based on diagnosis codes for glaucoma, POAG, or ocular hypertension.
Study Cohorts
Eligible patients were categorized into one of four mutually exclusive cohorts, based on their index treatment approaches (Figure 1). The index treatment approaches were defined as the date of first recorded antiglaucoma prescription or surgical procedure related to POAG during the selection window (July 1, 2014 to December 31, 2018). Patients could have received ≥1 antiglaucoma medication or undergone ≥1 surgical procedure in any of cohorts 1–3, as defined below.
- Cohort #1 (CH1): Eligible patients treated with ≥1 topical antiglaucoma medication only.
- Cohort #2 (CH2): Eligible patients who underwent ≥1 glaucoma-related procedure only.
- Cohort #3 (CH3): Eligible patients who were treated with ≥1 antiglaucoma medication and underwent ≥1 glaucoma-related procedure.
- Cohort #4 (CH4): Eligible patients who did not receive any antiglaucoma medications or undergo any glaucoma-related procedures.
More specifically: (1) If the index event was an antiglaucoma prescription, but the patient had no other medical claim for a POAG-related procedure during the selection window, the patient was allocated to CH1. (2) If the index event was a POAG-related procedure and the patient had no other medical claim for an antiglaucoma prescription during the selection window, the patient was allocated to CH2. (3): If the index event was a POAG-related procedure and the patient had ≥1 medical record for a POAG-related prescription during the selection window, the patient was allocated to CH3. (4) Patients who did not undergo any surgical procedure or fill any prescription for an antiglaucoma medication during the selection window were allocated to CH4.
Adherence and Persistence
Patient adherence and persistence with antiglaucoma medications were analyzed over the 1-year post-index period in patients treated with antiglaucoma medications (CH1 and CH3).
Adherence was measured using the medication possession ratio (MPR), and calculated as
- MPR = [(days with drug on-hand without removing overlap days between prescription fills during 360-day period)/360] × 100%.
MPR was summarized using point-estimates, along with 90% confidence intervals (CIs).
Persistence was measured by time to discontinuation and remaining persistent for the entire 1-year post-index period, according to
- Time to discontinuation = number of consecutive days from treatment initiation until discontinuation or end of the 1-year post-index period, whichever occurred first.
- Discontinuation was defined as gap between prescription fills in index therapy of at least 1.5 × days of the index therapy supply of the prior fill (discontinuation date defined as last day of supply prior to gap).
A full year’s persistence was defined when index antiglaucoma treatment was continued throughout the entire 1-year post-index period without discontinuation in therapy.
Statistical Analysis
Descriptive statistics were calculated. For continuous variables, mean and standard deviation were computed. For categorical variables, counts and percentages were computed. Missing data were not imputed.
Three pairwise two-sample comparisons were conducted across study cohorts, CH1 to CH4. These were prespecified analyses by clinical and demographic characteristics and by disease-specific HCRU. Adherence and persistence with therapy were compared between CH1 versus CH3. A subgroup analysis was carried out to compare adherence or persistence by antiglaucoma medication type (CH1 vs CH3), including patients on multiple antiglaucoma medications. For continuous variables, the two-sample t-test and/or Wilcoxon’s rank-sum test were used. For categorical variables, the Chi-square test or Fisher’s exact test was used. To account for multiple comparisons, a Bonferroni correction was used.
A binary logistic regression analysis was conducted to predict the odds of full-year’s persistence with antiglaucoma medications during 1-year post-index period as an outcome variable. Predictors of persistence were examined for CH1 vs CH3 using a backward elimination process via stepwise logistic regression. Odds ratio (OR) and two-sided P-value were calculated, with 95% CIs. Binary logistic regression analysis was also conducted for occurrence of any disease-specific diagnostic procedures in the 1-year post-index period, to identify predictors of these diagnostic procedures in each cohort (CH2 or CH3). In both logistic regression analyses, diabetes was excluded as a predictor due to collinearity with Charlson Comorbidity Index (CCI) score category. Hypertension was also excluded due to collinearity with dyslipidemia. A Kaplan–Meier survival analysis was finally conducted on persistence with index antiglaucoma medication to determine the probability of remaining persistent for the entire 1-year post-index period for patients in CH1 versus CH3. Two-sided P-value <0.05 was considered statistically significant for all analyses.
Results
Study Population
The database contained a total of 33,635,787 million patients during the selection window (July 1, 2014, to December 31, 2018), of whom 232,572 met all inclusion criteria and formed the study population (Figure 1). Patients who did not receive any antiglaucoma medications or undergo any glaucoma-related procedures (CH4) during the selection window formed the largest cohort (n = 161,320 [69.4%]), followed by patients who received ≥1 antiglaucoma medication only (CH1; n = 60,895 [26.2%]) and patients received ≥1 antiglaucoma medication and underwent ≥1 glaucoma-related procedure during the selection window (CH3, n = 6027 [2.6%]). Patients who underwent ≥1 glaucoma-related procedure only during the selection window formed the smallest cohort (CH2; n = 4330 [1.9%]) (Figure 1). Most patients in each cohort were female and aged ≥55 years (Table 1). The mean (±standard deviation [SD]) age ranged from 62.9±13.2 years in CH2 to 68.6±12.5 years in CH1. Regardless of study cohort, most patients were insured through either a Commercial or Medicare Risk insurance (Table 1). The most common comorbidities across cohorts were related to the cardiovascular system, including hypertension (from 47.3% [CH2] to 68.3% [CH1] of patients) and dyslipidemia (from 47.1% [CH2] to 61.2% [CH1] of patients) (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of Patients in the Four Study Cohortsa |
Primary Open-Angle Glaucoma Management
In patients who received antiglaucoma medications during the selection window, the most common medication(s) prescribed were prostaglandins (CH1: 69.7% and CH3: 62.7%), with latanoprost the most commonly prescribed specific antiglaucoma medication (51.3% and 46.1%, respectively), and prescribed to a significantly larger proportion of patients in CH1 versus CH3 (P<0.0001) (Table 2). Conversely, a significantly larger proportion of patients in CH3 versus CH1 received other therapies, including beta-blockers, cholinergic mimetics, and alpha agonists (all P≤0.0395; Table 2). For patients who underwent prespecified procedure(s) of interest for POAG, the most common procedure was gonioscopy-assisted transluminal trabeculotomy (GATT) (CH2 and CH3) (81.1% and 65.4%, respectively), with trabeculotomy the only other procedure undergone by >5% of the patients (20.0% and 16.3%, respectively). Both procedures were used by significantly more patients in CH2 vs CH3 (P<0.0001) (Table 2).
 |
Table 2 Medication and Procedures of Interest by Study Cohorta |
Healthcare Resource Utilization in the Post-Index Period
At least three-quarters of patients in CH2 and CH3 had disease-specific office visits in the post-index period (78.2% and 75.0%, respectively), both significantly higher than seen in CH1 and CH4 (26.3% and 23.8%, respectively; P<0.0002 for any comparison) (Table 3). Furthermore, 36.3% and 42.5% of patients in CH2 and CH3 reported disease-specific surgical services in the post-index period, significantly more than in CH1 and CH4 (3.8% and 3.3%, respectively; P<0.0001 for any comparison) (Table 3). Disease-specific hospitalizations were low in all cohorts (Table 3). Essential hypertension was a common cause of hospitalization alongside POAG.
 |
Table 3 Disease-Specific Healthcare Resource Utilization in the 1-Year Post-Index Period by Study Cohorta |
Adherence and Persistence in the Post-Index Period
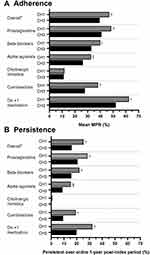
At least three-quarters of patients in either CH1 or CH3, discontinued therapy during the 1-year post-index period (74.6% vs 83.9%, respectively). Adherence was suboptimal in both CH1 and CH3 (MPR: 47.1% vs 39.4%, respectively), but significantly higher for patients in CH1 versus CH3 (P<0.0001) (Figure 2). When patients were stratified by medication class, patterns of adherence were similar to that seen overall, and generally higher in CH1 versus CH3 (Figure 2). Mean MPR was highest for prostaglandin medications, but still significantly higher for patients in CH1 vs CH3, respectively (48.5% vs 40.9%, respectively; P<0.0001).
A higher proportion of patients in CH1 achieved a full year’s persistence compared with CH3 (25.4% vs 16.1%, respectively; P<0.0001) (Figure 2). Mean number of continuous days on therapy before patients discontinued or reached end of the 1-year post-index period was approximately 37 days longer for patients in CH1 versus CH3 (148.9 days vs 111.8 days, respectively; P<0.0001). When stratified by individual medication class, the highest proportion of patients achieved a full year’s persistence with prostaglandin medications (Figure 2). However, patients in CH1 taking >1 type of medication had the longest number of continuous days on therapy before discontinuing (178.4 days, from the date of starting index prescription), and significantly longer than for patients in CH3 (132.9 days; P<0.0001).
Predictors of Remaining Persistent with Index Antiglaucoma Medication in the 1-Year Post-Index Period (Full-Year Persistence)
Significant, positive predictors of a full year’s persistence included being female, older age (≥55 vs <55 years), living in the West (vs South) region of the US, and having a history of dyslipidemia or thyroid disease (vs no history of comorbidities) (all P≤0.0003; Table 4). Negative predictors of a full year’s persistence included patients who had undergone procedure(s) in addition to taking medication(s) (ie, CH3) versus patients on a medication-only regimen (ie, CH1), and those living in the Northeast (vs South) (P<0.0001 for each; Table 4). Compared with patients prescribed prostaglandins as their antiglaucoma medication, patients were generally less likely to remain persistent with any other therapy regimen, including beta-blockers or alpha-adrenergic agonists (P<0.0001 for each; Table 4).
 |
Table 4 Logistic Regression Analysis for Odds Ratio of Persistence with Therapy for POAG During the 1-Year Post-Index Period |
Based on a survival analysis over the 1-year post-index period, patients in CH1 were significantly more likely to remain persistent with their medication regimen (vs CH3 P<0.0001).
Predictors of Undergoing a Surgical Procedure in the 1-Year Post-Index Period
Significant, positive predictors of patients undergoing a disease-specific diagnostic procedure in the post-index period included older age (≥55 vs <55 years) and patients who had already undergone a procedure (ie, patients in CH2 or CH3 vs CH1) (all P<0.0001; Supplemental Table S1). Significant, negative predictors of a patient undergoing disease-specific procedure included being female, patients who were uninsured (vs insured), and patients with a history of certain comorbidities, including depression, dyslipidemia, history of myocardial infarction or carotid artery disease, smoking or thyroid disease (vs no history of comorbidities) (all P≤0.0368; Supplemental Table S1). Patients in CH4 were also less likely to have undergone a disease-specific diagnostic procedure in the post-index period compared with patients in CH1 (P<0.0001).
Discussion
Glaucoma is a leading cause of blindness worldwide,3 and represents an enormous healthcare burden in both developed and developing countries.32,33
Primary open-angle glaucoma (POAG), accounting for more than 90% of US cases of glaucoma, is a chronic, progressive disease. Although this form of glaucoma is commonly associated with elevated intraocular pressure (IOP), more than two-thirds of patients with IOP exceeding 21 mm Hg do not have glaucoma. Since 15% of the patients with glaucoma have a normal IOP of 21 mm Hg or less on a consistent basis, there are factors other than IOP that likely contribute to disease development.34,35
The Cox model revealed a strong age effect, a significantly 19% higher incidence for women (P≤0.001), injuries of the eye and orbit (175%, P≤0.001), degeneration of iris and ciliary body (155%, P=0.022), myopia (155%, P≤0.001), retinal vascular occlusions (134%, P≤0.001), hypertension (13%, P≤0.001) and diabetes mellitus (23%, P≤0.001).35
However, this study of RWE from patients enrolled in US-managed care highlights that the majority of patients (70%) might not have received any kind of treatment for their POAG, despite having received a diagnosis. Furthermore, of patients who received antiglaucoma medication(s), adherence was suboptimal. Adherence was better among patients whose sole source of treatment was antiglaucoma medications compared with those who were on medications in addition to having undergone a procedure for POAG. Accordingly, persistence to antiglaucoma medications were less in patients who had undergone surgical procedures (ie, CH3) compared with patients on a medication-only regimen (ie, CH1), throughout the 1-year post-index period.
It would be possible that CH3 patients treated with antiglaucoma medication(s) and underwent procedure(s) might have several considerations. They might have stopped their medication(s) because they do not need the antiglaucoma medication(s) after their successful surgical procedure(s), or they might have stopped their medication(s) because of insufficient knowledge about the need to continue their medication(s) even after the surgical procedure(s). This suggests that it would be optimal if the information patients receive to be tailored to their needs to ensure that it is relevant.36
The exact reason for having cohort #4 (CH4) patients who did not receive any antiglaucoma medications or undergo any glaucoma-related procedures is unknown. Also, as treating ophthalmologists cannot base the decision to start treatment solely on IOP measurement as in (CH4), there might be good reasons for not treating those group as they might have a low risk of ever-developing visual impairment and with an acceptable IOP.37
In a prospective, cross-sectional survey, adults with glaucoma taking ≥1 glaucoma medication who received care in glaucoma clinics evaluated the frequency of 11 commonly cited barriers to optimal glaucoma medication adherence and identified barriers contributing to poor adherence.36
Majority of the subject population cited more than one barrier as important. The most prevalent barriers to adherence were forgetfulness, lack of self-efficacy, skepticism that glaucoma would lead to vision loss, skepticism that glaucoma medications would prevent vision loss and insufficient knowledge about glaucoma.36
The general lack of patient treatment and medication compliance for POAG is of major concern. As a result, every effort should be made to educate the POAG population of the importance of adhering and persisting with their therapy regimen, including after a surgical procedure.
This 6-year study of more than 200,000 eligible patients with POAG enabled an overview of glaucoma management in the US that included patients who had undergone common procedures to correct POAG and/or those who were prescribed commonly used antiglaucoma therapies. Of patients who underwent a surgical procedure, most underwent GATT rather than trabeculotomy. Surgery provides an alternative approach to antiglaucoma eye drops and may be a viable option to circumvent problems with medication adherence or incomplete response to topical therapy.9 However, more patients in our dataset were taking medication(s) in addition to having undergone surgical procedure(s) than those just having surgery alone. This is in line with observations by other researchers, which suggest the majority of patients still require treatment for IOP following a surgical procedure.38 As such, surgery may not be the answer to poor adherence with eye drops in this patient population.38
Despite availability of surgical and pharmacologic approaches to lower IOP, most patients with a diagnosis of POAG might not have received any therapy post-diagnosis (70%). Of those receiving some form of treatment, the largest proportion of patients were managed by medications alone. Clinical guidelines note that prostaglandin analogs are the most frequently prescribed first-line antiglaucoma eye drops for lowering IOP and are known to be safe and well-tolerated for once-daily application.9,22,39 The current study reflects these guidelines,9 with therapies, such as beta-blockers and alpha-adrenergic agonists, prescribed less often, and combination therapies prescribed to below 23% of the patients. If a single medication is effective for lowering IOP but the target pressure is not reached, combination therapy is recommended.9,40 The IOP can be lowered by medical treatment, laser therapy, or incisional surgery (either alone or in combination). Another study showed that patients who underwent trabeculectomy had a lower mean IOP on fewer medications.37
Using data from a nationally representative sample of Medicare beneficiaries, authors characterized longitudinal trends in laser and incisional glaucoma surgery utilization between 2008 and 2016. They learned that there continues to be a decline in trabeculectomy surgery coupled with a rise in GDI surgeries from 2008 to 2016.41
The total number of therapeutic glaucoma procedures performed overall increased 14.7% from 294,990 in 2008 to 338,230 in 2016. The total number of traditional incisional glaucoma surgeries decreased 11.7% from 37,225 in 2008 to 32,885 in 2016 (P=0.02). By contrast, the total number of MIGS procedures increased 426% from 13,705 in 2012 (the first year these procedures were captured in our dataset) to 58,345 in 2016 (P=0.001).41
The overall number of trabeculectomies performed on fee-for-service Medicare enrollees decreased from 25,610 in 2008 to 18,925 in 2016 (P=0.0001).41
The overall number of goniotomies performed increased 1911% from only 135 in 2008 to 2715 in 2016 (P=0.13). Most of this increase occurred from 2015 to 2016. Canaloplasty volume increased 797% from 180 in 2008 to 1615 in 2016 (P=0.13). The number of iStent trabecular micro-bypass (0191T, 0376T) procedures performed increased substantially from 350 in 2012 to 42,635 in 2016 (P=0.0003).41–43
The number of laser trabeculoplasties performed on Medicare enrollees remained stable from 156,185 in 2008 to 153,865 in 2016 (P=0.62). The number of iridotomies performed decreased slightly from 84,850 in 2008 to 80,720 in 2016 (P=0.09).41–43
Furthermore, with various IOP-lowering medications, lasers, and now MIGS as options, traditional incisional glaucoma surgeries are often performed on patients with more advanced disease, many of whom are under the care of a glaucoma subspecialist.41–43
Of the 71,252 patients who received treatment, 94% received medication(s), either alone or in addition to a surgical procedure.9 In the present study, adherence was poor (37–49%) and significantly below the 80% (ie, MPR >0.8) often used to signify acceptable adherence.44–46 However, the observed adherence levels are consistent with other studies of antiglaucoma medications,47–49 and highlight a complacency in adhering to eye drops. We did note that patients were less likely to remain persistent with antiglaucoma medications that were not prostaglandin analogs, despite all being topically applied, suggesting barriers to adherence are not solely due to the nature of eye drops.
A number of factors are known to influence adherence and persistence specifically with eye drops, such as complicated medication schedules (eg, once- vs twice- or more daily drops), side effects, and perceived benefit of treatment, alongside forgetfulness and difficulty with administration, all of which have been associated with poor adherence.50,51 Psychological variables have also been related to adherence, such as self-efficacy and overall motivation to improve the condition, intention or commitment to take the medication, and having the drops at hand when needed.52 Although we did not study specific reasons for nonadherence, patients who had undergone a procedure may feel the surgical procedure “solved” their POAG, and therefore do not feel the need to remain adherent with the postsurgical medication regimen. An association between medication nonadherence and glaucomatous vision loss has been confirmed elsewhere,53 highlighting the real-life consequences of nonadherence.
Available evidence suggests that a patient-centric approach to improve adherence is critically important to ensure patients are both motivated and empowered. Novel technology-based methods (eg, eHealth and mHealth) to promote and measure adherence and persistence are now available.54
Unmet Clinical Needs: How Can Management of POAG Be Improved?
This study highlights clear unmet clinical needs to improve glaucoma management, in order to lower risk of blindness14 and, associated healthcare costs and burden.55 We highlight that 70% of the patients might not have received treatment for their POAG, and furthermore, over half of all patients were nonadherent with their medications throughout the 1-year post-index period. At each examination, medication dosage and frequency of use should be recorded by the physician and behaviors regarding medication-taking be monitored and scheduling improved, when required. For example, administration of eye drops could be linked to a daily task to improve patient’s forgetfulness.9 Interventions that include a team-based approach to help co-ordinate care are often most successful.54
Comorbidities identified in this POAG patient population are in line with those reported elsewhere,10–13 and highlight the potential for targeted screening for glaucoma when patients are managed for common comorbidities such as hypertension or dyslipidemia, or with other common risk factors, such as thyroid disease. About 15% of the comorbidities included sleep disorders, which was 16.7% and 15.3% in CH1 and CH4 groups compared 13.0% in CH2 and CH3 groups (Supplementary Table S1). Indeed, POAG is an ideal disease that can be detected by targeted screening at outpatient appointments, given it is often asymptomatic until late stage,56 and early interventions can slow progression of visual field loss and prevent blindness.9
IOP was poorly represented in this database, and the natural language processing used to capture this information may become more refined. For example, we were not able to identify patterns in IOP and medication-taking behavior, and information on visual field loss was not available. Simple approaches, such as using standardized terms in EHR-keeping may improve capturing of IOP data using a more consistent methodology. There are many factors that influence adherence with a therapeutic regimen, including patient-related, physician-related, and socioeconomic-related factors,57 and an integrated program of measures will most likely be needed to address the problems highlighted.
Our study should be evaluated considering certain limitations. Our observations are naturally restricted by the population under study and known limitations around conducting analyses within managed care data.58 We did not include sleep apnea during a priori study designing, which could be a limitation. The 2022 ICD-10-CM diagnosis code for unspecified sleep apnea is G47.30, which is a billable/specific ICD-10-CM code that can be used to indicate diagnosis for reimbursement purposes.59
We are dependent on the accuracy of diagnostic codes to define our study sample and need to consider that diagnostic codes are collected for billing purposes. Also, patients may not have filled the prescriptions or taken the medications prescribed. Limited IOP data were available, and we were not able to draw any conclusions around the effectiveness of index therapy on IOP. Improving the documenting and reporting of IOP is a critical aspect for all physicians filling in EHRs, to allow conclusions to be drawn between medication effectiveness, adherence, and disease-specific HCRU.
The ICD-9/ICD-10 codes reflecting disease severity will allow for stratification of a patient population in a practice. For the unspecified open-angle glaucoma and primary open-angle glaucoma, the ICD-9 code by type of glaucoma shows that the staging codes for billing purpose are 365.10 and 365.11 respectively60 and the ICD-10 codes are H40.10 and H40.11, respectively.61 The ICD-9 staging definitions to determine the severity of the glaucoma show that the ICD-9 codes for mild, moderate and severe forms of glaucoma are 365.71, 365.72 and 365.73, respectively.60
A potentially modifiable risk factor, OSA, has been increasingly associated with glaucoma independent of intraocular pressure. OSA may alter blood flow to the optic nerve head and, in combination with other predisposing factors, lead to decreased ocular perfusion pressure.62
From prior studies, it is known that African Americans are predisposed to POAG,2,6,8,9 and there may be subgroups of African Americans who are more predisposed to disease progression than other subgroups.63 Although ethnicity data were available within the Market Clarity data, these were not captured in the present analysis and therefore we were not able to assess how ethnic origin may have impacted prevalence or outcomes assessed (adherence/persistence, HCRU) in the present study. Although this study only includes patients within Optum’s Market Clarity data, POAG and ocular hypertension are widespread problems, both within the US and worldwide, with elevated IOP as the basic etiology.1,2,6 Therefore, the results of this study should have implications for the treatment of POAG in other regions or healthcare settings with access to medical care.
Conclusions
Despite glaucoma being the second most common cause of blindness worldwide, this RWE study of US claims data highlights that 70% of the patients diagnosed with POAG might not have received antiglaucoma treatment. Pharmacologic management appears to be the major approach to POAG management, with prostaglandins used by more than half of all patients who received antiglaucoma medications. Adherence and persistence with antiglaucoma medication were, however, suboptimal especially in patients who had previously undergone a procedure for POAG or ocular hypertension. Targeted screening and educational approaches are thus needed to improve POAG management in the US.
Institutional Review Board (IRB)/Independent Ethics Committee (IEC)
The data used for this study did not involve the interaction or interview with any subjects and the data do not include any individually identifiable data (eg, did not include names, addresses, social security or medical record numbers or other obvious identifiers) and as such is not research involving human subject as defined at 45 CFR 46.102(f)(2). Furthermore, this study used existing fully de-identified data and the investigator(s) cannot be identified, directly or through identifiers linked to subjects and as such was exempt from 45 CFR 46.101(b)(4) from all 45 CFR part 46 requirements. Consequently, IRB approval was not required.
Ethical Conduct of the Study
The study was conducted in accordance with legal and regulatory requirements, as well as with scientific purpose, value and rigor and follow generally accepted research practices such as Good Pharmacoepidemiology Practices (GPP) issued by the International Society for Pharmacoepidemiology (ISPE), the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidance, Pharmaceutical Research and Manufacturers Association (PhRMA) guidelines and similar.
Subject Information and Consent
Informed consent was not applicable to this retrospective real-world database study.
Acknowledgment
Medical writing support was provided by Karen Burrows of Engage Scientific Solutions, a division of Envision Pharma Group (Horsham, UK), and was funded by Upjohn, a legacy division of Pfizer, now part of Viatris Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Upjohn, a legacy division of Pfizer, now part of Viatris Inc.
Disclosure
JSI was a full-time employee of Viatris Inc., at the time the study was conducted. KHZ, JZL, and TAH are full-time employees of Viatris Inc and hold stocks. The authors report no other conflicts of interest in this work.
References
1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi:10.1016/j.ophtha.2014.05.013
2. Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR. Global variations, and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100:86–93. doi:10.1136/bjophthalmol-2015-307223
3. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health. 2017;5:e1221–e1234. doi:10.1016/S2214-109X(17)30393-5
4. Allen D, Vasavada A. Cataract, and surgery for cataract. BMJ. 2006;333:128–132. doi:10.1136/bmj.333.7559.128
5. Haripriya A, Chang DF, Reena M, Shekhar M. Complication rates of phacoemulsification and manual small-incision cataract surgery at Aravind Eye Hospital. J Cataract Refract Surg. 2012;38:1360–1369. doi:10.1016/j.jcrs.2012.04.025
6. Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538.
7. National Eye Institute. Glaucoma data and statistics (open-angle glaucoma); 2019. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/glaucoma-data-and-statistics.
8. Doshi V, Ying-Lai M, Azen SP, Varma R; Los Angeles Latino Eye Study Group. Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension. The Los Angeles Latino Eye Study. Ophthalmology. 2008;115:639–647 e2. doi:10.1016/j.ophtha.2007.05.032
9. Prum BE, Lim MC, Gedde SJ. Primary open-angle glaucoma Preferred Practice Pattern® guidelines. American Academy of Ophthalmology® (AAO). Ophthalmology. 2016;123:P41–P111. doi:10.1016/j.ophtha.2015.10.053
10. Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122:72–78. doi:10.1016/j.ophtha.2014.07.051
11. Wang S, Bao X. Hyperlipidemia, blood lipid level, and the risk of glaucoma: a meta-analysis. Invest Ophthalmol Vis Sci. 2019;60:1028–1043. doi:10.1167/iovs.18-25845
12. Chung HJ, Hwang HB, Lee NY. The association between primary open-angle glaucoma and blood pressure: two aspects of hypertension and hypotension. Biomed Res Int. 2015;2015:827516. doi:10.1155/2015/827516
13. Jung Y, Han K, Park HYL, Lee SH, Park CK. Metabolic health, obesity, and the risk of developing open-angle glaucoma: metabolically healthy obese patients versus metabolically unhealthy but normal weight patients. Diabetes Metab J. 2020;44:414–425. doi:10.4093/dmj.2019.0048
14. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–2193. doi:10.1016/S0140-6736(17)31469-1
15. Rossetti L, Digiuni M, Montesano G, et al. Blindness and glaucoma: a multicenter data review from 7 academic eye clinics. PLoS One. 2015;10:e0136632. doi:10.1371/journal.pone.0136632
16. Sheybani A, Scott R, Samuelson TW, et al. Open-angle glaucoma: burden of illness, current therapies, and the management of nocturnal IOP variation. Ophthalmol Ther. 2020;9:1–14. doi:10.1007/s40123-019-00222-z
17. Whitson JT. Glaucoma: a review of adjunctive therapy and new management strategies. Expert Opin Pharmacother. 2007;8:3237–3249. doi:10.1517/14656566.8.18.3237
18. Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411–434. doi:10.2165/00003495-200059030-00003
19. Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118:1766–1773. doi:10.1016/j.ophtha.2011.01.047
20. Cheung CY, Li SL, Chan PP, et al. Intraocular pressure control and visual field changes in primary angle closure disease: the CUHK PACG Longitudinal (CUPAL) study. Br J Ophthalmol. 2020;104:629–635. doi:10.1136/bjophthalmol-2019-314322
21. Miglior S, Pfeiffer N, Torri V, Zeyen T, Cunha-Vaz J, Adamsons I. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9.
22. Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. doi:10.1016/S0140-6736(14)62111-5
23. Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105:209–215. doi:10.1016/S0161-6420(98)92665-3
24. Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi:10.1016/j.ophtha.2004.01.025
25. Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: proyecto VER. Arch Ophthalmol. 2001;119:1819–1826. doi:10.1001/archopht.119.12.1819
26. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. doi:10.1001/jama.1991.03470030069026
27. Stein JD, Ayyagari P, Sloan FA, Lee PP. Rates of glaucoma medication utilization among persons with primary open-angle glaucoma, 1992 to 2002. Ophthalmology. 2008;115:1315–9, 1319.e1. doi:10.1016/j.ophtha.2007.12.017
28. Bourne RR, Taylor HR, Flaxman SR, et al. Number of people blind or visually impaired by glaucoma worldwide and in world regions 1990–2010: a meta-analysis. PLoS One. 2016;11:e0162229. doi:10.1371/journal.pone.0162229
29. Mcmonnies CW. Glaucoma history and risk factors. J Optom. 2017;10:71–78. doi:10.1016/j.optom.2016.02.003
30. Optum Inc. Optum market clarity database (2007–2019). Available from: https://www.optum.com/business/solutions/life-sciences/explore-data/advanced-analytics/market-clarity-data.html.
31. Office for Civil Rights (OCR). Guidance regarding methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) privacy rule Available from: https://www.hhs.gov/sites/default/files/ocr/privacy/hipaa/understanding/coveredentities/De-identification/hhs_deid_guidance.pdf.
32. Atik A, Barton K, Azuara-Blanco A, Kerr NM. Health economic evaluation in ophthalmology. Br J Ophthalmol. 2020;105:602–607. doi:10.1136/bjophthalmol-2020-316880
33. Park I, Gale J, Skalicky SE. Health economic analysis in glaucoma. J Glaucoma. 2020;29:304–311. doi:10.1097/IJG.0000000000001462
34. Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141(1):24–30. doi:10.1016/j.ajo.2005.07.044
35. Kreft D, Doblhammer G, Guthoff RF, Frech S. Prevalence, incidence, and risk factors of primary open-angle glaucoma - A cohort study based on longitudinal data from a German public health insurance. BMC Public Health. 2019;19(1):851. doi:10.1186/s12889-019-6935-6
36. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi:10.1016/j.ophtha.2015.03.026
37. Baker ND, Barnebey HS, Moster MR, et al.; INN005 Study Group. Ab-Externo MicroShunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021;128(12):1710–1721. doi:10.1016/j.ophtha.2021.05.023
38. Schultz NM, Wong WB, Coleman AL, Malone DC. Predictors, resource utilization, and short-term costs of laser trabeculoplasty versus medication management in open-angle glaucoma. Am J Ophthalmol. 2016;168:78–85. doi:10.1016/j.ajo.2016.05.001
39. Van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112:1177–1185. doi:10.1016/j.ophtha.2005.01.042
40. Khouri AS, Realini T, Fechtner RD. Use of fixed-dose combination drugs for the treatment of glaucoma. Drugs Aging. 2007;24:1007–1016. doi:10.2165/00002512-200724120-00004
41. Rathi S, Andrews CA, Greenfield DS, Stein JD. Trends in glaucoma surgeries performed by glaucoma subspecialists versus nonsubspecialists on Medicare beneficiaries from 2008 through 2016. Ophthalmology. 2021;128(1):30–38. doi:10.1016/j.ophtha.2020.06.051
42. Radhakrishnan S, Chen PP, Junk AK, Nouri-Mahdavi K, Chen TC. Laser peripheral iridotomy in primary angle closure: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(7):1110–1120. doi:10.1016/j.ophtha.2018.01.015
43. Töteberg-Harms M, Meier-Gibbons F. Is laser trabeculoplasty the new star in glaucoma treatment? Curr Opin Ophthalmol. 2021;32(2):141–147. doi:10.1097/ICU.0000000000000732
44. Baumgartner PC, Haynes RB, Hersberger KE, Arnet I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front Pharmacol. 2018;9:1290. doi:10.3389/fphar.2018.01290
45. Haynes RB, Taylor DW, Sackett DL, Gibson ES, Bernholz CD, Mukherjee J. Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2:757–764. doi:10.1161/01.HYP.2.6.757
46. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi:10.1111/j.1524-4733.2007.00213.x
47. Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence, and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi:10.1016/j.ajo.2005.04.051
48. Wilensky J, Fiscella RG, Carlson AM, Morris LS, Walt J. Measurement of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims data. Am J Ophthalmol. 2006;141:S28–33. doi:10.1016/j.ajo.2005.09.011
49. Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once daily and adjunctive medication use. Am J Ophthalmol. 2007;144:533–540. doi:10.1016/j.ajo.2007.06.012
50. Sayner R, Carpenter DM, Robin AL, et al. How glaucoma patient characteristics, self-efficacy and patient-provider communication are associated with eye drop technique. Int J Pharm Pract. 2016;24:78–85. doi:10.1111/ijpp.12215
51. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316.
52. Cook PF, Schmiege SJ, Mansberger SL, Kammer J, Fitzgerald T, Kahook MY. Predictors of adherence to glaucoma treatment in a multisite study. Ann Behav Med. 2015;49:29–39. doi:10.1007/s12160-014-9641-8
53. Newman-Casey PA, Niziol LM, Gillespie BW, Janz NK, Lichter PR, Musch DC. The association between medication adherence and visual field progression in the collaborative initial glaucoma treatment study. Ophthalmology. 2020;127:477–483. doi:10.1016/j.ophtha.2019.10.022
54. Hassan TA, Sáenz JE, Ducinskiene D, Cook JP, Imperato JS, Zou KH. New strategies to improve patient adherence to medications for noncommunicable diseases during and after the COVID-19 era identified via a literature review. J Multidiscip Healthc. 2021;14:2453–2465. doi:10.2147/JMDH.S313626
55. Rees G, Leong O, Crowston JG, Lamoureux EL. Intentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucoma. Ophthalmology. 2010;117:903–908. doi:10.1016/j.ophtha.2009.10.038
56. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi:10.1001/jama.2014.3192
57. World Health Organisation (WHO). Adherence to long-term therapies. Evidence for action Available from: https://www.who.int/chp/knowledge/publications/adherence_report/en/.
58. Fishman PA, Wagner EH. Managed care data and public health: the experience of Group Health Cooperative of Puget Sound. Ann Rev Public Health. 1998;19:477–491. doi:10.1146/annurev.publhealth.19.1.477
59. ICD10Data.com. ICD-10-CM diagnosis code. Glaucoma data and statistics (open-angle glaucoma). 2022 ICD-10-CM Diagnosis Code G47.30: Sleep apnea, unspecified. Available from: icd10data.com.
60. Fellman RL, Mattox CG, Ross KM, Vicchrilli S. Know the new glaucoma staging codes. EyeNet Magazine; 2011. American Academy of Ophthalmology. Know the New Glaucoma Staging Codes - American Academy of Ophthalmology. Available from: aao.org.
61. American Academy of Ophthalmology. ICD-10 glaucoma staging definitions; 2015. ICD-10 Glaucoma Staging Definitions - American Academy of Ophthalmology. Available from: aao.org.
62. Faridi O, Park SC, Liebmann JM, Ritch R. Glaucoma and obstructive sleep apnoea syndrome. Clin Exp Ophthalmol. 2012;40(4):408–419. doi:10.1111/j.1442-9071.2012.02768.x
63. Pleet A, Sulewski M, Salowe RJ, et al. Risk factors associated with progression to blindness from primary open-angle glaucoma in an African American population. Ophthalmic Epidemiol. 2016;23:248–256. doi:10.1080/09286586.2016.1193207
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.