Back to Journals » Cancer Management and Research » Volume 14
Clinical Outcomes and Prognostic Factors in Soft Tissue Sarcoma Patients After Unplanned Excision
Authors Takemori T, Kawamoto T , Hara H, Fukase N, Fujiwara S, Kitayama K, Yahiro S, Miyamoto T, Mifune Y, Hoshino Y, Kakutani K, Matsumoto T, Matsushita T , Niikura T, Kuroda R, Akisue T
Received 10 March 2022
Accepted for publication 9 May 2022
Published 25 May 2022 Volume 2022:14 Pages 1815—1824
DOI https://doi.org/10.2147/CMAR.S364912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Toshiyuki Takemori,1 Teruya Kawamoto,1,2 Hitomi Hara,1 Naomasa Fukase,1 Shuichi Fujiwara,1 Kazumichi Kitayama,1 Shunsuke Yahiro,1 Tomohiro Miyamoto,1 Yutaka Mifune,1 Yuichi Hoshino,1 Kenichiro Kakutani,1 Tomoyuki Matsumoto,1 Takehiko Matsushita,1 Takahiro Niikura,1 Ryosuke Kuroda,1 Toshihiro Akisue1,3
1Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine, Kobe, Japan; 2Division of Orthopaedic Surgery, Kobe University Hospital International Clinical Cancer Research Center, Kobe, Japan; 3Department of Rehabilitation Science, Kobe University Graduate School of Health Sciences, Kobe, Japan
Correspondence: Teruya Kawamoto, Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine, 7-5-1, Kusunoki-cho, Chuo-ku, Kobe, 650-0017, Japan, Tel +81-783825985, Fax +81-783516944, Email [email protected]
Purpose: Soft tissue sarcomas (STSs) constitute a group of rare, heterogeneous tumors representing approximately 1% of all cancers. Owing to the rarity and pathological diversity of the disease, unplanned excision (UE) has often been performed for STS, resulting in an unfavorable prognosis. This study aimed to clarify clinical outcomes and prognostic factors in STS patients who underwent UE.
Patients and Methods: In a retrospective review of the medical records of patients with STS who underwent surgery at our institution between 1999 and 2015, patients were enrolled to either a UE group or a planned excision (PE) group. An analysis was then conducted to identify factors associated with prognosis after UE.
Results: Of 134 patients undergoing surgery for STS, 110 were enrolled to the PE group and 24 to the UE group. The median size of the primary tumor was significantly smaller, and more lesions were located in the superficial layer in the UE group than in the PE group. In addition, plastic reconstruction after additional radical resection was required significantly more often in the UE group than in the PE group. No significant difference in overall survival, local recurrence-free survival, or disease-free survival (DFS) between the UE and PE groups was observed; however, metastasis-free survival was significantly better in the UE group. In the UE group, poorer DFS was associated with older age (≥ 61 years) and a larger primary tumor (≥ 2.9 cm).
Conclusion: A prognosis similar to that in patients undergoing PE could be achieved by appropriate additional surgeries in patients initially undergoing UE. However, UE for STS should be avoided, especially in older patients and those with a larger primary tumor.
Keywords: unplanned excision, soft tissue sarcoma, survival, prognostic factor
Introduction
Soft tissue sarcomas (STSs) are rare malignant tumors, representing approximately 1% of cancers in the adult population.1 A soft tissue lesion larger than 5 cm in size or located in the deep layer can be suspected to be an STS, while a superficial lesion smaller than 3 cm is typically considered a benign tumor. Unplanned excision (UE), defined as tumor resection without appropriate preoperative diagnostic evaluation, has often been performed for smaller STS, especially those located in the superficial layer. The proportion of patients undergoing UE has slowly declined since the start of the 2000s; however, such patients still account for approximately 10% of all STS cases.2 Because UE for STS often results in inadequate margins of excision, the presence of residual tumor cells after UE has been reported to be as high as 83%3 and has been associated with poor prognosis.3–6 Moreover, achieving curative resection after UE could require much more extensive surgery, including amputation or plastic reconstruction such as skin grafting and musculocutaneous flaps for soft tissue coverage, because determining safe surgical margins can often be more complicated after a UE than after a planned excision (PE) by trained orthopedic oncologists.3,7–9 Nevertheless, several studies reported that the prognosis achieved in patients who undergo appropriate additional treatments after UE can be similar to that achieved in PE patients.3,6,7,10 However, factors affecting prognosis after UE have not been identified.
Herein, we aimed to clarify clinical outcomes and prognostic factors by comparing STS patients who underwent additional surgeries after UE with those who underwent PE.
Patients and Methods
Ethics Considerations
This retrospective review was approved by the Ethics Committee of Kobe University Hospital (no. B210126). Informed consent was obtained using an opt-out system. Patients who chose not to participate were excluded. All the processes of research were performed and secured in accordance with the relevant guidelines and regulations of Declaration of Helsinki.
Patients
We retrospectively reviewed the clinical records of 134 patients with STS who underwent surgery at our institution between January 1999 and December 2015. Patients with inadequate clinical records and/or who received any neo-adjuvant treatments were excluded from the study. All patients underwent definitive resection, with no distant metastasis detected before surgery and were followed for at least 5 years or until death, if that occurred within 5 years after their surgery at our institution.
Study Design
For the analysis, the patients were enrolled to either the PE or the UE group. The 110 patients in the PE group visited or were referred before any surgical treatment and underwent primary surgery after appropriate preoperative evaluations at our institution. The 24 patients in the UE group were referred after undergoing a UE for STS; they subsequently underwent a definitive resection. The UEs were performed by general surgeons, plastic surgeons, orthopedic surgeons, or dermatologists at a previous institution. All patients in the UE group underwent an additional resection to obtain negative surgical margins around the lesion. All lesions were diagnosed by pathology examination, and in the UE group, the specimen excised at the previous institution was reviewed and confirmed by pathologists at our institution.
The information collected from each patient’s medical records included age at the time of primary tumor diagnosis, sex, primary tumor size, primary tumor location and depth, histologic type of the primary tumor, follow-up period, detailed treatment information, local recurrence, distant metastasis, and status at last follow-up. Before the primary or additional resection, the tumor size was determined from magnetic resonance imaging (MRI) by measuring the largest diameter of the lesion on any axis. Tumor depth was classified as “superficial” when the lesion was located between the skin and the fascia, and “deep” when it was located deeper than the fascia. The indications for adjuvant chemotherapy and/or radiotherapy were determined during an institutional multidisciplinary discussion for each patient. In the UE group, contrast-enhanced MRI was performed before the additional surgery to evaluate any residual tumor, hematoma, and/or edema remaining from the UE. Local recurrence-free survival (LRFS) was defined as the period from the date of surgery at our institution to the date of any recurrence at or near the primary tumor location. Metastasis-free survival (MFS) was defined as the period from the date of surgery at our institution to the date of distant metastasis at any site. Disease-free survival (DFS) was defined as the period from the date of surgery at our institution to the date of local recurrence or distant metastasis at any site.
Statistical Analysis
Differences between the groups were evaluated using the Mann–Whitney U-test and the chi-squared test. OS, LRFS, MFS, and DFS were estimated using the Kaplan–Meier method, and the Log rank test was used to assess differences in survival.11 Validation of the optimal cut-off value was calculated by a receiver operating characteristic curve analysis. Differences and correlations were considered statistically significant at P < 0.05. All statistical analyses were performed using EZR (version 1.53: Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).12 More precisely, EZR is a modified version of R Commander, designed to add statistical functions that are frequently used in biostatistics.
Results
Clinical Characteristics of the Patients and Primary Tumors
Table 1 shows the patient characteristics of the PE (52 men, 58 women) and UE (17 men, seven women) groups. Median age at the time of primary tumor diagnosis was 58 years (range: 0.1–91 years) in the PE group and 61 years (range: 30–88 years) in the UE group. Median follow-up was 89.5 months (range: 1–258 months) in the PE group and 95.5 months (range: 10–258 months) in the UE group. No significant difference in either age at the time of primary tumor diagnosis (P = 0.183) or follow-up duration (P = 0.405) was observed.
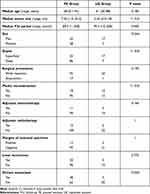 |
Table 1 Clinical Characteristics of the PE and the UE Patients |
Median primary tumor size was significantly smaller in the UE group than in the PE group (3.55 cm vs 7.50 cm, P < 0.01). The lesions were located in the superficial layer more often in the UE group than in the PE group (70.8% vs 21.8%, P < 0.01).
Regarding surgery, the proportion of amputations was comparable in both groups (UE, 4.2% vs PE, 15.5%; P = 0.195); however, plastic reconstruction was required in significantly more cases in the UE group than in the PE group (50.0% vs 16.4%, P < 0.01). No significant difference between the groups in the requirement for adjuvant chemotherapy and radiotherapy was observed.
The histologic diagnoses of the primary tumors were similar in both groups (Table 2). The most common subtype in the UE group was myxofibrosarcoma (six patients, 25.0%), followed by undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (five patients, 20.8%), leiomyosarcoma (four patients, 16.7%), and myxoid liposarcoma (four patients, 16.7%). The primary tumor sites in the PE and UE groups are listed in Table 3. The most common primary tumor site in the UE group was the thigh (eight patients, 33.3%), followed by the lower leg (six patients, 25.0%) and the trunk (five patients, 20.8%).
 |
Table 2 Histological Subtypes of the Primary Tumor in the PE and the UE Groups |
 |
Table 3 Primary Tumor Site in the PE and the UE Groups |
Prognosis in the PE and UE Groups
The OS, LRFS, MFS, and DFS are shown in Figure 1. Five-year OS (Figure 1A), LRFS (Figure 1B), and DFS (Figure 1D) were, respectively, 77.9%, 81.2%, and 57.3% in the PE group and 83.3%, 78.1%, and 74.2% in the UE group. No significant differences in OS (P = 0.385), LRFS (P = 0.953), and DFS (P = 0.155) between the groups were observed. The 5-year MFS rates were 63.3% and 83.3% in the PE and UE groups, respectively, a significant difference favoring the UE group (P = 0.021, Figure 1C).
Local recurrence was experienced by 20 patients (18.2%) in the PE group at a median duration of 33.5 months from the date of surgery at our institution (range: 2–246 months) and by five patients (20.8%) in the UE group at a median duration of 24.0 months (range: 9–59 months; Table 1). Neither the rate of local recurrence (P = 0.775) or nor the duration to local recurrence (P = 0.513) was significantly different between the two groups. Distant metastasis occurred in 48 patients (43.6%) in the PE group and in four patients (16.7%) in the UE group at median durations of 24.5 months (range: 1–179 months) and 9.5 months (range: 1–32 months) respectively. Distant metastasis occurred in significantly more patients in the PE group than in the UE group (P = 0.020, Table 1), but with no significant difference in the duration from the date of diagnosis to the date of distant metastasis (P = 0.220). After surgery at our institution, 33 patients (30.0%) in the PE group and five (20.8%) in the UE group died of their disease at medians of 30.0 months (range: 2–161 months) and 23.0 months (range: 10–91 months) respectively. Neither the number of deaths (P = 0.459) nor the time to death from the date of surgery at our institution (P = 0.800) was significantly different between the groups. Analyses of cases involving tumors located in the superficial layer showed no significant differences between the groups in OS (P = 0.750), LRFS (P = 0.613), MFS (P = 0.484), and DFS (P = 0.324). Thus, when an appropriate additional surgery follows a UE, the patient’s prognosis could be similar to that in patients undergoing PE.
Clinical Outcomes and Prognostic Factors After UE
Among the 24 additionally resected specimens after UE at our institution, positive margins and residual tumor cells were observed in three specimens (12.5%) and 15 specimens (62.5%), respectively. Local recurrence was observed in two of the three patients with positive margins in the additionally resected specimen (66.7%) and in three of the 21 patients with negative margins in the specimen (14.3%, P = 0.018).
The receiver operating characteristic curve analyses of the relationship between DFS and age at the time of primary tumor diagnosis or primary tumor size (Figure 2) revealed that 61 years and 2.9 cm were optimal cut-off values for age at the time of primary tumor diagnosis (n = 24; area under the curve: 0.703; sensitivity: 87.5%; specificity: 56.2%; 95% confidence interval [CI] 0.468–0.92) and primary tumor size (n = 24; area under the curve: 0.874; sensitivity: 100%; specificity: 43.8%; 95% CI: 0.731–1). In the UE group, patients 61 years and older at the primary tumor diagnosis and/or with a primary tumor 2.9 cm or greater in size had a significantly poorer DFS (Figure 3). Univariate analyses revealed that the factors prognostic for DFS in the UE group were age at primary tumor diagnosis, primary tumor size, and surgical procedure (Table 4).
 |
Table 4 Univariate Analyses for DFS in the UE Group |
Discussion
UE for STS is defined as tumor resection without appropriate preoperative evaluation, including radiographic and/or histologic examinations. Soft tissue tumors are rare tumors with a wide variety of histologic subtypes, and most are benign lesions.1 Generally, soft tissue tumors that are larger than 5 cm, rapidly increasing in size, located in the deep layer, or painful, should be considered to be STS. Moreover, if STS is suspected, the patient should be referred to a specialized facility for further evaluation and appropriate treatment. However, because of the rarity and histologic diversity of the disease, UEs are often performed in STS patients at non-specialized facilities, especially when the patient’s lesion is small and/or superficially located.7,10,13 Currently, UE in STS patients occurs in 10–53% of cases.2,14–16
The approach to the treatment of STS after UE is not well established. The effectiveness of radiotherapy, which is often used after UE, is unclear because radiotherapy alone for STS has been reported to be associated with a high recurrence rate.4,17 Re-resection with appropriately wide margins should therefore be recommended. Determining safe surgical margins after UE by computed tomography and/or MRI can be difficult because of postoperative reactive changes or hemorrhage,9 and even contrast-enhanced MRI has been reported to have only 78% accuracy for evaluating residual disease.14 The main objective of extensive re-resection is the achievement of negative margins in additionally resected specimens, because positive margins have been associated with a high rate of local recurrence.18,19 In the present study, a significantly higher rate of local recurrence was observed in two of the three additionally resected specimens with positive margins (66.7%), but in just three of the 21 specimens with negative margins (14.3%).
Amputation or plastic reconstruction has been reported at a higher rate after UE than after PE for primary STSs.3,7,8,10,18 In our study, although the rate of amputation did not significantly differ between the PE and UE groups, plastic reconstruction was required proportionally more often in the UE group than in the PE group, in line with previous reports.3,7,8,18
Various publications have reported on the prognosis of patients undergoing UE or PE.3,6,7,10,20 Smolle et al reported that, compared with PE, re-resection after UE was associated with improved OS and decreased distant metastasis, although the risk for local recurrence was similar.7 Other authors reported that OS, LRFS, and MFS were not significantly different in patients who underwent either UE or PE for high-grade sarcoma.3,21 Conversely, Saeed et al reported decreased LRFS and DFS for UE compared with PE in STS patients.20 In the present study, OS, LRFS, and DFS were not significantly different between the UE and PE groups. MFS was significantly better in the UE group than in the PE group; however, in the analysis of lesions arising in the superficial layer, no significant difference between the groups was observed. Based on the latter finding, the poorer MFS in the PE group might be attributable more lesions in the PE group being located in the deep layer. As in most previous reports, prognosis in the UE and PE groups was similar when additional resection after UE resulted in negative margins; the higher number of small and superficial lesions in the UE group may be related to that group’s relatively good prognosis.3,6,7,10
Residual tumor cells in the additionally resected specimens were reported at a rate of 58%– 83%,3,5,14,18,19 and have frequently been described as a poor prognostic factor in patients who underwent UE.4–6 In the present study, residual tumor cells in the additionally resected specimens were observed in 15 patients (62.5%), but did not affect their prognoses. Garnet et al reported that a primary tumor size larger than 5 cm was associated with a higher rate of metastasis in patients who underwent UE,17 and other authors reported that routine postoperative radiotherapy after UE could achieve better local control.6,22 In our study, univariate analysis revealed that an age of 61 years or greater at primary tumor diagnosis and a primary tumor size of 2.9 cm or greater were poor prognostic factors in the UE group; however, no factor was prognostic in the multivariate analysis.
Our study had several limitations. First, owing to the retrospective design, we cannot exclude the possibility of selection bias. Second, the UE group included all patients who underwent additional resection, but not those who received other therapies such as chemotherapy and radiotherapy. Third, because STS is a rare cancer, the sample size was small. Furthermore, there should be a bias of patients’ background between two groups due to the wide variety of histologic subtypes. Multicenter studies with larger samples, meta-analyses, and systematic reviews will therefore be helpful in elucidating prognostic factors in STS patients who undergo UE.
Conclusion
In the present study, when an appropriate additional resection for STS patients who had undergone UE was achieved, their prognoses were similar to that in patients who had undergone PE. Nevertheless, UE should be avoided, especially in older patients and in those with larger STS. We confirmed that STS patients after UE can benefit from appropriate additional surgeries by specialized orthopaedic oncologists. Therefore, if STS is suspected, the patients should be referred to a specialized sarcoma facility.
Acknowledgments
We would like to thank Editage for editing and reviewing this manuscript’ for the English language.
Disclosure
The authors report no conflicts of interest connected to this work.
References
1. Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200–229. doi:10.3322/caac.21605
2. Grimer R, Parry M, James S. Inadvertent excision of malignant soft tissue tumours. EFORT Open Rev. 2019;4(6):321–329. doi:10.1302/2058-5241.4.180060
3. Traub F, Griffin AM, Wunder JS, et al. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer. 2018;124(19):3868–3875. doi:10.1002/cncr.31648
4. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer. 2003;97(10):2544–2553. doi:10.1002/cncr.11367
5. Nakamura T, Kawai A, Sudo A. Analysis of the patients with soft tissue sarcoma who received additional excision after unplanned excision: report from the bone and soft tissue tumor registry in Japan. Jpn J Clin Oncol. 2017;47(11):1055–1059. doi:10.1093/jjco/hyx123
6. Bianchi G, Sambri A, Cammelli S, et al. Impact of residual disease after “unplanned excision” of primary localized adult soft tissue sarcoma of the extremities: evaluation of 452 cases at a single Institution. Musculoskelet Surg. 2017;101(3):243–248. doi:10.1007/s12306-017-0475-y
7. Smolle MA, Tunn PU, Goldenitsch E, et al. The prognostic impact of unplanned excisions in a cohort of 728 soft tissue sarcoma patients: a multicentre study. Ann Surg Oncol. 2017;24(6):1596–1605. doi:10.1245/s10434-017-5776-8
8. Tokumoto H, Akita S, Kubota Y, et al. Effect of unplanned excision of soft tissue sarcomas on skin defects and reconstructive procedures. J Plast Surg Hand Surg. 2020;54(6):372–376. doi:10.1080/2000656X.2020.1799817
9. Kaste SC, Hill A, Conley L, et al. Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop Relat Res. 2002;397:204–211. doi:10.1097/00003086-200204000-00025
10. Charoenlap C, Imanishi J, Tanaka T, et al. Outcomes of unplanned sarcoma excision: impact of residual disease. Cancer Med. 2016;5(6):980–988. doi:10.1002/cam4.615
11. Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53(282):457–462. doi:10.1080/01621459.1958.10501452
12. Kanda Y. Investigation of the freely available easy-to-use software `EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi:10.1038/bmt.2012.244
13. Pollack A, Zagars GK, Goswitz MS, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys. 1998;42(3):563–572. doi:10.1016/S0360-3016(98)00277-6
14. Gingrich AA, Elias A, Michael Lee CY, et al. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J Surg Res. 2017;208:26–32. doi:10.1016/j.jss.2016.08.096
15. Lewis JJ, Brennan MF. Soft tissue sarcomas. In: Sabiston DC, editor. The Biological Basis of Modern Surgical Practice. New York: Saunders; 1999:528–534.
16. Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78(5):650–655. doi:10.2106/00004623-199605000-00003
17. Garnet P, Nelson J, Wallace N, et al. Analysis of the effect of the size and grade of soft tissue sarcoma on rates of unplanned resection, metastatic disease, mortality, and morbid re-resection over 20 years. Orthopedics. 2021;44(3):166–171. doi:10.3928/01477447-20210104-05
18. Potter BK, Adams SC, Pitcher JD Jr, et al. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res. 2008;466(12):3093–3100. doi:10.1007/s11999-008-0529-4
19. Chandrasekar CR, Wafa H, Grimer RJ, et al. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90(2):203–208. doi:10.1302/0301-620X.90B2.19760
20. Saeed H, King DM, Johnstone CA, et al. Preoperative radiation therapy followed by reexcision may improve local control and progression-free survival in unplanned excisions of soft tissue sarcomas of the extremity and chest-wall. Int J Surg Oncol. 2016;2016:5963167. doi:10.1155/2016/5963167
21. Morattel B, Mustaki L, Montemurro M, et al. Oncological outcome, functional results and costs after unplanned excision of musculoskeletal soft tissue sarcoma. Eur J Surg Oncol. 2020;46(5):898–904. doi:10.1016/j.ejso.2020.01.025
22. Beane JD, Yang JC, White D, et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014;21(8):2484–2489. doi:10.1245/s10434-014-3732-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



