Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Clinical Outcomes, Costs, and Healthcare Resource Utilization in Patients with Metastatic Merkel Cell Carcinoma Treated with Immune Checkpoint Inhibitors vs Chemotherapy
Authors Zheng Y, Yu T, Mackey RH, Gayle JA, Wassel CL, Phatak H, Kim R
Received 7 November 2020
Accepted for publication 23 February 2021
Published 23 March 2021 Volume 2021:13 Pages 213—226
DOI https://doi.org/10.2147/CEOR.S290768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Ying Zheng,1 Ting Yu,2 Rachel H Mackey,3,4 Julie A Gayle,3 Christina L Wassel,3 Hemant Phatak,1 Ruth Kim5
1 EMD Serono, Inc., Rockland, USA; An Affiliate of Merck KGaA, Darmstadt, Germany; 2Global Medical Affairs, EMD Serono, Inc., Rockland, MA, USA; An Affiliate of Merck KGaA, Darmstadt, Germany; 3Premier Applied Sciences, Premier, Inc, Charlotte, NC, USA; 4Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA; 5Pfizer Inc., New York, NY, USA
Correspondence: Ruth Kim
Pfizer Inc., New York, NY 10017, USA
Tel +1 212 733-7637
Email [email protected]
Purpose: Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer with poor prognosis. This study compared patient characteristics, comorbidities, adverse events (AEs), treatment persistence, healthcare resource utilization (HRU) and costs in patients with metastatic MCC (mMCC) treated with immune checkpoint inhibitors (ICIs) or recommended chemotherapy per 2018 National Comprehensive Cancer Network (NCCN) Guidelines.
Patients and Methods: A retrospective, observational study was conducted using data from 3/1/2015 through 12/31/2017 from the Premier Healthcare Database, a US hospital discharge database. The study included patients aged ≥ 12 years with International Classification of Diseases Codes for MCC and metastasis, categorized by their first treatment (index) during the study period (ICI or NCCN-recommended chemotherapy [chemotherapy]). Patient, hospital, and visit characteristics were assessed at the index date and Charlson Comorbidity Index (CCI) score and comorbidities during a 6-month look-back period. Clinical outcomes, including AEs and treatment persistence were assessed over 90 days and HRU and costs over 180 days post-index.
Results: Of 75 patients with mMCC receiving ICIs (n=37) or chemotherapy (n=38), mean age was ≈73 years, and 21.3% had a history of immune-related (IR) conditions. Overall, ICI- and chemotherapy-treated patients were similar in most baseline characteristics, IR comorbidities, and CCI score. However, more ICI patients (46%) than chemotherapy patients (26%) persisted on treatment over 90-day follow-up, odds ratio (95% CI): 2.04 (0.93, 4.47), P=0.07. Over 180-day follow-up, 33% of patients had an inpatient admission with mean length of stay (LOS) ≈2 days shorter for ICI vs chemotherapy (not statistically significant). Total costs, primarily driven by pharmacy costs, were higher for ICIs than chemotherapy; other departmental costs were similar between treatment groups.
Conclusion: In a real-world setting, patients with mMCC receiving ICIs had higher treatment persistence over 90 days, shorter inpatient LOS and similar departmental cost (excluding pharmacy cost) than those receiving chemotherapy.
Keywords: metastatic Merkel cell carcinoma, immune checkpoint inhibitors, chemotherapy
Plain Language Summary
Merkel cell carcinoma (MCC) is a rare but aggressive skin cancer that often spreads (metastasizes) throughout the body. Patients with MCC have poor survival. Clinical trials have shown that “immune checkpoint inhibitors” (ICIs) improve survival in advanced (metastatic) MCC (mMCC), but because mMCC is rare, there is limited information on real-world treatment outcomes. Therefore, this study used data from a large US hospital database (from 03/01/2015 to 12/31/2017) to compare characteristics and 3 month healthcare use of 75 patients aged 12 years and older with mMCC who were either treated with ICIs (37 patients) or with specific chemotherapy (38 patients). More than 20% of patients had immune-related conditions before treatment, which was related to more side effects during treatment. Overall, most characteristics and outcomes were similar for patients treated with ICIs vs. chemotherapy, but during the 90 days after initial treatment, ICI-treated mMCC patients were more likely to continue to receive ICI treatment (46%) than those receiving chemotherapy (26%). ICI-treated mMCC patients had higher total costs than chemotherapy-treated patients, due to higher pharmacy costs, since costs were similar for other aspects of treatment. These results suggest that ICIs were tolerable and effective in most of these mMCC patients.
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive form of skin cancer with poor prognosis and outcomes, including poor survival.1–4 At diagnosis, approximately one-third of patients with MCC have distant metastases.5 Treatment options for advanced MCC have been very limited.4 Responses to chemotherapy are generally poor, with relatively short duration,6,7 and are worse in second or later lines of therapy.8 More recently, better clinical responses have been shown with immune checkpoint inhibitors (ICIs), including the anti-programmed cell death-ligand 1 (PD-L1) antibody avelumab and the anti-programmed cell death-protein 1 (PD-1) antibody pembrolizumab. In 2017, avelumab became the first treatment approved by the US Food and Drug Administration for adults and pediatric patients aged ≥12 years with metastatic MCC (mMCC).9–11 In 2018, pembrolizumab was approved for adult and pediatric patients with recurrent locally advanced or metastatic MCC. For disseminated (metastatic) MCC, the 2018 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) recommended ICIs (avelumab, pembrolizumab, nivolumab), and for patients with contraindications to ICIs (including lack of durable response), recommended chemotherapies included cisplatin ± etoposide, carboplatin ± etoposide, topotecan, and combination therapy with the CAV regimen (cyclophosphamide, doxorubicin [or epirubicin], and vincristine), as clinical judgment dictates.12 However, as noted by the NCCN 2018 Guidelines12 and other publications,13 despite the increasing use of ICIs, there is a lack of published literature on real-world outcomes in patients with mMCC treated with ICIs and specific NCCN-recommended chemotherapy. Real-world evidence to assess treatment efficacy, tolerability, and adverse events (AEs) is especially needed for rare forms of cancer, due to limited data from randomized clinical trials (RCTs). Furthermore, because ICIs may contribute to immune-related AEs (irAEs) and there is concern about exacerbating existing autoimmune disease,14 clinical trials generally exclude patients with autoimmune-related conditions, leaving another important gap in knowledge.
This real-world evidence study used a US hospital discharge database to compare patient and hospital characteristics, comorbid conditions, and clinical and economic outcomes, including AEs, healthcare resource utilization (HRU), and costs, for mMCC patients initially treated with ICIs vs those initially treated with chemotherapy.
Patients and Methods
The Premier Healthcare Database
The Premier Healthcare Database (PHD)15 was used to conduct this retrospective, observational study of patients with mMCC treated with ICIs or chemotherapy. The PHD is a large hospital-based, service-level, all-payer database containing discharge information from inpatient and hospital-based outpatient visits. It represents approximately 25% of all US admissions from geographically diverse non-governmental community and teaching hospitals and rural and urban health systems. The PHD contains data from standard hospital discharge files, including patient demographics and disease states; health insurance type; admission and discharge diagnoses; admission source and type; discharge status and disposition; and hospital pharmacy medication use. Information on billed services includes overall departmental- and service-level costs (adjusted to 2018 US Dollars) for inpatient and outpatient encounters. Unique masked identifiers allow patients to be tracked in the same hospital across inpatient and hospital-based outpatient settings. All data in the PHD are statistically de-identified and compliant with the Health Insurance Portability and Accountability Act.15
Study Population
Patients aged ≥12 years with mMCC and initial treatment with an ICI or chemotherapy per NCCN Guidelines during the main study period of March 1, 2015, through December 31, 2017, were eligible for the study. mMCC was defined using primary or secondary International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) or 10th Revision, Clinical Modification (ICD-10-CM), codes (Supplementary Table 1). Patients who were pregnant were excluded from the study (Figure 1).
 |
Figure 1 Study timeline. Abbreviations: mMCC, metastatic Merkel cell carcinoma; AEs, adverse events; HRU, healthcare resource utilization. |
Treatment group was based on the first ICI or chemotherapy treatment received during the main study period, which was identified using text searches of both generic and brand names in the hospital discharge chargemaster data. ICI treatments included PD-L1 inhibitors avelumab, atezolizumab, or durvalumab and PD-1 inhibitors nivolumab or pembrolizumab. Per NCCN 2018 Guidelines, for patients with mMCC with contraindications to ICIs, chemotherapy options include cisplatin ± etoposide, carboplatin ± etoposide, topotecan, and combination therapy with the CAV regimen.12
Study Timeline
The main study period was March 1, 2015, through December 31, 2017, with a 90-day follow-up through March 31, 2018, for clinical outcomes, including AEs, and a 180-day follow-up for economic outcomes (Figure 1). From the time of the first ICI or chemotherapy treatment in the main study period (index date), there was a 6-month look-back period to assess comorbidities.
Patient, Visit, and Hospital Characteristics
Patient, visit, and hospital characteristics were examined at the index visit. Patient demographics included age, sex, race, ethnicity, and primary payer (ie, Medicare, Medicaid, commercial.) Tumor location was also assessed using ICD-9-CM/ICD-10-CM codes (Supplementary Table 1). Hospital characteristics included admission status (inpatient vs outpatient), admission type (elective, emergency, urgent, or information unavailable), and hospital setting (bed size, geographic location, urban vs rural, and teaching status.) The Deyo-modified Charlson Comorbidity Index (CCI) score16,17 was calculated using characteristics at the index visit (see Supplementary Table 2 for ICD-9-CM/ICD-10-CM codes). Individual comorbidities (assessed during 6-month look-back period from the index date) were identified by ICD-9-CM and ICD-10-CM codes (Supplementary Table 3). Immune-related and immunocompromised comorbidities included celiac disease, colitis, thyroid disorders, rheumatoid arthritis, and solid organ transplant (complete list in Supplementary Table 3); other comorbidities included anemia, peripheral edema, diabetes, nephropathy, pneumonitis, and venous thromboembolism (complete list in Supplementary Table 3). Differences in CCI scores and patient, hospital, and visit characteristics at the index date, as well as comorbid conditions during the look-back period, were assessed for the ICI vs chemotherapy groups.
Clinical Characteristics, Outcomes, Costs, and HRU
To assess clinical outcomes, patients were followed up for 90 days after the index visit (through March 31, 2018), to reduce bias when comparing between treatment groups. Clinical outcomes assessed included “treatment persistence”, AEs, and all-cause in-hospital mortality. Treatment persistence was defined as the percentage of patients persisting on their initial treatment category (ICI or recommended chemotherapy) within each predefined follow-up period of 1–30, 31–60 and 61–90 days after discharge from their index treatment.
AEs were defined using ICD-9-CM/ICD-10-CM codes (Supplementary Table 4) and included irAEs that were selected using the American Society of Clinical Oncology Clinical Practice Guideline recommendations for irAE management.18 A total of 45 AEs commonly associated with ICIs and chemotherapies were assessed.
For calculations of costs and HRU, patients were followed up for 180 days (6 months, through June 30, 2018), to align with the 6-month assessment period for reimbursement per the Center for Medicare & Medicaid Innovation’s Oncology Care Model practices. The number and percentage of inpatient stays, length of stay (LOS), total costs and departmental costs (cardiology, hospice, emergency, laboratory, pharmacy, operating room, radiology, respiratory, occupational therapy, central supply, room and board) were calculated for the ICI and chemotherapy groups.
Statistical Analysis
Descriptive statistics were calculated with continuous variables expressed as means and standard deviations (SDs) and categorical variables expressed as counts and percentages. Patient characteristics and outcomes were compared between initial treatment groups (ICI vs chemotherapy) and P values were calculated using the χ2 test, Fisher’s exact test, t test, analysis of variance test, Wilcoxon rank-sum test, or Kruskal–Wallis test, as appropriate.
To compare 90-day treatment persistence for ICI vs chemotherapy groups, the odds ratio (95% CI) was quantified with repeated measures logistic regression using generalized estimating equations (GEE), specifically a generalized linear model with a binomial distribution and logit link.
Results
Patient, Visit, and Hospital Characteristics
A total of 3418 patients with MCC were initially identified, of whom 3408 were aged ≥12 years and 539 had mMCC (Figure 2). Among the 539 patients with mMCC, 75 whose initial therapy during the study period was ICI (n=37) or chemotherapy (n=38) were included in the current study. The remaining patients (n=464) were excluded from the study as they had received other non-NCCN-recommended chemotherapy (n=8) or other treatment (n=456), which included surgery/radiation or no treatment. Of patients in the chemotherapy group, 84% received platinum-based chemotherapy.
Baseline characteristics were similar between the ICI and chemotherapy groups in terms of age (mean, ≈73 years), race (≥89% White), and ethnicity (≤5% Hispanic), but there was a higher proportion of men in the ICI group than the chemotherapy group (73% vs 53%, respectively; P=0.07) (Table 1). Hospital characteristics were also similar between ICI and chemotherapy groups, but the ICI group included more patients with commercial insurance (30% vs 13%; P=0.11) and had a lower proportion of inpatient admissions (16% vs 32%; P=0.12) than the chemotherapy group (Table 1). Finally, the ICI group had a higher proportion of trunk (32% vs 13%; P=0.047) tumor locations than the chemotherapy group (Table 1).
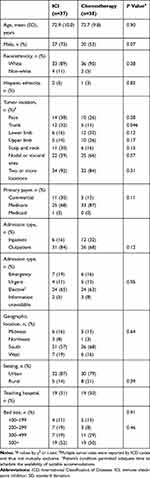 |
Table 1 Patient and Hospital Characteristics at Baseline |
Overall, 53.3% (40 of 75) of patients with mMCC had a history of comorbid conditions, which was more common in chemotherapy (65.8%, 25 of 38) than ICI (40.5%, 15 of 37) treated patients, P=0.04 for difference (Figure 3). This difference was due to higher proportions of anemia (34.2% vs 8.1%, p=0.01) and probably diabetes (34.2% vs 13.5%, p=0.057) in the chemotherapy than ICI group, since other comorbidities, including immune-related or immunocompromised conditions, were not significantly different between groups (p≥0.43 for all.) In the ICI group, the most frequent comorbidities were diabetes, peripheral edema, and thyroid disorders; in the chemotherapy group, the most frequent were diabetes, peripheral edema, and anemia (Figure 3). For history of immune-related or immunocompromised comorbid conditions (Supplementary Table 3), which was found for 21.3% (16 of 75) of the study cohort, there was no difference between ICI (19%, 7 of 37) and chemotherapy (23.7%, 9 of 38), P=0.78. Average CCI score and average number of immune-related and immunocompromised comorbid conditions were also similar in the ICI and chemotherapy groups (Table 2).
 |
Table 2 Comorbid Conditions Among Patients with mMCC Receiving ICI vs Chemotherapya |
Treatment Persistence
A higher proportion of patients in the ICI group than in the chemotherapy group persisted on treatment in their original treatment category during the 90-day follow-up period: 68% vs 53% during days 1–30, 54% vs 42% during days 31–60, and 46% vs 26% during days 61–90, respectively (Figure 4). Using data from all 3 time periods, the odds ratio (95% CI) for persisting on original treatment across the 90-day follow-up period is 2.04 (0.93, 4.47), P=0.0742 for patients originally on ICI vs chemotherapy treatment. This odds ratio suggests that in our study cohort, patients on ICI were twice as likely as patients on chemotherapy to persist on their original treatment over 90-day follow-up.
Adverse Events and in-Hospital Mortality
During the 90-day follow-up period, the average (SD) number of AEs per patient was 1.2 (1.5) in the ICI group vs 1.6 (1.7) in the chemotherapy group, (P=0.89). Overall, 58.7% (44 of 75) patients had any AE during the 90-day follow-up, including 54.1% (20 of 37) ICI patients and 63.2% (24 of 38) chemotherapy patients (P=0.49 for difference). Individual AEs (including individual immune-related AEs) were also not statistically different between ICI and chemotherapy patients, except for anemia, which was approximately twice as high in chemotherapy (44.7%) than ICI (21.6%) patients, P=0.0497 for difference (Figure 5). Most patients with any AEs experienced irAEs (95.5%, 42 of 44 patients); irAEs occurred in 51.4% (19/37) of patients receiving ICI. Among all patients with mMCC (without regard to initial treatment group), observed AEs during follow-up were more likely among patients with previous immune-related or immunocompromised comorbid conditions (81.3%, 13 of 16 patients) than among patients with no identified immune-related or immunocompromised comorbid conditions (53%, 31 of 59 patients, P=0.048 for difference).
In both groups, the most frequent AEs were anemia, peripheral edema, and thrombocytopenia Pancytopenia (5%), adrenal insufficiency (3%), fatigue (3%), pituitary conditions (3%), and pruritis (3%) were present only in the ICI group, while constipation (8%), neutropenia (8%), mucositis (5%), and diarrhea (3%) were present only in the chemotherapy group (Figure 5). No inpatient mortality was reported up to 90 days after initiation of mMCC treatment in either the ICI or chemotherapy groups.
Costs and HRU
Over 180 days of follow-up, total costs (including all the individual departmental costs in Figure 6) were higher in the ICI group, with average an (SD) of $74,124.02 ($193,964.50) in the ICI group and $28,236.19 ($32,932.62) in the chemotherapy group (P=0.005). The median (interquartile range [IQR]) total costs were $34,311.70 ($23,515.20-$64,212.26) in the ICI group compared with $20,811.40 ($5,390.61-$32,788.53) in the chemotherapy group. This difference was partly due to some high-cost outliers/variability (much higher SD) in the ICI group. The difference in average total costs was also driven by higher pharmacy cost, which was the highest departmental cost in both treatment groups (Figure 6). In contrast, average costs for other departments were similar or slightly lower (cardiology, laboratory, respiratory, room and board) in the ICI group than in the chemotherapy group (Figure 6). In both the ICI and chemotherapy groups, emergency, cardiology, central supply, and therapy costs all averaged less than $1000 over the 180-day follow-up (Figure 6).
For HRU, overall, 25 patients (33%) had an inpatient stay within 180 days after their initial treatment, with a mean (SD) LOS of 9.4 (8.5) days and a median (IQR) LOS of 8.0 (4.0–12.0) days (Table 3). The percentages of patients with inpatient stays during the 180-day follow-up were not significantly different between the ICI and chemotherapy groups (41% vs 26%, respectively; P=0.23) (Table 3). Among patients with an inpatient stay (n=25), the mean number of inpatient stays was not statistically different between the ICI and chemotherapy groups (1.53 vs 1.30, respectively; P=0.55). However, the LOS was, on average, about 2 days shorter in the ICI group than in the chemotherapy group (mean, 8.4 vs 10.8 days; median [IQR], 6 [3.0–11.0] vs 9.5 [6.0–12.0] days, respectively), although this difference was not statistically significant (P=0.45).
 |
Table 3 Healthcare Resource Utilization During 180 Days of Follow-Up for Patients with mMCC Receiving ICI vs Chemotherapya |
Discussion
In this real-world setting of 75 patients with mMCC in a US hospital discharge database, treatment persistence (staying in their initial treatment category) over 90-day follow-up was approximately 2-fold higher (odds ratio [95% CI]: 2.04 [0.93, 4.47], P=0.0742) for patients whose initial treatment was ICIs compared with patients whose initial treatment was NCCN-recommended chemotherapy. Overall, ICI and chemotherapy groups were similar for baseline patient, visit, and hospital characteristics, and history of all comorbidities and immune-related comorbidities, except for higher proportion of anemia and (possibly) diabetes for chemotherapy compared to ICI groups. Average CCI score and average number of immune-related or immunocompromised conditions, was also similar in the ICI and chemotherapy groups. Overall, 59% of patients had any AE during a 90-day follow-up period, which was also similar for ICI and chemotherapy groups, except for anemia, which was twice as high in chemotherapy than ICI patients. However, approximately 20% of the patients in both treatment groups had a history of immune-related or immunocompromised conditions, and those patients were more likely to have AEs during 90-day follow-up than patients without immune-related comorbidities. Among patients with inpatient admissions during 180-day follow-up, LOS was approximately 2 days shorter for patients receiving ICIs than for those receiving chemotherapy, although the difference did not reach statistical significance. Finally, higher average pharmacy costs drove the average total costs higher for ICI than chemotherapy-treated patients with mMCC, but average costs for departments other than pharmacy were similar or slightly lower in the ICI group than in the chemotherapy group.
As previously noted, published studies of patients with mMCC are relatively scarce, partly due to the rarity of MCC. As in prior studies, the patients with mMCC in our study were older and predominantly White and male.6,9,19 In this study, the ICI group had a slightly higher proportion of trunk tumor locations, although numbers were small and these differences may be due to chance. Head/neck and trunk tumor locations have been reported to be associated with worse survival than upper and lower extremity tumor locations,3 so we speculate that ICI use might be partly related to consideration of tumor location. However, other studies would need to confirm this observation.
In this real-world observational study, patients who were initially receiving ICIs were more likely than those initially receiving chemotherapy to persist in the same treatment category over 90 days after treatment initiation. Although this difference failed to reach statistical significance in our small sample size, the magnitude (a twofold higher persistence for ICI-treated patients) suggests a clinical difference. This agrees with another recent study of patients with MCC that reported a substantially longer median treatment duration for patients treated with ICIs (300 days) compared to chemotherapy (91 days.)19 However, since there are no FDA-approved chemotherapy options for mMCC, the treatment duration used in real-world varies considerably, with one large study reporting median time to discontinuation of 1.8 months, with a range of 0.1 to 15.9 months on chemotherapy treatment.6 Another study reported median progression-free-survival (PFS) of 94 days from start of chemotherapy.7 All of these studies have longer chemotherapy duration than in our study, in which only 53% of chemotherapy patients persisted in their original treatment category by 30 days after treatment, and only 26% persisted during the 61–90 days after their initial treatment. We speculate that the higher treatment persistence for ICI than chemotherapy in our study may be driven by both efficacy and toxicity differences between ICI and chemotherapy that have been reported in other studies.20
The current study evaluated immune-related and immunocompromised comorbidities and other comorbid conditions in the 6 months prior to the first ICI or chemotherapy treatment, and immune-related AEs and other AEs during the 90 days following the first treatment. As noted, in our real-world study more than 20% of the patients with mMCC in both chemotherapy and ICI-treated groups had a history of immune-related or immunocompromised conditions. These patients are typically excluded from clinical trials due to concern that they have a higher risk of irAEs. However, the cautious use of ICIs in patients with underlying autoimmune disease is supported by several studies.14,20–23 Our study demonstrated that over 90-day follow-up, AEs were more likely to be observed in patients with previous immune-related conditions than in those without. Overall, the ICI group was similar compared to the chemotherapy group in the proportion of total comorbidities and in average number of prior comorbidities, but ICI patients had similar, or in some cases, lower AEs. Direct comparison of AEs in our study to AEs in clinical trials is difficult because of differing definitions of AEs. However, since our study included patients with immune-related comorbidities, who are excluded from most clinical trials,24 it is not surprising that in our study, the proportions of patients with mMCC with some irAEs (eg, thyroid disorders [≈10%] and colitis [≈7%]) were higher or similar to those reported in ICI RCTs (thyroid disorders, 5%-12%; colitis, <2%).9,10,25–28 Notably, in our study the ICI and chemotherapy groups had similar percentages of these AEs, which also suggests that the higher proportions compared with RCTs are due to inclusion of mMCC patients with immune-related conditions.
In this study, ICI-treated patients with mMCC had higher average and median total costs through 180 days of follow-up than chemotherapy-treated patients did, which appeared to be due to higher pharmacological treatment costs, although the costs were unadjusted for potential differences in patient population. The higher pharmacological treatment costs could possibly be associated with the higher treatment persistence rate with ICI than with chemotherapy, as identified in this study. Published studies have demonstrated the value of ICI treatment. For example, a cost-effectiveness analysis was conducted to assess the lifetime costs and effects of avelumab, a newly available treatment option for mMCC, vs standard care, from a UK National Health Service perspective. The results suggested that avelumab is likely to be a cost-effective treatment option for UK patients with mMCC.29 To fully assess ICI value in mMCC, additional factors such as clinical efficacy measures, safety, and patient-reported outcomes must be considered.30–32 Previously published studies examining the costs related to the use of ICIs in patients with mMCC are not fully comparable to the current study due to different study criteria4,19,33 or different healthcare systems.29 However, extrapolating median total costs over 6 months in our study to 1 year, they were roughly similar to those previously reported for patients with advanced MCC over 1 year.4
The current study had several strengths and limitations, primarily related to its retrospective, observational descriptive design and use of real-world data. Strengths include providing information on treatment persistence, costs, and HRU among patients with the rare but severe mMCC using ICI or chemotherapy treatments in a real-world setting. The study also examined and compared a wide array of AEs of varying severity between patients with mMCC receiving ICIs vs chemotherapy treatments. A primary limitation is the relatively small sample size, which limits the ability to find statistically significant differences. However, the small sample size is due to the rarity of mMCC, and as such, this study provides hypothesis-generating information for future studies on treatment patterns in mMCC. However, results must be cautiously interpreted due to the observational study design and limited sample size. Another limitation is that the PHD is not a random sample and thus may not be generalizable to the US population; however, the PHD does include healthcare providers from all areas of the country and 1 in 5 hospital discharges in the United States. The study was limited to a set time period (Sep 2014–June 2018), which may also affect generalizability outside this study window. While the study includes follow-up for clinical outcomes and look-back periods for comorbidities in patients with mMCC receiving ICI and chemotherapy treatments, this study did not formally assess confounding. Furthermore, the study did not assess the subsequent therapy for patients who switched treatment. Another limitation of our study is the potential underreporting of patients requiring hospital admissions in the follow-up period if they did not return to the same hospital for subsequent treatment. Finally, the short follow-up for clinical outcomes may not be extrapolated into longer term and studies with larger sample size and longer follow-up are needed to understand the long-term outcomes of mMCC patient receiving ICI or chemotherapy.
Conclusions
The availability of ICIs for patients with mMCC is changing clinical paradigms and treatment patterns. Among these real-world patients with mMCC, of whom >20% had existing immune-related or immunocompromised conditions, patients with ICI treatment had higher total costs, driven partly by high-cost outliers and partly by higher pharmacy costs, but costs were similar for departmental costs other than pharmacy. There was a trend toward shorter LOS in the ICI group than in the chemotherapy group. Patients receiving ICI treatment had greater treatment persistence and therefore longer time on treatment over 90 days than did patients receiving chemotherapy, suggesting that ICIs may be tolerable and effective among real-world patients with mMCC.
Abbreviations
AE, adverse event; CAV, cyclophosphamide, doxorubicin (or epirubicin), + vincristine; CCI, Charlson Comorbidity Index; HRU, healthcare resource utilization; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; ICD-10-PCS, International Classification of Diseases, 10th Revision, Procedure Coding System; ICI, immune checkpoint inhibitor; IQR, interquartile range; irAE, immune-related adverse event; LOS, length of stay; MCC, Merkel cell carcinoma; mMCC, metastatic Merkel cell carcinoma; NCCN, National Comprehensive Cancer Network; PD-1, programmed cell death-protein 1; PD-L1, programmed cell death-ligand 1; PHD, Premier Healthcare Database; RCT, randomized controlled trial; SD, standard deviation.
Data Sharing Statement
This data from this study is not available for sharing as it is from the Premier Health Database, PHD, which is a proprietary database.
Ethics Approval and Informed Consent
Institutional review board approval for this study was not required, based on title 45 of the Code of Federal Regulations part 46 because the study used existing de-identified hospital discharge data, and recorded information could not be identified directly or through identifiers linked to individuals. Given the use of deidentified data only in this study, it was deemed not to require ethics board review based on the policy of the Office of Human Subjects Research Protections, National Institutes of Health, under the revised Common Rule. Furthermore, all data were compliant with the Health Insurance Portability and Accountability Act and no informed consent was pursued.
Acknowledgments
The authors thank Mo Yang for assistance with the manuscript. Christina Wassel’s current affiliation is at Genesis Research, Hoboken, NJ, USA.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. In addition to the above contributions, additional specific contributions included the following: Ying Zheng, Ting Yu, Hemant Phatak, and Ruth Kim made substantial contributions to conception and design and interpretation of data; participated in revising the article critically for important intellectual content; and gave final approval of the version to be submitted and any revised version. Julie A Gayle and Christina L Wassel made substantial contributions to conception and design and analysis and interpretation of data; participated in drafting the article and revising it critically for important intellectual content; and gave final approval of the version to be submitted and any revised version.
Rachel H Mackey made substantial contributions to the analysis and interpretation of data; participated in drafting the article and revising it critically for important intellectual content; and gave final approval of the version to be submitted and any revised version.
Funding
This study was funded by EMD Serono, an affiliate of Merck KGaA, Darmstadt, Germany, as part of an alliance between Merck KGaA, Darmstadt, Germany, and Pfizer Inc., New York, NY, USA.
Disclosure
Rachel H Mackey and Julie A Gayle are full-time employees of Premier, Inc. which received payment from EMD Serono to conduct the study, and have no competing interests against this study. Christina L Wassel is a former full-time employee of Premier, Inc., and has no competing interests against this study. Hemant Phatak, Ting Yu, and Ying Zheng are former full-time employee of EMD Serono, Inc., an affiliate of Merck KGaA, Darmstadt, Germany, who, in the course of this employment, received stock options exercisable for, and other stock awards of, ordinary shares of EMD Serono. Ruth Kim is a full-time employee of Pfizer Inc. who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Pfizer Inc. She also reports ownership of stocks from Exelixis and BMS. The authors report no other conflicts of interest in this work.
References
1. Hughes MP, Hardee ME, Cornelius LA, Hutchins LF, Becker JC, Gao L. Merkel cell carcinoma: epidemiology, target, and therapy. Curr Dermatol Rep. 2014;3(1):46–53. doi:10.1007/s13671-014-0068-z
2. Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102(11):793–801. doi:10.1093/jnci/djq120
3. Liang E, Brower JV, Rice SR, Buehler DG, Saha S, Kimple RJ. Merkel cell carcinoma analysis of outcomes: a 30-year experience. PLoS One. 2015;10(6):e0129476. doi:10.1371/journal.pone.0129476
4. Steuten L, Garmo V, Phatak H, Sullivan SD, Nghiem P, Ramsey SD. Treatment patterns, overall survival, and total healthcare costs of advanced Merkel cell carcinoma in the USA. Appl Health Econ Health Policy. 2019;17(5):733–740. doi:10.1007/s40258-019-00492-5
5. Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3(1):17077. doi:10.1038/nrdp.2017.77
6. Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13(19):1699–1710. doi:10.2217/fon-2017-0187
7. Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5(9):2294–2301. doi:10.1002/cam4.815
8. Becker JC, Lorenz E, Ugurel S, et al. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget. 2017;8(45):79731–79741. doi:10.18632/oncotarget.19218
9. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, Phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi:10.1016/S1470-2045(16)30364-3
10. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4(9):e180077. doi:10.1001/jamaoncol.2018.0077
11. Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6(1):7. doi:10.1186/s40425-017-0310-x
12. Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel cell carcinoma, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(6):742–774. doi:10.6004/jnccn.2018.0055
13. Nghiem P, Kaufman HL, Bharmal M, Mahnke L, Phatak H, Becker JC. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017;13(14):1263–1279. doi:10.2217/fon-2017-0072
14. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168(2):121–130. doi:10.7326/M17-2073
15. Premier Applied Sciences. Premier Healthcare Database: Data That Informs and Performs. Available from: https://products.premierinc.com/applied-sciences. Accessed January 11, 2021
16. Deyo RA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
18. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385
19. Chandra S, Zheng Y, Pandya S, et al. Real-world outcomes among US Merkel cell carcinoma patients initiating immune checkpoint inhibitors or chemotherapy. Future Oncol. 2020;16(31):2521–2536. doi:10.2217/fon-2020-0453
20. NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. v1.2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf.
21. Haanen J, Ernstoff MS, Wang Y, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol. 2020;31(6):724–744. doi:10.1016/j.annonc.2020.03.285
22. La-beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy. 2015;35(10):963–976. doi:10.1002/phar.1643
23. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi:10.1038/s41571-019-0218-0
24. Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM. Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol. 2018;25(5):403–410. (). doi:10.3747/co.25.4047
25. Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. doi:10.1056/NEJMoa1603702
26. D’Angelo SP, Bhatia S, Brohl AS, et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer. 2020;8:1.
27. Bavencio (avelumab) Prescribing information. EMD Serono. 2020.
28. Keytruda (pembrolizumab). Prescribing Information. Whitehouse Station, NJ: Merck & Co., Inc; 2020.
29. Bullement A, Nathan P, Willis A, et al. Cost effectiveness of avelumab for metastatic Merkel cell carcinoma. Pharmacoecon Open. 2019;3(3):377–390. doi:10.1007/s41669-018-0115-y
30. Kelly ZR, Davar D. The Financial and Physical Toxicity of Immune Checkpoint Inhibitors in Cancer. ASCO Daily News; 2019. Available fromhttps://dailynews.ascopubs.org/do/10.1200/ADN.19.190405/full/.
31. Kaufman HL, Atkins MB, Dicker AP, et al. The value of cancer immunotherapy summit at the 2016 society for immunotherapy of cancer 31st anniversary annual meeting. J Immunother Cancer. 2017;5:38. doi:10.1186/s40425-40017-40241-40426
32. Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. Journal Immunother Cancer. 2018;6(1):128. doi:10.1186/s40425-018-0442-7
33. Bharmal M, Kearney M, Zheng Y, Phatak H. Budget impact model of avelumab in patients with metastatic merkel cell carcinoma in the US. Clinicoecon Outcomes Res. 2019;11:349–359. doi:10.2147/CEOR.S202642
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





