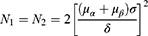Back to Journals » Risk Management and Healthcare Policy » Volume 16
Clinical Pathway for Enhanced Recovery in the Management of Non-Variceal Upper Gastrointestinal Bleeding: A Randomized Controlled Trial
Authors Zhang YY , Zhang QX, Li JT, Wang Y, Zhuang ZH, Zhuang JY
Received 11 September 2023
Accepted for publication 2 November 2023
Published 24 November 2023 Volume 2023:16 Pages 2579—2591
DOI https://doi.org/10.2147/RMHP.S433068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gulsum Kubra Kaya
Yan-Yan Zhang,1,2,* Qiao-Xian Zhang,3,* Jun-Ting Li,1 Yan Wang,1 Ze-Hao Zhuang,4,5 Jia-Yuan Zhuang1
1School of Nursing, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 2School of Nursing, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China; 3Department of Nursing, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 4Endoscopy Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 5Department of Gastroenterology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ze-Hao Zhuang, Endoscopy Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China, Tel +8613178032412, Email [email protected] Jia-Yuan Zhuang, School of Nursing, Fujian Medical University, No. 1 Xueyuan Road, Fuzhou, Fujian, People’s Republic of China, Tel +8613860669060, Email [email protected]
Purpose: To explore the effects of the clinical pathway on the outcomes of patients with non-variceal upper gastrointestinal bleeding.
Materials and Methods: Randomized controlled trial. The study was conducted in two medical centers in China from 1 June 2022 to 31 December 2022. Patients with a diagnosis of non-variceal upper gastrointestinal bleeding who provided written informed consent were consecutively assigned to the intervention group. The patients in the intervention group were treated using the clinical pathway, while the control group received routine care and follow-up. Time, cost, complications, and prognostic indicators were analyzed. Intentional-to-treat analysis and per-protocol analysis were used for data analysis.
Results: A total of 114 eligible patients with non-variceal upper gastrointestinal bleeding were randomly divided into two groups and included in the intention-to-treat analysis. In addition, 106 patients were included in the per-protocol analysis. The median age of the 106 patients was 57 years (range, 18– 92 years) and 83.0% were male. There were no significant differences between groups regarding the baseline characteristics. The intervention group demonstrated a statistically significantly shorter length of stay, lower hospital cost (ie, cost during hospitalization, cost in the emergency room, and cost in the ward), significantly fewer cases of complications, and a higher level of patient satisfaction when compared with the control group. There was no significant difference between the two groups in the rates of transfusion, repeat endoscopy, rebleeding readmission, and mortality.
Conclusion: The implementation of the clinical pathway for patients with non-variceal upper gastrointestinal bleeding may help improve patient outcomes and satisfaction.
Trial Registration Number: ChiCTR2200060316.
Registration Link: https://www.chictr.org.cn/.
Keywords: clinical pathway, medical-nursing integration model, non-variceal upper gastrointestinal bleeding, patient, outcomes
Introduction
Non-variceal upper gastrointestinal bleeding (NVUGIB) is one of the most common acute and critical diseases, with an incidence of 19.4–57.0 per 100,000 individuals.1 Although the incidence of NVUGIB appears to decrease with improvements in modern medical techniques, the mortality rate related to upper gastrointestinal bleeding (UGIB) in China is estimated to be 4–14%.2 The medical resources are limited owing to the large population of China, and the management of NVUGIB is complex and challenging; thus, physicians and hospitals face the increasingly difficult challenge of reducing the length of stay (LOS) and healthcare costs while maintaining quality of care.
The management of NVUGIB has evolved over the past decade with the development of endoscopic treatment and proton-pump inhibitor (PPI) acid-suppressive therapy.3 Evidence-based guidelines have highlighted that most patients with NVUGIB should receive PPI therapy immediately after admission and endoscopy within 24 h.4,5 Previous studies suggested that low-risk patients with UGIB can be safely managed in an ambulatory setting or discharged early.6,7 Ideally, physicians should identify patients who are less likely to require intensive care and select them as outpatients after initial hemostasis; targeted nursing should be carried out according to the patient’s condition, reducing unnecessary treatment and allowing for earlier discharge, thereby resulting in substantial resource savings. However, evidence-based guidelines and previous findings that we recognize as appropriate are not sufficiently applied in clinical routines, the management of NVUGIB mainly focuses on condition monitoring and therapeutic care. Thus, the potential benefit of guidelines or practice norms may be lost, which might affect the treatment effect and management quality.8,9 A previous study suggested that physicians tend to be conservative in the management of low-risk UGIB, resulting in higher levels of care and longer hospital LOS.10 Consequently, the LOS of patients is likely to be significantly prolonged. Additionally, the traditional nursing mode has no obvious job duty division, which leads to a situation in which the nursing staff has no sense of working predictably and proactively, and nursing quality is lowered. Therefore, prolonged LOS, limited medical resources, and a lack of guidelines or practice norms related to nursing partially support the need for evidence-based enhanced recovery protocols.
The clinical pathway (CP) has been promoted for diseases that are frequent causes of hospitalization, expensive to treat, and have a high variation in approaches to diagnosis and treatment.11 It provides a way to promote cooperation and coordination between doctors and nurses, providing best practice guidance on the management of patients with particular diagnoses, such as geriatric trauma,12 macular degeneration,13 and thyroid nodular disease.14 A retrospective cohort study showed that the implementation of CP in patients who underwent total knee arthroplasty, with a focus on early rehabilitation, contributed to a reduction in the LOS and costs during hospitalization, with no increase in the readmission rate.15 A CP has been found to effectively reduce costs while maintaining the quality of care in the management of patients with UGIB.16,17 Nevertheless, there has been very little evidence to date examining the effectiveness of CPs with a focus on early rehabilitation for the management of NVUGIB in Eastern Asian populations in the most recent 10 years. Furthermore, the implementation of CPs requires medical and nursing integration. Involving multiple disciplines is beneficial for promoting cooperation between doctors and nurses, heightening the quality of nursing, and achieving the requirements and goals of effectively promoting high-quality treatment and care.18 Therefore, at the current treatment level, it is necessary to incorporate CPs into clinical practice using the input of a multidisciplinary team.
This study aimed to construct a CP for enhanced recovery under the medical–nursing integration model, reducing the hospital LOS and costs while maintaining the quality of care. We then compared the effectiveness of CP-directed management with that of routine practice in improving the outcomes of patients with NVUGIB. We present the following article in accordance with the CONSORT reporting checklist (see Appendix 1).
Materials and Methods
This study adopted a prospective, multicenter, randomized controlled design and was conducted at two medical centers in South-East China. The two centers are urban tertiary-care medical institutions for emergency endoscopy for NVUGIB in the Fujian province and receive a high number of referral emergency cases, with 2000 beds and 1500 beds respectively.
The study enrolled eligible patients according to the following inclusion criteria: (1) patients aged ≧18 years; (2) patients with overt signs of UGIB (hematemesis, melena, or both) at admission; (3) patients who met the diagnostic criteria for NVUGIB, as defined in the European Society of Gastrointestinal Endoscopy (ESGE) Guideline (2021);5 (4) patients with basic Chinese reading and writing skills, without mental disorders; (5) and patients (or their relatives on their behalf) who agreed to provide written informed consent to participate in this study. The exclusion criteria were as follows: (1) patients who were discharged from the emergency room (ER) automatically; (2) patients whose condition did not stabilize after initial resuscitation. Considering the study’s hypotheses and outcomes, the formula for calculating the two sample sizes was as follows:
Notes: N1 and N2 are the sample contents required by the two groups; δ represents the difference between the mean of two populations; σ represents the standard deviation; α = 0.05 (two-tailed test), μα = 1.96 and μβ = 1.28.
The sample size was based on the data derived from a Phase II pilot study. The standard deviation of the LOS was 9.39 days, and the difference between the means of the two groups was 4.09 days. We determined that 16 patients were required for each group. After allowing for a 20% loss to follow-up, ultimately, 20 patients were required for each group.
The study comprised four phases: (1) development and dissemination of the CP for NVUGIB, (2) pilot testing of the CP and study protocol, (3) examination of the effectiveness of the CP, and (4) continuous follow-up.
Phase I of the study (ie, the development and dissemination of the CP for patients with NVUGIB) began with a review of the relevant literature. A comprehensive search of PubMed, Web of Science, Embase, Cochrane Library, CINAHL, WANGFANG (Chinese), CNKI (Chinese), VIP (Chinese), and CBM (Chinese) from inception to December 2021 was undertaken to identify articles that concerned evidence-based assessment, diagnosis, and management of patients with NVUGIB (using PubMed as an example, the detailed search strategy is shown in Appendix 2, Supplementary Table 1). All the search results were exported to EndNote version 20 (Thomson Reuters, Toronto, Canada). The relevant literature was reviewed to develop a CP for NVUGIB.
Following the literature review, a retrospective study was conducted between January 2021 and December 2021, and consecutive patients with a first diagnosis of NVUGIB were included. The clinical data of these patients were analyzed to determine their LOS, cost of hospitalization, and treatment and nursing measures. The results of the literature review have been supplemented and adjusted. From January 2022 to April 2022, a multidisciplinary committee of ten experts chaired by one of the authors, including representatives from the ER, endoscopy unit, and gastroenterology department, held two meetings to reach a consensus on the CP. The work responsibilities of the medical staff in each department were also clarified. The CP is presented and reviewed. Special attention was paid to the “hand-offs” approach and to overcoming the major barriers to implementation.
The CP is an 23-page, double-sided, A4 paper-based document that comprised two parts: 1) the hospitalization procedure, and 2) a checklist containing three parts of treatment and care from admission to discharge. Residents and nurses filled in the blanks and marked the boxes on the checklists. The checklists were then given back to the chief residents when the enrolled patients were discharged (the CPs are shown in Appendix 3).
Dissemination of the CP and education of all necessary personnel in each department was continued for the duration of the study through 1) a weekly multidisciplinary committee during the first 4 weeks to make adjustments as necessary; 2) a monthly ward staff meeting to assess feedback regarding recommendations for improving the CP; 3) an orientation meeting at the beginning of every month when interns/residents/chief residents changed; and 4) printed sheets documenting the CP, which were clipped to the outside front cover of the in-patient chart as a formal part of the medical records, to avoid omissions caused by different levels and abilities of personnel.
Phase II of the study (ie, pilot testing of the CP) was conducted between 20 May 2022 and 20 June 2022. The purpose of phase II was to determine the feasibility of the study protocol, CP, and data collection instruments. The pilot study was similar to the main study in terms of the study setting, participant selection criteria, intervention duration, and outcome measures. Four avenues were identified and targeted for intervention in CP: 1) early endoscopy for early diagnosis in the ER (within 24 h); 2) reduction in LOS in the ER (within 24 h); 3) early feeding after endoscopy (within 24 h); and 4) early discharge (within 5–7 days).
Phase III of the study will be conducted between 1 July 2022 and 31 December 2022, recruiting all consecutive patients with NVUGIB from the two centers. Participants were numbered individually. A research nurse, independent of the trial, randomly assigned the patients in a 1:1 ratio using the website software program Research Randomizer (http://www.random.org). Treatment assignments were concealed in numbered, sealed envelopes. The researchers of the investigation team obtained written informed consent from eligible patients, and the research nurse opened the sealed envelope with the assigned intervention. Due to the nature of the intervention, patients, clinicians, and the study team could not be blinded to the treatment allocation.
Both groups underwent endoscopy and were closely monitored. In the UC group, patients with NVUGIB underwent routine practice at our institution. The patients in the CP group were treated using CP-directed management, which started in the ER and ended after the patients were discharged, requiring nursing, medical, and ancillary staff to observe and manage the patient in the ER, endoscopy unit, and gastroenterology department. Patients with no need for endoscopic hemostasis or who underwent successful endoscopic treatment for high-risk stigmata (Forrest I and IIa) were classified as low-risk. For low-risk patients, oral feeding (a liquid diet that did not require chewing) was initiated within 24 h of endoscopy. A soft diet (eg, rice gruel) was administered for 4–5 days, followed by a regular diet thereafter.
The CP involved education about the preparations before, during, and after endoscopy, providing patients and their relatives with the necessary knowledge about the disease or procedure as well as psychological care. For improving physicians’ and nurses’ adherence to the CP, the directors of the ER and ward, nurse managers of the ER and ward, and staff established weekly case review meetings to discuss and monitor the progress of the implementation. Any variances to the pathway or complications were reported.
Phase IV continuous follow-up: In this study, a person is identified as responsible for continuous follow-up (nurse manager or charge nurse in the ward). We shared the result from the first evaluation for future continued monitoring and evaluation by the institution during the 30-day follow-up period.
Data Collection
The following data of the enrolled patients were blinded and collected by two research assistants who used a pre-designed data collection form: demographic data (sex and age), medical insurance, education level, occupation, marital status, original residence, smoking and alcohol history, medication on admission, history of UGIB, time from the onset of symptoms to the admission to the hospitals, presenting symptoms, vital signs (blood pressure and pulse), laboratory findings, estimated blood loss, mental status, skin condition, endoscopic diagnosis, and treatment. The updated Charlson Comorbidity Index (updated CCI) was used to evaluate the health status of patients, which has been validated in patients with UGIB (Supplementary Table 2). As the prognosis might be worse if the updated CCI was 3 points or more, we divided patients into two groups (score 0–2 and score 3 or more).
The adverse outcomes (complications during hospitalization, repeat endoscopy, 30-day rebleeding, unplanned readmission, and mortality) and transfusion of the patients were recorded. All data were entered into Excel and checked to ensure the accuracy of the data collection.
Statistical Analysis
Categorical variables were described using numbers and percentages. Chi-square (χ²) tests were used to measure associations between categorical variables. The rate difference and 95% confidence interval (CI) were calculated. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test and Q-Q Plots. Continuous variables were described using means and standard deviations and then compared between the groups with a Student’s t-test and a Mann–Whitney U-test for parametric and nonparametric data, respectively. The median difference and 95% CI were also calculated. Differences in demographic characteristics between groups were reported at baseline.
Two analysis sets were defined in this study: the intention-to-treat (ITT) and per-protocol (PP) sets. The ITT population included all patients who met the inclusion criteria and were randomized. Patients who did not follow the protocol were excluded from the PP analysis. A PP analysis is one with no adjustment for confounding, will be valid only if adherence occurred completely at random.19 We performed analyses both in the ITT and PP populations. Data were analyzed using SPSS Statistics for Mac (version 28.0; IBM Corp., Armonk, NY, USA) and the level of significance was set at a P value of < 0.05 for all analyses.
Results
Demographic Characteristics of the Sample
A total of 180 patients with NVUGIB were enrolled consecutively, of whom 114 were randomized (56 patients were included in the CP group, and 58 patients were included in the UC group); 66 patients were excluded based on the exclusion criteria. A further eight patients were excluded from our analysis (Figure 1). Follow-up was completed for 106 patients, and these data were included in the PP analysis. The median age of the 106 patients was 57 years (range, 18–92 years) and 83.0% were male. The proportion of patients with peptic ulcer at the time of admission was 82.1%, and 44.3% of patients received endoscopic hemostasis; 20.4% (11/54) of patients were transferred from other hospitals in the CP group, and 34.6% (18/52) of patients referred in the UC group. All patients in the present study received endoscopy. We compared the difference in demographic characteristics between those eight patients who were excluded from our analysis (Supplementary Table 3).
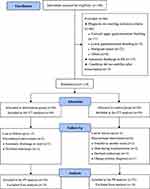 |
Figure 1 Patient flow chart. Abbreviations: ITT, intention-to-treat; PP, per-protocol. |
The baseline demographic and clinical characteristics of the study population are summarized in Tables 1 and 2. The study sample overall was mostly male and married. In the UC group, the median age was 59 years (range, 18–92 years); 19.6% (11/56) of patients were transferred from other hospitals in the CP group, and 32.8% (19/58) of patients were referred. Melena and hematemesis were the most common symptoms at presentation. Endoscopic hemostasis was most commonly performed using electrocoagulation or argon plasma (n = 36).
 |
Table 1 Baseline Characteristics of Patients |
 |
Table 2 Clinical Characteristics Associated with Bleeding |
Outcome Measures
As shown in Table 3, the time from presentation to endoscopy, the time from endoscopy to feeding, and the LOS in the CP group were significantly shorter when compared with the corresponding times in the UC group (all P < 0.05). The cost during hospitalization also demonstrated reductions in the CP group: total cost in the ER (CP 4137.5 vs UC 5324.7 yuan) (P = 0.001), total cost in the ward (CP 4678.9 vs UC 8254.8 yuan) (P < 0.001), and total cost during hospitalization (CP 9323.3 vs UC 13657.5 yuan) (P < 0.001) (Table 3). The results of the PP analysis were in line with the ITT analysis (Supplementary Table 4). As displayed in Table 4, patient satisfaction in the CP group after initiation of the CP was higher than that it was in the UC group (P < 0.05).
 |
Table 3 Length of Stay and Cost Variables Measured After Initiation of the Clinical Pathway |
 |
Table 4 Patient Satisfaction with Care Process and Outcome |
Transfusion and adverse events measured after initiation of the CP were not significantly different between the two groups, except for the rate of complications (Table 5). No patients in the CP group had complications, and eight patients in the UC group had complications. The complications of patients in the CP group are shown in Supplementary Table 5. The variances of CP are listed in Table 6 (for the intervention group only). The most common reason for the variations to the CP was action not required.
 |
Table 5 Transfusion and Adverse Events Measured After Initiation of the Clinical Pathway |
 |
Table 6 Occurrence of Variances to the Clinical Pathway in the Intervention Group (n = 56) |
Discussion
The drive to implement a CP was born out of the necessity of ensuring patient safety and finding avenues for the conservation of scarce healthcare resources.20 To the best of our knowledge, this is the first report of a CP for enhanced recovery in the management of NVUGIB, which encourages medical staff to deliver evidence-based practice.
Many clinical studies on NVUGIB have used hospital LOS as a healthcare performance indicator.21,22 The hospital LOS for patients with NVUGIB varied greatly among institutions, ranging from 3.8 to 8.7 days.17,21,23 The mean hospital LOS for NVUGIB patients is 8 days in China,24 which is higher than that in Korea (LOS: 6 days)25 and the USA (LOS: 4.5 days).26 Hay’s study showed that LOS could be lowered by having dedicated personnel to monitor patients with UGIB on a daily basis; however, this intervention is cumbersome and costly.27 In addition, Hay’s study excluded patients who did not become low-risk by the endoscopic criteria, and even then, the LOS was only reduced to 2.9 days.27 Our study included almost all patients with a first diagnosis of NVUGIB in the ER. The LOS during hospitalization was lower in the CP group than in the UC group (CP: 5.1 days vs UC: 7.8 days). The LOS in the CP group was also lower than our previous crude LOS estimates for patients with UGIB at our institutions (8–13 days), indicating that the CP was able to ensure timely treatment of patients with NVUGIB, which prompted this study. These findings were consistent with those of other enhanced recovery protocols.28,29 Overall, however, compared to one study in the USA over 20 years ago, when the LOS for NVUGIB was 3.5 days,30 the LOS for our CP group was 5.1 days. The difference between the non-Chinese study and ours, apart from demographic differences, may reflect differences in healthcare systems.
In patients with NVUGIB, deterioration occurs rapidly after disease onset. Therefore, patients should be treated as soon as possible to improve their clinical efficacy and prognosis. Nevertheless, there is no day ward or bleeding care unit for the management of UGIB in China; therefore, the problem of delaying treatment in the ER is common. To address this serious condition, we sought to avoid delaying beyond 24 h in the ER through fast medical procedures and clarifying team roles, which resulted in the LOS in the ER of patients in the CP group being decreased (CP: 7.4 days vs UC: 20.7 hours) (P = 0.001). In addition, the CP shortened the time from presentation to endoscopy. Following hemodynamic resuscitation, early endoscopy (≤24 h) performed after admission has been suggested by several guidelines.5,31 At present, no randomized controlled trial has assessed endoscopy within 24 h versus > 24 h. However, early endoscopy is a routine procedure for our CP. Therefore, the time from presentation to endoscopy was shorter in the CP group than in the UC group (CP: 8 h vs UC: 17 h). The achievement of a single outcome can have a significant impact on clinical practice. We achieved success in reducing the LOS in the ER and the time from presentation to endoscopy, which resulted in an improvement in the LOS and secondarily resulted in more cost-efficient care. Furthermore, complications during hospitalization are the main reasons for delayed discharge and prolonged hospital stay in patients.32 An important issue for all enhanced recovery protocols or CPs is the increased rate of complications and hospital readmission. In our study, the CP used in patients was safe and did not cause complications. There were no complications in the CP group during the study, suggesting that the CP in our study may be applicable in clinical practice.
Treatment cost places a serious financial burden on patients. According to one study, the annual median total hospitalization charges for NVUGIB increased from $9249 in 1989 to $20,370 in 2009 in the United States.33 Our results indicated that patients in the UC group incurred higher hospitalization costs than those in the CP group, with a longer LOS and higher rate of complications. As hospitalization costs are mostly dependent on LOS and procedures, the possible lower costs may be because our CP for enhanced recovery focused on optimizing treatment and care measures for patients from consultation to discharge, aimed at reducing the LOS and costs by bridging the CP in the ER and the CP in the ward. The complication rate was lower in the CP group than in the UC group (P < 0.05). Thus, the need for intensive and expensive care may decrease.
According to our CP plan, oral feeding was initiated within 24 h of endoscopy. The recommendations were difficult for physicians and nurses to follow; however, the time from endoscopy to feeding in the CP group (21.1 h) was shorter than that in the UC group (43.1 h) (P < 0.05). This underlines the importance of CP dissemination and education. Moreover, the results indicated that patients receiving early feeding were discharged earlier with no adverse events, which is consistent with the results of another study.34 Early feeding can improve nutrition and mobilization levels in patients with NVUGIB. These, in turn, can factor in early discharge since the criteria for discharge include the ability to eat and demonstrate bowel function. In our study, with active cooperation and good communication between doctors and nurses, most patients were safely fed earlier than usual. We also found that we were unable to achieve a reduction in the transfusion rate, but there was a trend toward a reduction (from 19.2% to 14.8%). Restrictive packed red blood cell transfusion practices are recommended in major guidelines.5,31 The trend toward a reduction might be due to its introduction at our institution during the study period. Consequently, our study reduced unnecessary treatment and allowed for the earlier discharge of patients, thereby resulting in substantial resource savings.
Satisfaction is a key component of healthcare quality assurance.35 In our study, there was a statistically significant difference in patient satisfaction between the two groups. Several factors may have contributed to the differences in this outcome: the active involvement of physicians and nursing experts in developing the CP, the involvement of all relevant healthcare providers in implementing the CP, clarification of the team roles, local development of the CP, a comprehensive approach to improve patient safety during medical procedures, and effective nurse telephone follow-up. In our study, time and economic costs were effectively reduced by achieving four targets (early endoscopy, reduction in LOS in the ER, early feeding, and early discharge) in the CP group. Physicians are primarily responsible for hospitalized patients with NVUGIB; nurses are involved in the management of patients with NVUGIB, and effective nursing interventions play an important role in improving the curative effect. In our study, a better doctor–nurse–patient relationship was established through the implementation of CP from admission to discharge. The primary goal of CP is early discharge,29 and early discharge of patients has been shown to be safe. Following the introduction of the CP, the rate of repeat endoscopy decreased from 15.4% to 9.3%, the rate of rebleeding decreased from 38.5% to 27.8%, and the rate of readmission decreased from 11.5% to 5.6%, although these were not statistically significant (P > 0.05); however, the patients in the CP group were lower than those in the UC group, indicating that early discharge of patients following closer monitoring under the guidance of the CP did not lead to an increase in adverse events and ensured the safety of patients. Taken together, these results contribute to improvements in patient satisfaction.
CPs aim to reduce inconsistencies in clinical practice and facilitate evidence-based practice. There are diverse reasons for these variations in clinical practice. Understanding the factors that contribute to variations in a CP is the focus of correlational studies and can be used as a reference for further improvements in a CP. In our study, the most common reason for variations in the CP was that action was not required, which is consistent with the results of another study.20 These variations did not require special processing. Furthermore, four patients did not transfer to the ward within 24 h on weekends or holidays because of inadequate bed space. Another study showed that patients with UGIB admitted on holidays had a lower rate of early endoscopy, longer time to endoscopy, and higher admission costs than those admitted on non-holidays.36 Therefore, the findings of our study suggest that if sufficient resources and services are available, patients should be transferred to a specialized ward faster, and medical staff are more likely to be able to adopt best practices. Moreover, two patients did not start oral feeding within 24 h, suggesting that the clinical judgment of the physician and the patient’s own situation should be combined to comprehensively assess whether an open diet within 24 h is beneficial to the patient. One patient requested to be discharged from the hospital. One patient did not undergo endoscopy. For these patients, along with providing safe and quality services through the CP, medical staff need to adopt serious measures to ensure patient/family health education and enhance their disease-related and endoscopy-related knowledge to facilitate patients’ adherence to the CP.
This study has some limitations. First, without participant/family and clinician blinding, we cannot exclude the possibility that expectation bias may have affected the results. Given that the two study groups were similar across all clinical and demographic variables, the likelihood of selection bias was very small. Second, the two centers are both the main regional facilities for emergency endoscopic treatment of NVUGIB and receive a high number of referral emergency cases; therefore, they contain a deficient sample representation. In addition, the sample sizes were small, and expanding the sample size and sources is necessary. Third, a computerized clinical pathway might be an easier way to provide timely information and better monitoring; however, it cannot directly communicate with or convince physicians to change their orders. Furthermore, the two centers offer around-The-clock endoscopy services to fellow and senior endoscopists. Our results cannot be generalized to hospitals that do not have such support.
Conclusion
CP for enhanced recovery in the management of NVUGIB is an effective method to meet the needs of both patient care and a cost-conscious healthcare system. In our study, it improved the primary outcome (LOS) and many secondary outcomes that promoted early discharge. Hence, providing adequate stimuli and incentives to encourage clinical physicians and nurses to participate in CPs can promote interdisciplinary care and effective patient management.
Data Sharing Statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Ethical Statement
All patients signed informed consent to participate in this study. The study was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University (MRCTA, ECFAH of FMU[2022]243) and followed the Declaration of Helsinki. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Acknowledgments
We would like to thank Ji-Zhen Wang and Kai-Yan Wei for assistance with data collection. We also like to thank those staff who assisted us in data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Fujian Provincial Health Technology Project [2021RKA006].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102–113. doi:10.1159/000323958
2. Zhong M, Chen WJ, Lu XY, Qian J, Zhu CQ. Comparison of three scoring systems in predicting clinical outcomes in patients with acute upper gastrointestinal bleeding: a prospective observational study. J of Dig Dis. 2016;17(12):820–828. doi:10.1111/1751-2980.12433
3. Kanno T, Yuan Y, Tse F, Howden CW, Moayyedi P, Leontiadis GI. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane gut group. Cochrane Database Sys Rev. 2022;2022(1). doi:10.1002/14651858.CD005415.pub4
4. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. 2019;171(11):805. doi:10.7326/M19-1795
5. Gralnek IM, Stanley AJ, Morris AJ, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline – update 2021. Endoscopy. 2021;53(03):300–332. doi:10.1055/a-1369-5274
6. Lanas A, Dumonceau JM, Hunt RH, et al. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018;4(1):18020. doi:10.1038/nrdp.2018.20
7. Laursen SB, Dalton HR, Murray IA, et al. Performance of new thresholds of the Glasgow Blatchford score in managing patients with upper gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2015;13(1):115–121.e2. doi:10.1016/j.cgh.2014.07.023
8. Vogt C, Allo G, Buerger M, et al. Assessing guideline adherence in patients with non-variceal upper gastrointestinal bleeding receiving antiplatelet and anticoagulant therapy. Scand J Gastroenterol. 2019;54(11):1357–1363. doi:10.1080/00365521.2019.1688384
9. Osman S, Hassanin A, Salama H. Establishing Nursing Guideline For Nurses Caring With Haematemsis Patients Undergoing Upper Gastrointestinal Endoscopy. Mansoura Nurs J. 2019;6(1):1–10. doi:10.21608/mnj.2019.154323
10. Targownik LE, Gralnek IM, Dulai GS, Spiegel BM, Oei T, Bernstein CN. Management of acute nonvariceal upper gastrointestinal hemorrhage: comparison of an American and a Canadian medical centre. Can J Gastroenterol. 2003;17(8):489–495. doi:10.1155/2003/264595
11. Spiegel TF, Wassermann TB, Neumann N, et al. A clinical pathway for heart failure reduces admissions from the ED without increasing congestion in the ED. Am J Emerg Med. 2018;36(7):1202–1208. doi:10.1016/j.ajem.2017.12.012
12. Pivalizza EG, Sen S, Hernandez N. Implementation of a Geriatric Trauma Clinical Pathway. JAMA Surg. 2023;158(1):104–105. doi:10.1001/jamasurg.2022.4822
13. Hogg HDJ, Brittain K, Teare D, et al. Safety and efficacy of an artificial intelligence-enabled decision tool for treatment decisions in neovascular age-related macular degeneration and an exploration of clinical pathway integration and implementation: protocol for a multi-methods validation study. BMJ Open. 2023;13(2):e069443. doi:10.1136/bmjopen-2022-069443
14. Sifontes-Dubón M, García-López JM, González-Ortega N, Pazos-Couselo M. Evaluation of a clinical pathway for thyroid nodular disease: timings and delays in the diagnosis and treatment of thyroid cancer. J Clin Med. 2021;10(23):5681. doi:10.3390/jcm10235681
15. Foni NO, Costa LAV, Paião ID, et al. Clinical pathway improves medical practice in total knee arthroplasty. PLoS One. 2020;15(5):e0232881. doi:10.1371/journal.pone.0232881
16. Mumtaz K, Kamani L, Hamid S, Abid S, Shah HA, Jafri W. Impact of a bleeding care pathway in the management of acute upper gastrointestinal bleeding. Indian J Gastroenterol. 2011;30(2):72–77. doi:10.1007/s12664-011-0089-5
17. Pfau PR, Cooper GS, Carlson MD, et al. Success and Shortcomings of a clinical care pathway in the management of acute nonvariceal upper gastrointestinal bleeding. Am J Gastroenterol. 2004;99(3):425–431. doi:10.1111/j.1572-0241.2004.04090.x
18. Zhang J, Gu LL, Xu Y, Zhao BB, Li D, Xiao C. Integrated medical care and the continuous 4C nursing model to improve nursing quality and clinical treatment of patients with acute stroke: based on a retrospective case-control study. Contrast Media Mol Imaging. 2022;2022:1–9. doi:10.1155/2022/4810280
19. Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med. 2017;377(14):1391–1398. doi:10.1056/NEJMsm1605385
20. Mohamed WRA, Leach MJ, Reda NA, Abd-Ellatif MM, Mohammed MA, Abd-Elaziz MA. The effectiveness of clinical pathway-directed care on hospitalisation-related outcomes in patients with severe traumatic brain injury: a quasi-experimental study. J Clin Nurs. 2018;27(5–6):e820–e832. doi:10.1111/jocn.14194
21. Gong EJ, Lee SJ, Jun BG, et al. Optimal timing of feeding after endoscopic hemostasis in patients with peptic ulcer bleeding: a randomized, noninferiority trial (CRIS KCT0001019). Am J Gastroenterol. 2020;115(4):548–554. doi:10.14309/ajg.0000000000000584
22. Güven İE, Başpınar B, Durak MB, Yüksel İ. Comparison of urgent and early endoscopy for acute non-variceal upper gastrointestinal bleeding in high-risk patients. Gastroenterol Hepatol. 2022;5. doi:10.1016/j.gastrohep.2022.05.002
23. Kim MS, Moon HS, Kwon IS, et al. Validation of a new risk score system for non-variceal upper gastrointestinal bleeding. BMC Gastroenterol. 2020;20(1):193. doi:10.1186/s12876-020-01346-4
24. Lu M, Sun G, Zhang XM, et al. Peptic ulcer is the most common cause of Non-Variceal Upper-Gastrointestinal Bleeding (NVUGIB) in China. Med Sci Monit. 2018;24:7119–7129. doi:10.12659/MSM.909560
25. Kim J, Gong EJ, Seo M, et al. Timing of endoscopy in patients with upper gastrointestinal bleeding. Sci Rep. 2022;12(1):6833. doi:10.1038/s41598-022-10897-3
26. Saraiva RO, Loureiro RV, Coimbra JMG. An uncommon cause of recurrent upper gastrointestinal bleeding. Gastroenterology. 2022;S0016508522010745. doi:10.1053/j.gastro.2022.09.018
27. Hay JA, Maldonado L, Weingarten SR, Ellrodt AG. Prospective evaluation of a clinical guideline recommending hospital length of stay in upper gastrointestinal tract hemorrhage. JAMA. 1997;278(24):2151–2156. doi:10.1001/jama.1997.03550240041031
28. Forsmo HM, Erichsen C, Rasdal A, Tvinnereim JM, Körner H, Pfeffer F. Randomized controlled trial of extended perioperative counseling in enhanced recovery after colorectal surgery. Dis Colon Rectum. 2018;61(6):724–732. doi:10.1097/DCR.0000000000001007
29. Sánchez-Iglesias JL, Gómez-Hidalgo NR, Pérez-Benavente A, et al. Importance of Enhanced Recovery After Surgery (ERAS) protocol compliance for length of stay in ovarian cancer surgery. Ann Surg Oncol. 2021;28(13):8979–8986. doi:10.1245/s10434-021-10228-2
30. Podila PV, Ben-Menachem T, Batra SK, Oruganti N, Posa P, Fogel R. Managing patients with acute nonvariceal gastrointestinal hemorrhage: development and effectiveness of a clinical care pathway. Am J Gastroenterol. 2001;96(1):208–219. doi:10.1111/j.1572-0241.2001.03477.x
31. Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116(5):899–917. doi:10.14309/ajg.0000000000001245
32. McAleese P, Odling-Smee W. The effect of complications on length of stay. Ann Surg. 1994;220(6):740–744. doi:10.1097/00000658-199412000-00006
33. Abougergi MS, Travis AC, Saltzman JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastro Endosco. 2015;81(4):882–888.e1. doi:10.1016/j.gie.2014.09.027
34. Khoshbaten M, Ghaffarifar S, Jabbar Imani A, Shahnazi T. Effects of early oral feeding on relapse and symptoms of upper gastrointestinal bleeding in peptic ulcer disease: FEEDING IN PEPTIC ULCER WITH BLEEDING. Digestive Endoscopy. 2013;25(2):125–129. doi:10.1111/j.1443-1661.2012.01347.x
35. Kamyabi N, Nakhaei M, Nasiri A, Akbari E, Sharifzadeh G. Effects of video- and pamphlet-based patient educations on anxiety and satisfaction among candidates for gastroscopy. Modern Care Journal. 2016;In Press(In Press). doi:10.17795/modernc.10647
36. Chang A, Ouejiaraphant C, Pungpipattrakul N, Akarapatima K, Rattanasupar A, Prachayakul V. Effect of holiday admission on clinical outcome of patients with upper gastrointestinal bleeding: a real-world report from Thailand. Heliyon. 2022;8(8):e10344. doi:10.1016/j.heliyon.2022.e10344
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.