Back to Journals » Cancer Management and Research » Volume 12
Clinical Outcomes and Safety of Different Treatment Modes for Local Recurrence of Rectal Cancer
Authors Tang Z, Liu L , Liu D, Wu L, Lu K, Zhou N, Shen J, Chen G, Liu G
Received 1 September 2020
Accepted for publication 2 November 2020
Published 30 November 2020 Volume 2020:12 Pages 12277—12286
DOI https://doi.org/10.2147/CMAR.S278427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zhongzhu Tang,1,2 Luying Liu,1,2 Dong Liu,1,2 Lie Wu,1,2 Ke Lu,1,2 Ning Zhou,1,2 Jinwen Shen,1,2 Guiping Chen,3 Guan Liu1,2
1Department of Abdominal Radiotherapy, Cancer Hospital of the University of Chinese Academy of Sciences & Zhejiang Cancer Hospital, Hangzhou, People’s Republic of China; 2Institute of Cancer and Basic Medicine (IBMC), Chinese Academy of Sciences, Hangzhou, People’s Republic of China; 3Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Guan Liu
Department of Abdominal Radiotherapy, Cancer Hospital of the University of Chinese Academy of Sciences & Zhejiang Cancer Hospital, 1 Banshan East Road, Hangzhou 310022, People’s Republic of China
Tel +86 57188122030
Email [email protected]
Guiping Chen
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310022, People’s Republic of China
Email [email protected]
Objective: Optimal approaches to patients with local recurrence of rectal cancer are unclear in China. This study aimed to evaluaty -30te the clinical outcomes and toxicity associated with different treatment regimens for patients with local recurrence of rectal cancer.
Methods: A retrospective chart review of patients with local recurrence of rectal cancer and previous radical surgical treatment between March 2010 and December 2017 with curative intent was performed. Disease-related endpoints included treatment progression-free survival (PFS) and overall survival (OS) using the Kaplan–Meier method. Toxicities were assessed using Common Terminology Criteria for Adverse Events, version 5.0, and complications were scored according to the Clavien-Dindo classification.
Results: A total of 71 patients met the inclusion criteria in this study. The recurrence sites were mainly local recurrence in the pelvic cavity and regional lymph node metastasis. Twenty patients received chemoradiotherapy combined with surgery, 10 underwent surgery alone, and others received chemoradiotherapy-alone (n = 27) and chemotherapy-alone (n = 14) treatment. A clear difference was found in PFS between surgery/chemoradiotherapy with surgery and chemoradiotherapy/chemotherapy groups (26.6 months vs 14.1 months, P = 0.033). The PFS of patients in the surgery combined with chemoradiotherapy, surgery alone, and chemotherapy/chemoradiotherapy groups was 65.2 months, 20.2 months, and 14.2 months, respectively (P = 0.042). The multivariate analysis of PFS demonstrated that surgery was an independent factor. The proportion of patients with distant metastases after chemoradiotherapy/chemotherapy was higher than that of patients undergoing surgery (36.6% vs 21.4%, P = 0.179). The OS of patients in the surgery combined with chemoradiotherapy, surgery alone, and chemotherapy/chemoradiotherapy groups was 89.4 months, 66.0 months, and 62.8 months, respectively (P = 0.189). Radiation treatment and surgery did not increase extra severe toxicities.
Conclusion: Surgery combined with chemoradiotherapy was a beneficial treatment mode for managing patients with locally recurrent, nonmetastatic rectal cancer. It was associated with better local disease control, no increase in toxicity, and prolonged survival among patients with locally recurrent rectal cancer.
Keywords: chemoradiotherapy, chemotherapy, locally recurrent rectal cancer, outcome, surgical treatment
Introduction
Despite total mesorectal excision (TME) and neoadjuvant radiotherapy and significant advances in the multidisciplinary management, local recurrence rates after radical surgery for rectal cancer have drastically decreased over the last decades. However, local recurrence of rectal cancer (LRRC) still occurs in 5–10% of these patients.1–3 The management of patients with LRRC is often complicated and requires multidisciplinary surgical and nonsurgical treatments.4 If microscopically complete (R0) resection is possible, the recommended therapeutic strategy in this cohort of patients is surgical resection with 5-year survival rates of about 50%.5,6
Radiotherapy for patients with LRRC may be an effective treatment. Nowadays, chemoradiation (CRT) combined with various anticancer drugs is used to improve treatment outcomes. In a study of unresectable T4 rectal cancer or local recurrence, 5-fluorouracil (5-FU)/leucovorin administered concurrently with radiation therapy was superior to radiation alone with regard to local control and overall survival (OS).7 Therefore, it is considered that CRT might provide better outcomes for the LRRC.
However, only a few previous studies have reported on the efficacy and outcomes of curative surgery combined with CRT. On the contrary, surgery is performed based on several factors for patients with LRRC: recurrence pattern, involvement of adjacent organs, medical unfitness, or patients’ refusal due to the considerable risk of morbidity and mortality. Management and choosing treatment modes for LRRC in the pelvic region remain a challenge and need further exploration.
Therefore, this retrospective study was performed to clarify the treatment outcomes in patients with LRRC in the pelvic region, and explore outcomes according to the different treatment modes.
Methods
Patients
A retrospective chart review of patients with LRRC and surgical treatment between March 2010 and December 2017 with curative intent was performed at Zhejiang Cancer Hospital. All patients enrolled in this study underwent curative resection for primary rectal cancer and were evaluated with abdominal computed tomography as part of routine follow-up after primary surgery. The criterion for pelvic local recurrence included presacral (posterior), anterior (central), lateral, inferior (perineal) anastomotic recurrence and (or) lymph node metastasis in the pelvic cavity. Patients with extensive distant metastatic disease were excluded from the study. Approval was obtained from the institutional review board at Zhejiang Cancer Hospital and was in accordance with the guidelines of the Helsinki Declaration (as revised in 2013). We only collect clinical data and prognosis of patients retrospectively and did not interfere with treatment, so Individual consent for this retrospective analysis was waived. In addition, our research data was confidential.
Treatment Procedure and Regimens
Surgical Treatment
Surgical treatment was considered feasible in patients with resectable pelvic local recurrence by a multidisciplinary team. The resection of rectal cancer after recurrence depended on the specific location of the recurrence to determine the surgical methods, mainly including laparoscopic Hartmann procedure, abdominoperineal resection (APR), pelvic tumor resection plus bilateral oophorectomy, pelvic lymphadenectomy, abdominoperineal resection combined with pelvic lymphadenectomy, and extended resection of the sacrococcygeal mass. Surgical complications were scored according to the Clavien-Dindo classification.8
Nonsurgical Treatment
Patients receiving nonsurgical regimens usually had an unresectable local recurrence or a poor clinical condition. No standard therapies regarding the choice of nonsurgical regimens were available. Nonsurgical treatment consisted of radiotherapy, chemotherapy, or chemoradiotherapy. Generally, patients with symptomatic pelvic local recurrence were treated with radiotherapy or chemoradiotherapy and those with asymptomatic unresectable pelvic local recurrence were treated with chemotherapy. The dose and fractioning of radiotherapy were based on the clinical judgment of the radiation oncologists, resulting in heterogeneity in radiotherapy management. Briefly, all patients received 25–28 times of 45–50.4 Gy radiation therapy (RT). Chemotherapy was mainly based on standard regimens and doses according to oncologists. Regimens included XELOX (capecitabine combined with oxaliplatin), FOLFOX (leucovorin, fluorouracil, and oxaliplatin), and FOLFIRI (leucovorin, fluorouracil, and irinotecan).
Assessment of Tumor Response
The assessment included tumor response, resection margin, disease-free survival, and progression-free survival (PFS), as well as toxicity and complications caused by the treatment regimen. Tumor responses were evaluated in accordance with the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1) to monitor objective tumor responses, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease. The disease control rate (DCR) was defined as the sum of the objective response and stabilization rates (CR + PR + SD). OS was calculated from the date of starting treatment until the date of death from any cause or censored at last follow-up. Local recurrence-free survival was calculated from the date of first surgery until the date of pelvic local recurrence detected by imaging or histology. Disease-free survival (DFS) was calculated from the date of second surgery to the date of local recurrence or distant metastases or censored at last follow-up or death. PFS was defined as the period from the date of treatment of recurrence to the date of disease progression evaluated using RECIST (version 1.1) or death. Survival and follow-up were calculated from the date of the diagnosis of the local recurrence of rectal cancer till death or the last follow-up. Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0),9 and complications were scored according to the Clavien-Dindo classification.8
Statistical Analysis
Continuous data were reported as median (interquartile range or 95% confidence interval), and categorical data were reported as count (percentage). Group comparisons were made using the chi-square or Mann–Whitney U-test as appropriate. Survival was calculated by the (reversed) Kaplan-Meier method, and comparisons were made using the Log rank test. Statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corp, NY, USA).
Results
Patient Characteristics
A total of 71 patients [38 male (53.5%) and 33 female (46.5%)] with rectal cancer were identified to have local recurrence after radical surgery. After the first surgery for the primary tumor, 71 patients were operated for rectal adenocarcinoma. According to the postoperative stage, 11 patients were in stage I, 27 were in stage II, and 33 patients were in stage III. The median age was 58 years (34–78 years) for patients who had a recurrence. The recurrence sites were mainly local recurrence in the pelvic cavity and regional lymph node metastasis.
After local recurrence, 71 patients were all treated with chemotherapy, chemoradiotherapy, or surgery. Treatment modes included chemotherapy alone, surgery alone, chemoradiotherapy combined with surgery, or chemoradiotherapy. The treatment modes were surgery/subsequent chemoradiotherapy with surgery (n = 30), chemoradiotherapy (n = 27), and chemotherapy (n = 14). In the surgery/subsequent chemoradiotherapy with surgery mode, 20 patients received chemoradiotherapy combined with surgery and surgery-alone treatment. Further, R0 resections were performed in 23 patients (76.7%), R1 resections in 5 patients (16.7%%), and R2 resections in 2 patients (6.7%). The characteristics of patients with recurrence according to the three treatment modes are presented in Table 1.
 |
Table 1 Clinical Characteristics of Patients with Local Recurrent Rectal Cancer According to Treatment Regimens After Recurrence |
Treatment Models After Local Recurrence and Efficacy
The median time from primary tumor resection to the diagnosis of local recurrence was 17.4 months (95% CI 15.2–19.6 months). Furthermore, 69% of patients developed local recurrence within 2 years and almost all (93.0%) patients within 5 years. DFS for the primary tumor stage from recurrence to first surgery was compared. The results showed that the DFS was 23.3 months, 16.8 months, and 17.2 months in stages I, II, and III, respectively (P = 0.199).
Patients with local recurrence were divided into three groups according to the treatment mode: surgery/subsequent chemoradiotherapy surgery, chemoradiotherapy-alone, and chemotherapy-alone groups. The overall response rate (ORR) and DCR were compared between chemoradiotherapy-alone (n = 27) and chemotherapy-alone groups (n = 14). The ORR in the two groups was 33.3% (9/27) and 35.7% (5/14), respectively (P = 1.000). The DCR in the two groups was 100%. No difference in mPFS was observed between the chemoradiotherapy-alone and chemotherapy-alone groups (16.9 months vs 10.0 months, P = 0.585). For surgery/subsequent chemoradiotherapy with surgery, the mPFS was 26.6 months. A clear difference in PFS was noted between groups with surgery/subsequent chemoradiotherapy with surgery versus chemoradiotherapy/chemotherapy (26.6 months vs 14.1 months, P = 0.033) (Figure 1A). The PFS in the three groups were compared. The results showed that the PFS was 26.6 months, 16.9 months, and 10.4 months, respectively (P = 0.118) (Figure 1B). The PFS of patients according to the treatment mode, including surgery combined with chemoradiotherapy, surgery alone, and chemotherapy/chemoradiotherapy, was 65.2 months, 20.2 months, and 14.2 months, respectively (P = 0.042) (Figure 1C). The results of multivariate analysis for PFS of patients with LRRC are shown in Figure 4. The results demonstrated that surgery was an independent factor influencing PFS for treatment of locally recurrent rectal cancer patients (P<0.001, 95% CI 0.08–0.63).
After these treatments, 19 patients (19/69, 27.5%) had disease control and 50 patients (50/69, 72.5%) had re-recurrence. Two patients lost to follow-up. The analysis of patterns of recurrence in patients with re-recurrence revealed that 29 patients (29/59, 42.0%) still had local recurrence and 21 (21/69, 30.4%) had metastasis. In the surgery/subsequent chemoradiotherapy with the surgery group, 10 patients (10/28, 35.7%) had disease control and 64.3% (18/28) patients had re-recurrence. Also, 12 patients (12/28, 42.9%) still had local recurrence and 6 (6/28, 21.4%) had metastasis. In the chemoradiotherapy/chemotherapy group, 9 patients (9/41, 22.0%) were stable and 32 patients (32/41, 78.0%) had re-recurrence. The proportion of distant metastases in patients receiving chemoradiotherapy/chemotherapy was higher than that in patients undergoing surgery, despite no statistically significant difference (36.6% vs 21.4%, P = 0.179).
Adverse Effects
In 30 patients undergoing surgery, postoperative complications were registered, and 9 (30%) patients experienced complications (Clavien-Dindo grades 1–3). The most common complications were wound infection (n = 4), urinary tract infections (n = 3), and presacral abscess (n = 2). Among nine patients with major postoperative complications, only one patient had Clavien-Dindo Grade 3 complications, requiring intensive care unit admission (wound infection). No in-hospital mortality was reported. The complications for surgically treated patients are shown in Table 2.
 |
Table 2 Surgical Complications for Surgically Treated Patients |
Data on toxicity caused by induction chemotherapy and chemoradiotherapy were available for 41 patients. Grade 3–4 toxicity was observed in seven of 40 patients (17.5%), including leukopenia, anemia, thrombocytopenia, and gastrointestinal reaction. No grade 5 toxicity was seen. Details on surgery with chemoradiotherapy toxicity were available for 25 patients. Grade 3–4 toxicity was reported in three of 25 patients (12.0%), and no grade 5 toxicity was seen. The types of toxicities are shown in Table 3.
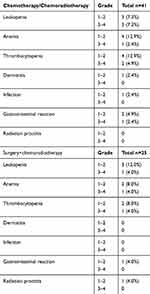 |
Table 3 Toxicities Graded by CTCAE (Version 5.0) in Patients Undergoing Chemotherapy and Chemoradiotherapy |
Follow-Up and Survival Analysis
In the follow-up, two patients were lost and 69 patients were evaluated. Furthermore, 24 patients survived, and 45 deaths (45/69, 65.2%) occurred. The median OS after the initial diagnosis of rectal cancer from the date of the diagnosis of the local recurrence of rectal cancer was 46.1 months (95% CI: 24.4–67.8). In analysis, patients in the surgery/subsequent chemoradiotherapy with surgery group had a prolonged median survival of 50.7 months compared with 32.0 months of the patients treated with chemoradiotherapy/chemotherapy, despite no statistically significant difference (P = 0.208) (Figure 2A). The OS of patients in the three groups (surgery/subsequent chemoradiotherapy with surgery vs chemoradiotherapy vs chemotherapy) was 50.7 months, 32.0 months, and 36.0 months, respectively (P = 0.452) (Figure 2B). The OS of patients with surgery combined with chemoradiotherapy, surgery alone, or chemotherapy/chemoradiotherapy was 64.4 months, 46.0 months, and 32.0 months, respectively (P = 0.282) (Figure 2C). Finally, the study also analyzed survival by resection margin and nonsurgical treatment. PFS for R0, R1, and R2 resection and nonsurgery was 35.8 months, 17.8 months, 10.0 months, and 14.2 months, respectively (P = 0.019) (Figure 3A). The OS of the four statuses was 64.4 months, 17.8 months, 10.0 months, and 32.0 months, respectively (P = 0.078) (Figure 3B).
 |
Figure 4 Multivariate analysis for progression free survival (PFS) in the rectal cancer with local recurrence. |
Discussion
This retrospective study was the largest report on patients with LRRC in China. It was novel in evaluating clinical outcomes of patients with locally recurrent rectal cancer treated with surgery/chemoradiotherapy with surgery and chemoradiotherapy/chemotherapy. Regarding clinical outcomes, chemoradiotherapy with surgery might be a favorable regimen for use after LRRC. In addition, surgery combined with chemoradiotherapy was safe, acceptable, and tolerable and could improve the quality of life.
Salvage surgical resection is the best option for long-term disease control in patients with local recurrence.10–12 Radiotherapy is also a salvage treatment for patients with LRRC.12–14 Hagemans et al conducted a retrospective study to evaluate the long-term outcomes of surgical and nonsurgical treatments of patients with LRRC.15 They demonstrated that the survival rates of patients undergoing surgery were significantly longer compared with the rates of patients receiving nonsurgical treatment. However, R2 resections did not result in a survival benefit compared with nonsurgical treatment in this series. These findings were similar to the results of the present study, which analyzed survival by resection margin and nonsurgical treatment. PFS and OS for R0 resection indicated the best prognosis; however, R2 resection was associated with a worse prognosis compared with nonsurgical treatment. This finding confirmed that the resection margin status for LRRC was an important prognostic factor, and not all patients with LRRC were eligible for curative surgery. In addition, Lee et al performed a systematic review to evaluate the efficacy of re-irradiation and determine an optimal treatment of LRRC.16 Re-irradiation with or without surgery for LRRC showed oncologic and palliative efficacy. Re-irradiation and surgery were associated with higher survival rates. However, reports on salvage surgery for LRRC in China are still relatively few. The present study evaluated the treatment outcomes in patients with LRRC in the pelvic region and explored differences in outcomes based on the combined regimens. The results showed that chemoradiotherapy combined with surgery might increase disease control and lead to a higher OS rate. These results were similar to previous findings. In addition, this study analyzed the patterns of recurrence in patients with re-recurrence. For chemoradiotherapy combined with surgery or surgery-alone regimen, 42.9% of patients still had local recurrence and 21.4% had metastasis. The proportion of distant metastases after chemoradiotherapy/chemotherapy was higher than that after surgery (36.6% vs 21.4%). The results showed that chemoradiotherapy combined with surgery or surgery alone was the best option for rectal cancer with local recurrence. The data showed that the prognosis of patients treated with chemoradiotherapy/chemotherapy combined with surgery was better. A previous study showed that the addition of induction or consolidation chemotherapy to standard neoadjuvant chemoradiotherapy resulted in a higher pCR rate for locally advanced rectal cancer.17 Similarly, neoadjuvant therapy can also be tried for patients with LRRC to increase the chances of surgery so that patients have a longer survival time. Neoadjuvant treatment can be considered first to increase the chance of surgery for a patient who cannot be operated on temporarily in the case of recurrence but resection is possible. This needs to be explored in the future. Of course, an appropriate treatment plan should be chosen depending on the individual situation of the patient.
In terms of quality of life, LRRC can cause severe impairment due to severe pain, obstruction, or bleeding. The risk of intestinal obstruction, perforation, and pain caused by metastatic lesions was reduced because the metastatic lesions were removed by surgery.18 Surgically treated patients had a better quality of life, and radiotherapy could relieve pain.19,20 On the contrary, whether treatment-related complications might lead to a decline in the quality of life and increase the risk of death should be considered. The study implied that the complications of the surgery itself were relatively small. Regarding the adverse reactions of radiotherapy and chemotherapy, hematological toxicity, and nonhematological toxicity of grade ≥3 did not increase. Therefore, the choice of chemoradiotherapy combined with surgical treatment was acceptable for patients with LRRC.
The present study had some limitations. The retrospective nature of this study might have influenced some results, such as treatments and response assessments. The choice of treatment consisting of radiotherapy, chemotherapy, or surgery was judged by oncologists because of the limitations of this retrospective study. The follow-up data of patients were limited because treatment was usually performed in the referring hospitals. Prospective studies with a larger sample size should be performed to explore the most suitable treatment options for patients with LRRC. Further, previous drugs and radiotherapy technologies might have some shortcomings, and hence new technologies are needed.
In conclusion, this study showed a better disease control and prolonged survival among patients with LRRC undergoing surgery combined with chemoradiotherapy. No additional adverse reactions occurred, and toxicities were tolerable. Future studies should identify patients who can benefit most from these local treatments. In addition, exploring the improvement in the quality of life in a larger cohort is necessary. Moreover, more convincing prospective studies are needed.
Disclosure
The authors declare no conflicts of interest.
References
1. Ikoma N, You YN, Bednarski BK, et al. Impact of recurrence and salvage surgery on survival after multidisciplinary treatment of rectal cancer. J Clin Oncol. 2017;35(23):2631–2638. doi:10.1200/JCO.2016.72.1464
2. Van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicenter, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi:10.1016/S1470-2045(11)70097-3
3. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi:10.1056/NEJMoa060829
4. Westberg K, Palmer G, Hjern F, et al. Management and prognosis of 373 locally recurrent rectal cancer – a national population-based study. Eur J Surg Oncol. 2018;44(1):100–107. doi:10.1016/j.ejso.2017.11.013
5. Ogunbiyi OA, McKenna K, Birnbaum EH, et al. Aggressive surgical management of recurrent rectal cancer – is it worthwhile? Dis Colon Rectum. 1997;40(2):150–155. doi:10.1007/BF02054979
6. Wanebo HJ, Koness RJ, Vezeridis MP, et al. Pelvic resection of recurrent rectal cancer. Ann Surg. 1994;220(4):586–597. doi:10.1097/00000658-199410000-00017
7. Braendengen M, Tveit KM, Berglund A, et al. Randomized Phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26(22):3687–3694. doi:10.1200/JCO.2007.15.3858
8. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934
9. Common terminology criteria for adverse events (CTCAE) v.5.0. National Cancer Institute; 2009. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
10. Caricato M, Borzomati D, Ausania F, et al. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32(2):126–132. doi:10.1016/j.ejso.2005.11.001
11. Saito N, K oda K, Takiguchi N, et al. Curative surgery for local pelvic recurrence of rectal cancer. Dig Surg. 2003;20(3):192–200. doi:10.1159/000070385
12. Ichihara M, Ikeda M, Uemura M, et al. Feasibility and safety of laparoscopic lateral pelvic lymph node dissection for locally recurrent rectal cancer and risk factors for re-recurrence. Asian J Endosc Surg. 2019. doi:10.1111/ases.12778
13. Tao R, Tsai CJ, Jensen G, et al. Hyperfractionated accelerated reirradiation for rectal cancer: an analysis of outcomes and toxicity. Radiother Oncol. 2017;122(1):146–151. doi:10.1016/j.radonc.2016.12.015
14. Susko M, Lee J, Salama J, et al. The use of re-irradiation in locally recurrent, non-metastatic rectal cancer. Ann Surg Oncol. 2016;23(11):3609–3615. doi:10.1245/s10434-016-5250-z
15. Hagemans JAW, van Rees JM, Alberda WJ, et al. Locally recurrent rectal cancer; long-term outcome of curative surgical and non-surgical treatment of 447 consecutive patients in a tertiary referral centre. Eur J Surg Oncol. 2020;46(3):448–454. doi:10.1016/j.ejso.2019.10.037
16. Lee J, Kim CY, Koom WS, Rim CH. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: a meta-analysis and systematic review. Radiother Oncol. 2019;140:10–19. doi:10.1016/j.radonc.2019.05.021
17. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–448. doi:10.1097/SLA.0000000000003471
18. Esnaola NF, Cantor SB, Johnson ML, et al. Pain and quality of life after treatment in patients with locally recurrent rectal cancer. J Clin Oncol. 2002;20(21):4361–4367. doi:10.1200/JCO.2002.02.121
19. Bouchard P, Efron J. Management of recurrent rectal cancer. Ann Surg Oncol. 2010;17(5):1343–1356. doi:10.1245/s10434-009-0861-2
20. Ronnekleiv-Kelly SM, Kennedy GD. Management of stage IV rectal cancer: palliative options. World J Gastroenterol. 2011;17(7):835–847. doi:10.3748/wjg.v17.i7.835
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



