Back to Journals » Infection and Drug Resistance » Volume 17
Clinical Outcomes and Risk Factors for Death in Critically Ill Patients with Carbapenem-Resistant Klebsiella pneumoniae Treated with Ceftazidime-Avibactam: A Retrospective Study
Authors Zhang L, Ma Y, Zhao C, Zhao S, Zhao L, Yang Y, Wang Y, Meng H , Sun J
Received 18 October 2023
Accepted for publication 19 January 2024
Published 25 January 2024 Volume 2024:17 Pages 239—248
DOI https://doi.org/10.2147/IDR.S445243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lingchun Zhang,1,* Yani Ma,2,* Chenglong Zhao,1 Shujuan Zhao,1 Lulu Zhao,3 Yuxin Yang,4 Yuhan Wang,5 Haiyang Meng,6 Jun Sun1
1Department of Pharmacy, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, People’s Hospital of Henan University, Zhengzhou, People’s Republic of China; 2Department of Pharmacy, the First Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 3Department of Pharmacy, Gongyi People’s Hospital, Zhengzhou, People’s Republic of China; 4Department of Pharmacy, Anyang Ophthalmic Hospital, Anyang, People’s Republic of China; 5Department of Pharmacy, Henan Integrative Medicine Hospital, Zhengzhou, People’s Republic of China; 6Department of Pharmacy, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Sun, Department of Pharmacy, Henan Provincial People’s Hospital, No. 7 Weiwu Road, Zhengzhou, People’s Republic of China, Tel +86 13683827203, Email [email protected]
Purpose: Carbapenem-Resistant Klebsiella pneumoniae (CRKP) is a significant public health threat, because it is associated with substantial morbidity and mortality. However, the risk factors associated with treatment failure of ceftazidime-avibactam (CAZ-AVI) and the need for CAZ-AVI-based combination remain unclear.
Methods: We conducted a retrospective study of critically ill patients (age: > 18 years) diagnosed with CRKP infections and treated with CAZ-AVI for at least 24 h between June 2020 and December 2022 at Henan Provincial People’s Hospital.
Results: This study included a total of 103 patients who received CAZ-AVI. Of these, 91 (88.3%) patients received the standard dosage of 2.5 g every q8h, while only 20 (19.4%) received monotherapy. The Kaplan–Meier curves showed that the all-cause 30-day mortality was significantly higher among patients who experienced septic shock than those who did not. There was no significant difference in mortality between monotherapy and combination therapy. Dose reduction of CAZ-AVI was associated with a significantly increased mortality rate. Independent risk factors for the 30-day mortality included higher APACHE II score (HR: 1.084, 95% CI: 1.024– 1.147, p = 0.005) and lower lymphocyte count (HR: 0.247, 95% CI: 0.093– 0.655, p = 0.005). Conversely, a combination therapy regimen containing carbapenems was associated with lower mortality (HR: 0.273, 95% CI: 0.086– 0.869, p = 0.028).
Conclusion: Our study suggests that CAZ-AVI provides clinical benefits in terms of survival and clinical response in critically ill patients with CRKP infection. A higher APACHE II score and lower lymphocyte count were associated with 30-day mortality, while the combination therapy regimen containing carbapenems was the only protective factor. CAZ-AVI dose reduction was associated with an increased mortality rate. Futher large-scale studies are needed to validate these findings.
Keywords: carbapenem-resistant Klebsiella pneumoniae, ceftazidime-avibactam, retrospective study, combination therapy
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE), particularly Carbapenem-Resistant Klebsiella pneumoniae (CRKP), pose a significant public health threat because of their contribution to substantial morbidity and mortality.1,2 Data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) showed a progressive increase in the proportion of CRKP isolates from clinical specimens, increasing from 2.2% in 2009 to 19.4% in 2012.3 Similarly, in China, CRKP has emerged as a major concern, with K. pneumoniae resistance to imipenem swiftly escalating from 15.6% in 2015 to 29% in 2023, according to information from the China Antimicrobial Surveillance Network (CHINET, www.chinets.com). However, treating CRKP infections in developing countries presents significant challenges owing to limited access to new therapeutic agents. Ceftazidime-avibactam (CAZ-AVI), a novel β-lactam/β-lactamase inhibitor combination, was approved for use in China in September 2019.4 Avibactam is a non-β-lactam, β-lactamase inhibitor, which exhibits activity against Ambler class A (eg, extended-spectrum-β-lactamases [ESBLs], K. pneumoniae carbapenemases [KPCs]) and class C (AmpC) and some class D(OXA) enzymes.5,6 In China, CAZ-AVI has been approved for the treatment of complicated intra-abdominal infection (cIAI), hospital-acquired pneumonia (HAP), and other infections caused by multidrug-resistant gram-negative organisms in adult patients with limited treatment options.
Several studies have demonstrated that CAZ-AVI is more effective against CRKP than polymyxins and tigecycline.7–10 However, the risk factors associated with treatment failure of CAZ-AVI remain unclear. Additionally, previous in vitro studies have shown that CAZ-AVI, when combined with other agents, exhibits high synergistic activity against CRKP.11–13 Nonetheless, it is currently unclear whether there is a benefit from combined medication. Hence, we conducted a retrospective study to evaluate the outcomes and risk factors for death in patients with severe CRKP infections treated with CAZ-AVI, as well as the effects of combined therapy.
Materials and Methods
Patients
This study was conducted from June 2020 to December 2022 at Henan Provincial People’s Hospital, a central China tertiary care hospital with 5000 beds. Critically ill patients diagnosed with CRKP infections, aged >18 years, and treated with CAZ-AVI for at least 24 h were included in the study. Medical ethics approval was granted by the medical ethics committee of Henan Provincial People’s Hospital. As patient data were analyzed anonymously and patient confidentially maintained, the need for patient consent was waived. This study was performed in accordance with the tenets of Declaration of Helsinki.
Clinical Data and Outcomes
Demographic information, comorbidities, laboratory data, microbiological data, treatment regimen, and infection outcomes were collected from the hospital’s electronic medical records system by clinical pharmacists. Patients received either a standard dose or a reduced dose based on their level of renal impairment. For patients with moderate-to-severe renal impairment (eCrCL ≤ 50 mL/min), the dosage of CAZ-AVI was based according to the drug’s instructions. CRKP infection was defined as a positive clinical specimen together with signs and symptoms of infection in a patient. Clinical response was defined as a documented improvement in the signs and symptoms of infection as reported by clinicians.14 Microbiologic eradication was defined as a negative culture after CAZ-AVI therapy, when repeat cultures were available.15 The primary outcome was 30-day mortality following the initiation of CAZ-AVI treatment.
Microbiology
CRKP was defined as bacteria that tested resistant to any carbapenem (meropenem, ertapenem, or imipenem) or were positive for carbapenemase production.16 The Vitek 2 system (bioMérieux, Marcy l’Étoile, France) and the Phoenix100 automated system (Becton Dickinson Co., Sparks, MD, USA) were used for identification of CRKP isolates. Matrix-assisted lasers desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (Bruker Corporation, Karlsruhe, Germany) was used to confirm the identity of the isolate. Minimum inhibitory concentration (MIC) values for antimicrobial agents were determined by an automated broth microdilution method (Becton Dickinson Co., Sparks, MD, USA). Antibiotic susceptibility testing results were compared using the agar disk diffusion method (Becton Dickinson Co.), in accordance with the Clinical and Laboratory Standards Institute (CLSI) breakpoints.
Statistical Analysis
Categorical variables were expressed as frequencies (percentages) and evaluated with the chi-squared test or two-tailed Fisher’s exact test. Non-normally distributed continuous variables were expressed as the median with interquartile range (IQR) and evaluated with the Mann–Whitney non-parametric test. Multivariate Cox regression analysis was used to identify independent risk factors for 30-day mortality. Hazard ratios (HR) and 95% confidence intervals were calculated for all associations. All statistical analyses were carried out with SPSS software (version 25.0; IBM Corporation, Armonk, NY, USA) and Prism 7.0 (GraphPad) software. A two-sided P-value <0.05 was considered to indicate statistically significant differences.
Results
Patient Characteristics
A total of 103 patients (median age: 61 years; 74.8% male) who received CAZ-AVI were included in this study. The most common comorbidity was hypertension (n = 43, 41.7%), followed by cardiovascular disease (n = 27, 26.2%), and diabetes mellitus (n = 25, 24.3%). A total of 41 patients (39.8%) were admitted to the ICU, and 68 patients (66%) had previous exposure to carbapenems within 3 months before infection. Pneumonia was the most prevalent type of infection, accounting for 84.5% of all cases, followed by bloodstream infections, which accounted for 40.8% cases. Of the 103 patients, only 11 isolates showed carbapenemase production, whereas all the isolates showed serine β-lactamases (SBL) production. More than 90% of the patients were admitted to the ICU during their hospitalization. Among them, 83 patients received mechanical ventilation, and 21 patients received continuous renal replacement therapy. The median APACHE II score was 16 (Table 1).
 |
Table 1 Characteristics of Patients Treated with CAZ-AVI* |
Microbiological Characteristics
Table 2 summarizes the results of drug sensitivity testing for 92 CRKP strains. All strains were susceptible to polymyxin and CAZ-AVI. Of the isolates tested, 95.6% (87/91) were susceptible to tigecycline, followed by TMP-SMX (33.7%) and amikacin (27.2%). Most strains showed resistance to imipenem (92.4%), meropenem (93.5%), and ciprofloxacin (96.7%).
 |
Table 2 Antibiotic Susceptibility Results for CRKP Isolates |
Treatment and Outcomes
Among the patients who received treatment with CAZ-AVI, 91 (88.3%) received the standard dosage of 2.5 g every q8h, and only 20 (19.4%) received monotherapy. The most commonly used drugs in combination were carbapenems and tigecycline, followed by polymyxin B. The overall clinical response rate was 62.1% and the 30-day mortality rate was 28.2%, with a significant difference in the 30-day mortality rate between septic patients (shocked and non-shocked) (Table 1). The Kaplan–Meier curves showed that all-cause 30-day mortality was significantly higher in septic shock patients than non-septic shock patients (Figure 1A). No difference in mortality was found between monotherapy and combination therapy (Figure 1B). With regard to CAZ-AVI dosage, dose reduction significantly increased mortality (Figure 1C). Receiver operating characteristic (ROC) analysis showed that lymphocyte count had an area under the curve of 0.71, an optimal cut-off value of 0.52, with a sensitivity of 69%, and specificity of 75.7% (Figure 2).
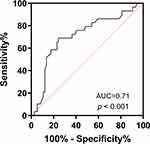 |
Figure 2 ROC curve of lymphocyte count. |
Risk Factors for 30-Day Mortality
In the multivariable analysis, the independent risk factor for 30-day mortality included higher APACHE II score (HR: 1.084, 95% CI: 1.024–1.147, p = 0.005) and lower lymphocyte count (HR: 0.247, 95% CI: 0.093–0.655, p = 0.005). Conversely, the combination therapy regimen containing carbapenems was related to low mortality (HR: 0.273, 95% CI: 0.086–0.869, p = 0.028) (Table 3).
 |
Table 3 Multivariable Cox Regression of Risk Factors for 30-Day Mortality |
Discussion
In this study, we evaluated the outcomes and risk factors associated with 30-day mortality in 103 critically ill patients who received CAZ-AVI for CRKP. Previous studies have reported a mortality rate of 8–37.5% among patients treated by CAZ-AVI for CRKP.8,17–20 In this study, we report a 30-day mortality rate of 28.2%. The difference may be because of the patients’ age, site of infection, and severity of disease. In our study, >90% patients were admitted to the ICU, and almost half of the patients had septic shock. Besides, we found a higher mortality in patients with septic shock, which is consistent with previous reports.16,21
Severity of illness of CRKP in most studies was assessed with the APACHE II scoring system at infection onset.22–27 A meta-analysis conducted by Qian et al reported that the average APACHE II score at the time of diagnosis of CRKP infection was considerably higher in patients who did not survive than in those who survived.28 Zheng et al identified that a higher APACHE II score was a risk factor for mortality.27 Similarly, in our study, a higher APACHE II score was independently associated with increased mortality. Hence, APACHE II score is a crucial and useful scoring system for clinicians to evaluate the severity of diseases and predict the outcome of patients with CRKP.
Among carbapenem-resistant Enterobacteriaceae-infected patients, previous studies revealed that higher mortality has observed in immunosuppressed patients, including the presence of hematological malignancies and immunosuppressive therapy.29,30 This implies that the patient’s immune function may play a crucial role in carbapenem-resistant infection. Lin et al reported that impaired lymphocyte function was a critical factor in influencing individual outcomes in patients with carbapenem-resistant infection.31 Cheng et al reported that the CD4+CD28+ T cell count was significantly lower in CRE than non-CRE septic patients and a lower cell count was significantly associated with a higher 28-day mortality.32 In our study, lower lymphocyte count was independently associated with increased mortality, which indicated that lymphocyte function might be a useful marker for early diagnosis of CRKP infection and outcome prediction.
We found combination therapy regimen containing carbapenems significantly reduced mortality; this finding was consistent with the previous study by Zheng et al.27 Subgroup analysis in their study showed that carbapenems were recognized as effective concomitant agents to decrease the 30-day mortality. This might be because of the synergistic effect of CAZ-AVI and carbapenems in vitro.11,33 In our study, we found CAZ-AVI dose reduction significantly increased mortality. This finding was consistent with those reported in Phase III pivotal trials of CAZ-AVI, wherein a significantly lower clinical cure rate was reported in patients treated with CAZ-AVI exhibiting moderate renal impairment than those treated with meropenem.34 Additionally, a meta-analysis conducted by Gatti et al revealed that renal dosing adjustments of CAZ-AVI were associated with a higher risk of mortality.35 This could be because implementing recommended dosing adjustments in renal patients may result in suboptimal pharmacokinetic/pharmacodynamic (PK/PD) targets, thereby increasing the risk of clinical failure in patients receiving CAZ-AVI. It should be noted that these findings require confirmation through larger studies in the future to further validate their implications.
This study has some limitations. First, it was a single-center study and the antibiotic susceptibility and treatment regimen of CRKP in other regions and populations may not be consistent. Second, we used retrospective data that could not fully record clinical variables. Third, this study focused on ICU patients, and the conclusions may therefore not be applicable to other patients.
Conclusion
This study identified the clinical outcomes and risk factors associated with 30-day mortality in critically ill patients with CRKP infection who received CAZ-AVI treatment. Our study suggests CAZ-AVI provides clinically benefits in terms of survival and clinical response in patients with CRKP infection. A higher APACHE II score, and lower lymphocyte count were associated with the 30-day mortality, while the combination therapy regimen containing carbapenems was the only protective factor. Compared to the full dose, a reduced dose of CAZ-AVI was associated with an increased mortality rate. These findings may help clinicians treat CRKP infections and reduce mortality in the future.
Abbreviations
CLSI, Clinical and Laboratory Standards Institute; CRE, Carbapenem-resistant Enterobacteriaceae; CRKP, Carbapenem-Resistant Klebsiella pneumoniae; HAP, Hospital-acquired pneumonia; HR, Hazard ratios; MIC, Minimum inhibitory concentration; ROC, Receiver operating characteristic; APACHE, Acute Physiology and Chronic Health Evaluation; IQR, Interquartile range.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval and Informed Consent
This study was conducted following the Declaration of Helsinki and obtained approval from the clinical research ethics committee of Henan Provincial People’s Hospital, and the need for obtaining written informed consent was waived due to the retrospective nature of this study.
Author Contributions
All authors made a significant contribution to the work reported in the conception, study design, execution, acquisition of data, analysis, and interpretation. All authors took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, and have agreed on the journal to which the article has been submitted. All authors agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mohd Asri NA, Ahmad S, Mohamud R, et al. Global prevalence of nosocomial multidrug-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. Antibiotics. 2021;10(12):1508. doi:10.3390/antibiotics10121508
2. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi:10.1093/infdis/jiw282
3. Sisto A, D’Ancona F, Meledandri M, et al. Carbapenem non-susceptible Klebsiella pneumoniae from micronet network hospitals, Italy, 2009 to 2012. Euro Surveill. 2012;17(33):20247. doi:10.2807/ese.17.33.20247-en
4. Wang Q, Xu P, Zhou Y. Analysis of the clinical application of ceftazidime-avibactam in China. J Infect Public Health. 2022;15(4):455–459. doi:10.1016/j.jiph.2022.02.003
5. Falcone M, Paterson D. Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections. J Antimicrob Chemother. 2016;71(10):2713–2722. doi:10.1093/jac/dkw239
6. Shirley M. Ceftazidime-avibactam: a review in the treatment of serious Gram-negative bacterial infections. Drugs. 2018;78(6):675–692. doi:10.1007/s40265-018-0902-x
7. Shi Y, Hu J, Liu P, et al. Ceftazidime-avibactam-based versus tigecycline-based regimen for the treatment of carbapenem-resistant Klebsiella pneumoniae-induced pneumonia in critically ill patients. Infect Dis Ther. 2021;10(4):2721–2734. doi:10.1007/s40121-021-00542-3
8. van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi:10.1093/cid/cix783
9. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883–17. doi:10.1128/AAC.00883-17
10. Fang J, Li H, Zhang M, et al. Efficacy of ceftazidime-avibactam versus polymyxin B and risk factors affecting clinical outcomes in patients with carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. 2021;12:780940. doi:10.3389/fphar.2021.780940
11. Gaibani P, Lewis RE, Volpe SL, et al. In vitro interaction of ceftazidime-avibactam in combination with different antimicrobials against kpc-producing Klebsiella pneumoniae clinical isolates. Int J Infect Dis. 2017;65:1–3. doi:10.1016/j.ijid.2017.09.017
12. Jayol A, Nordmann P, Poirel L, Dubois V. Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(2):542–544. doi:10.1093/jac/dkx393
13. Wang F, Zhou Q, Yang X, Bai Y, Cui J. Evaluation of ceftazidime/avibactam alone and in combination with amikacin, colistin and tigecycline against Klebsiella pneumoniae carbapenemase-producing k. Pneumoniae by in vitro time-kill experiment. PLoS One. 2021;16(10):56.
14. Meng H, Zhao Y, An Q, Zhu B, Cao Z, Lu J. Use of ceftazidime-avibactam for suspected or confirmed carbapenem-resistant organisms in children: a retrospective study. Infect Drug Resist. 2023;16:5815–5824. doi:10.2147/IDR.S426326
15. Meng H, Yang J, Niu M, Zhu H, Zhou Y, Lu J. Risk factors and clinical outcomes of carbapenem-resistant Klebsiella pneumoniae bacteraemia in children: a retrospective study. Int J Antimicrob Agents. 2023;62(4):106933. doi:10.1016/j.ijantimicag.2023.106933
16. Lu J, Zhang A, Han L, et al. Clinical outcomes and risk factors for death following carbapenem-resistant Klebsiella pneumoniae infection in solid organ transplant recipients. Microbiol Spectr. 2023;11(1):e0475522. doi:10.1128/spectrum.04755-22
17. Falcone M, Daikos GL, Tiseo G, et al. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-beta-lactamase-producing Enterobacterales. Clin Infect Dis. 2021;72(11):1871–1878. doi:10.1093/cid/ciaa586
18. Hakeam HA, Alsahli H, Albabtain L, Alassaf S, Al Duhailib Z, Althawadi S. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1–7. doi:10.1016/j.ijid.2021.05.079
19. Caston JJ, Cano A, Perez-Camacho I, et al. Impact of ceftazidime/avibactam versus best available therapy on mortality from infections caused by carbapenemase-producing Enterobacterales (cavicor study). J Antimicrob Chemother. 2022;77(5):1452–1460. doi:10.1093/jac/dkac049
20. Sree RA, Gupta A, Gupta N, et al. Ceftazidime-avibactam alone or in combination with aztreonam versus polymyxins in the management of carbapenem-resistant Klebsiella pneumoniae nosocomial infections (CAPRI study): a retrospective cohort study from south India. Infection. 2023. doi:10.1007/s15010-023-02094-9
21. Li J, Ren J, Wang W, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis. 2018;37(4):679–689. doi:10.1007/s10096-017-3160-z
22. Brescini L, Morroni G, Valeriani C, et al. Clinical and epidemiological characteristics of kpc-producing Klebsiella pneumoniae from bloodstream infections in a tertiary referral center in Italy. BMC Infect Dis. 2019;19(1):611. doi:10.1186/s12879-019-4268-9
23. Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis. 2017;36(7):1125–1131. doi:10.1007/s10096-017-2899-6
24. Su CF, Chuang C, Lin YT, et al. Treatment outcome of non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae infections: a multicenter study in Taiwan. Eur J Clin Microbiol Infect Dis. 2018;37(4):651–659. doi:10.1007/s10096-017-3156-8
25. Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by kpc-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–2143. doi:10.1093/jac/dkv086
26. Luan YY, Chen YH, Li X, et al. Clinical characteristics and risk factors for critically ill patients with carbapenem-resistant Klebsiella pneumoniae (CRKP): a cohort study from developing country. Infect Drug Resist. 2021;14:5555–5562. doi:10.2147/IDR.S343489
27. Zheng G, Zhang J, Wang B, et al. Ceftazidime-avibactam in combination with in vitro non-susceptible antimicrobials versus ceftazidime-avibactam in monotherapy in critically ill patients with carbapenem-resistant Klebsiella pneumoniae infection: a retrospective cohort study. Infect Dis Ther. 2021;10(3):1699–1713. doi:10.1007/s40121-021-00479-7
28. Qian Y, Bi Y, Liu S, Li X, Dong S, Ju M. Predictors of mortality in patients with carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis and a systematic review. Ann Palliat Med. 2021;10(7):7340–7350. doi:10.21037/apm-21-338
29. Wang Q, Zhang Y, Yao X, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis. 2016;35(10):1679–1689. doi:10.1007/s10096-016-2710-0
30. Meng H, Han L, Niu M, et al. Risk factors for mortality and outcomes in hematological malignancy patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Infect Drug Resist. 2022;15:4241–4251. doi:10.2147/IDR.S374904
31. Lin Q, Wang Y, Luo Y, et al. The effect of host immunity on predicting the mortality of carbapenem-resistant organism infection. Front Cell Infect Microbiol. 2020;10:480. doi:10.3389/fcimb.2020.00480
32. Cheng W, Wang H, Zhang J, et al. Lymphocyte subset counts as diagnostic and prognostic markers for carbapenem-resistant Enterobacteriaceae (CRE) infection in critically ill patients. Int J Infect Dis. 2020;96:315–322. doi:10.1016/j.ijid.2020.04.072
33. Kuai J, Zhang Y, Lu B, et al. In vitro synergistic activity of ceftazidime-avibactam in combination with aztreonam or meropenem against clinical Enterobacterales producing bla(KPC) or bla(NDM). Infect Drug Resist. 2023;16:3171–3182. doi:10.2147/IDR.S408228
34. Li J, Lovern M, Riccobene T, et al. Considerations in the selection of renal dosage adjustments for patients with serious infections and lessons learned from the development of ceftazidime-avibactam. Antimicrob Agents Chemother. 2020;64(4). doi:10.1128/AAC.02105-19
35. Gatti M, Fornaro G, Viale P, Pea F, Giannella M. Clinical efficacy of renal dosing adjustments of ceftazidime-avibactam in patients affected by carbapenem-resistant Gram-negative infections: a systematic review and meta-analysis of observational studies. Br J Clin Pharmacol. 2023;89(2):617–629. doi:10.1111/bcp.15586
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

