Back to Journals » International Journal of General Medicine » Volume 16
Clinical Observation of Uninterrupted Thrombolytic Therapy via Indwelling Catheter for Lower Limb Deep Vein Thrombosis
Authors Liu H, Wang R, Zhang L, Shi J, Yao J
Received 12 April 2023
Accepted for publication 10 June 2023
Published 15 June 2023 Volume 2023:16 Pages 2493—2501
DOI https://doi.org/10.2147/IJGM.S416814
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Woon-Man Kung
Haoyuan Liu,1 Rurong Wang,1 Liang Zhang,1 Jingming Shi,1 Jiaxi Yao2
1Department of Intervention, Hexi University Affiliated Zhangye People’s Hospital, Zhangye City, Gansu Province, 734000, People’s Republic of China; 2Institute of Urology, Hexi University, Zhangye City, Gansu Province, 734000, People’s Republic of China
Correspondence: Jingming Shi, Department of Intervention, Hexi University Affiliated Zhangye People’s Hospital, 67 Xihuan Road, Zhangye, Gansu, 734000, People’s Republic of China, Email [email protected] Jiaxi Yao, Institute of Urology, Hexi University Affiliated Zhangye People’s Hospital, 67 Xihuan Road, Zhangye, Gansu, 734000, People’s Republic of China, Email [email protected]
Purpose: Observe uninterrupted thrombolytic therapy via indwelling catheter for lower limb deep vein thrombosis.
Methods: We retrospectively studied data from 32 patients with lower extremity deep vein thrombosis who received comprehensive treatment, consisting of general treatment, inferior vena cava filter implantation, interventional thrombolysis, angioplasty, stenting, and post-operative monitoring.
Results: The efficacy and safety of the comprehensive treatment were observed for a follow-up period of 6– 12 months. The treatment was 100% effective; patient results indicated no serious bleeding, acute pulmonary embolism, or death after surgery.
Conclusion: The combination of intravenous and healthy side femoral vein puncture and directed thrombolysis to treat acute lower limb deep vein thrombosis is safe, effective, and minimally invasive while still achieving a good therapeutic effect.
Keywords: deep vein thrombosis, uninterrupted thrombolytic, interventional, stent implantation
Introduction
Deep vein thrombosis (DVT) of the lower extremity refers to abnormal coagulation of blood in the deep vein cavity, which leads to obstruction of blood return, swelling, pain and dysfunction of the lower limb.1,2 Abscission of a thrombus can cause pulmonary embolism (PE). If DVT is not effectively treated in the early acute stage, venous valve dysfunction is often a lasting result, called post-thrombotic syndrome (PTS).3 The clinical efficacy and safety of the classical treatment of lower limb DVT, such as simple anticoagulation, surgical thrombectomy, and systemic thrombolysis, are not satisfactory. Anticoagulation can inhibit the spread of a thrombus and reduce the risk of recurrence of PE and DVT but it cannot directly accelerate thrombolysis. In recent years, with the development of interventional technology, catheter directed thrombolysis (CDT) for DVT has become a more developed and commonly used treatment method. CDT can promote the directional clearance of a lower limb DVT, restore venous patency, and reduce the risk of venous valve insufficiency. CDT can also reduce bleeding complications and increase thrombolysis efficiency, which is more effective than systemic thrombolysis.4,5
Meta analysis shows that CDT has a higher thrombolytic rate than anticoagulation alone and can reduce the incidence of DVT recurrence and PTS. CDT approach veins include the contralateral femoral vein, jugular vein, tibial vein, great saphenous vein, and small saphenous vein.6–8 There are few reports on the efficacy of CDT, and there are differing reports on the application of the technology, thrombolytic dosage and method, postoperative monitoring indicators, and complications. To further evaluate the difference in efficacy between conventional treatment and CDT for patients with lower limb DVT, we reviewed data from 32 patients who received comprehensive treatment of acute lower extremity DVT and interventional catheter insertion at our hospital between January 2016 and March 2017.
Materials and Methods
General Information
Data was collected from 32 patients with DVT, including 11 males and 21 females, ranging in age from 38 to 80 years with an onset time from 4 h to 15 days. Within the selected DVT patient group, there were eight postpartum cases, seven cases with malignant tumors, eight cases without obvious inducement, three cases with recent traumatic fractures, and six cases involving surgical operations (hip and knee joint replacement, abdominal surgery, and gynecological myomectomy). Plasma D-dimer, plasma fibrinogen (FIB), plasma activated partial thrombo time (APTT), vascular ultrasound, and lower limb deep vein angiography were assessed before surgery. Patients were evaluated and treated per the process portrayed in Figure 1. All patients provided written informed consent.
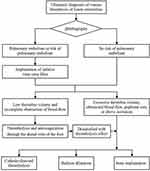 |
Figure 1 Flow chart of treatment for lower limb venous thrombosis. |
General Treatment
Patients were instructed to rest in bed, raise the affected limb by 30 cm, perform foot and ankle exercises, and massage the soft tissues to promote venous return and reduce the swelling of the affected limb.
Inferior Vena Cava Filter (IVCF) Implantation
To prevent pulmonary embolism during the operation or thrombolysis treatment, an IVCF was implanted in all patients before thrombolysis. Thirteen patients were diagnosed with acute pulmonary embolism by chest-enhanced computed tomography before thrombolysis, three patients were diagnosed with a floating thrombosis in the iliofemoral vein by intraoperative angiography, and two patients were found to have common iliac vein thrombosis and distal inferior vena cava thrombosis. Five patients were not received IVCF due to age (>70 years), and one patient was not successfully received IVCF due to excessive tortuosity of the vena cava.
Interventional Thrombolytic Therapy
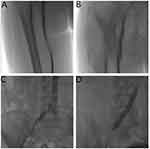
Before the operation, tourniquets were tied at the ankle and above the knee of the affected side, a contrast agent was injected into the dorsal foot vein, and deep and superficial veins were displayed in lower limb phlebography to visualize valve function and the specific location and scope of thrombosis. During the operation, 24 patients underwent popliteal vein puncture. Of these, eight patients had a large number of popliteal vein thrombosis and no popliteal vein access was found, so an opposite femoral vein puncture was performed. During the operation, a guide wire and 4F catheter were used to go up the popliteal vein to the superficial femoral vein, common iliac vein, and the lower part of inferior vena cava. Alternatively, the opposite femoral vein was used and the guide wire and catheter crossed the common iliac bifurcation to enter the affected limb and slowly entered the thrombotic segment of the affected limb through the venous valve. Angiography was performed section by section to further define the location and scope of the thrombus. After measuring the length of the thrombus, the corresponding length of thrombolytic catheter was placed in the thrombotic segment and urokinase was injected. The perfusion segment was retained in the area of the thrombosis, bound, and fixed (Figure 2).
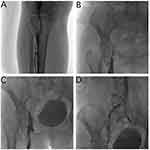 |
Figure 2 Radiographic image before thrombolysis. (A) Popliteal vein thrombosis. (B and C) Iliac vein occlusion. (D) Guide wire recanalization angiography showed numerous thrombi in the iliac vein. |
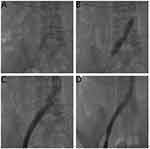
Uninterrupted thrombolysis consisted of a continuous urokinase micro pump of 40,000 IU/h and continuous pumping of heparin sodium into the vascular sheath at 500 U/h. FIB concentration and APTT were monitored every 4 hours. The thrombolytic effect was observed through thrombolytic catheter angiography every 24 h, and the catheter perfusion section was promptly adjusted to maintain its location in the thrombus. In the thrombolytic process, when FIB was <1.5 g/l, urokinase was reduced by half to 20,000 IU/h, and when FIB was <1 g/l, urokinase was stopped. Cold precipitation was infused when FIB was <0.5 g/l, FIB was supplemented, and bleeding was prevented. When APTT was >60 s, heparin sodium was halved to 250 U/h; when APTT was >90 s, heparin sodium was stopped. When re-examination angiography showed an unobstructed deep vein or no progress in thrombolysis in two consecutive angiographies, thrombolysis and anticoagulation were suspended (Figure 3). The thrombolytic treatment time lasted 48–72 h.
Angioplasty and Stent Implantation
After 48–72 h of thrombolysis, venography was rechecked, and balloon dilation or stent implantation for the stenosis and occluded segments were performed, including 21 cases of simple balloon dilation with a balloon diameter of 8–12 mm. In cases where an angiogram after balloon dilation showed that the stenosis of the venous lumen was still >50%, stents were implanted. All patients with stents were treated with WALLSTENT™ closed-loop dense mesh stents, with a diameter of 10–14 mm and a length of 9–12 cm (Figure 4).
Postoperative Treatment
A dose of 4000 U of low-molecular-weight heparin sodium was administered once every 12 h after discontinuation of the heparin sodium provided during surgery. Initial anticoagulant doses of warfarin, aspirin, and clopidogrel (Plavix) were 3 mg, 100 mg, and 75 mg, respectively; oral doses of these medications were taken concurrently to prevent platelet aggregation. Three days after surgery, the international standardized ratio of prothrombin time (PT-INR) was reviewed; when it reached 1.5–2.5 times the normal value, the dose of warfarin was adjusted according to the measured value. When the INR reached 2–3 times the normal value, the use of low-molecular-weight heparin sodium was discontinued but warfarin, aspirin, and Plavix were continued. Plavix was discontinued after 3 months but the dosages of warfarin and aspirin were maintained. Re-examination of lower limb phlebography indicated the discontinuation of warfarin in the absence of thrombosis recurrence or progression; however, aspirin was recommended for more than 12 months. For those over 50 years of age or with risk factors of thrombotic disease, lifelong aspirin use was recommended. For patients over 70 years of age, obvious bleeding or thrombotic recurrence during the period of taking warfarin, 15 mg/day of rivalsaban was administered orally for anticoagulation. All patients wore medical elastic stockings for 2 years after their operation.
Criteria for Efficacy Determination
Patient results were divided into four categories based on the efficacy of their thrombolysis procedure: cured, remarkably effective, effective, and ineffective.
A patient was considered cured when the circumferential diameter difference at 15 cm below the knee of both lower limbs was less than 1 cm, the affected limb swelling had disappeared, the skin color was normal, and activity was unrestricted. Also, lower limb phlebography showed that the deep vein wall was smooth and unobstructed.
A remarkable effect was considered when the circumference difference at 15 cm below the knees of both lower limbs was 1–2 cm, the swelling of the affected limb was significantly reduced, and the sense of heaviness and fatigue was significantly alleviated. Venography of the lower limbs showed that most of the deep veins were reopened, and the pipe wall was only slightly unsmooth.
The thrombolysis procedure was considered effective when the circumference difference at 15 cm below the knee of both lower limbs was more than 2 cm, the swelling of the affected limb was slightly relieved, additional symptoms were slightly relieved, phlebography indicated that part of the deep veins of the lower limb were reopened, and a small amount of collateral circulation was established.
Ineffective thrombolysis was defined as the circumference difference at 15 cm below the knee of both lower limbs was more than 2 cm, the swelling of the affected limb was not relieved, additional symptoms were not relieved, phlebography indicated that the deep veins of the lower limb were not obviously reopened, and the collateral circulation was not obvious.
Results
Among 32 patients, 21 underwent balloon dilation alone and 11 underwent balloon dilation combined with stent placement, and the technical success of all procedures was 100%. The plasma D-dimers continued to increase 24 and 48 hours after admission for treatment, and decreased after 72 hours. FIB decreased 24 and 48 hours after treatment, and increased after 72 hours. APTT continued to increase 24 and 48 hours after treatment, and slightly decreased after 72 hours (Figure 5).
 |
Figure 5 Changes in plasma D-dimer, FIB, and APTT levels within three days after patient admission for treatment. (A) D-dimer level change. (B) FIB level change. (C) APTT level change. |
After one, three, and six months post-operative periods, intravenous angiography was performed again and treatment results were classified; 28 cases were cured, three cases were considered remarkably effective, one case was deemed effective, resulting in a total effective rate of 100%. There were no cases of serious bleeding, acute pulmonary embolism, death, or other complications after each operation. Four patients had gingival bleeding and epistaxis during thrombolysis, which disappeared after adjustment of their medication. Subsequent follow-up with 30 patients (two patients lost the follow-up) with color ultrasound reexamination found two patients with thrombosis in the stent, which was determined to be caused by poor compliance, and insufficient anticoagulation and antiplatelet after the operation. In both cases, the thrombus disappeared after repeated catheter thrombolysis.
Discussion
At present, the incidence rate of DVT is increasing year over year, with more than 600,000 new DVT patients in the United States annually.9,10 The prevalence and diagnosis rates of DVT in China have also been increasing in recent years. Thus, it is important to use effective measures to diagnose and treat this condition to improve the prognosis of the growing number of DVT patients. At present, there is no uniform standard for effective thrombolytic methods and drug dosage for the treatment of acute DVT. The focus of standard DVT treatment is the use of anticoagulants to prevent pulmonary embolism and dissolve the thrombosis. However, anticoagulants alone have no direct thrombolytic effect. At present, conventional DVT treatment often fails to restore venous patency, and venous valve function can be permanently damaged.11,12
Due to the poor efficacy of anticoagulant therapy alone and systemic thrombolytic therapy, and the possible serious complications for patients with DVT, it was proposed in the 1990s that a thrombosis should be directly exposed to thrombolytic agents through CDT. Compared with peripheral venous thrombolysis and surgical thrombectomy, seamless catheter thrombolysis and anticoagulation treatment for acute DVT have high thrombolytic efficiency and a low bleeding risk. This method continuously gives thrombolytic drugs in the thrombotic segment, reducing the thrombolytic exposure time. Regular monitoring and timely adjustment of medication mitigates the bleeding risk. It has the advantages of a high local drug concentration, rapid thrombolysis, low postoperative thrombosis recurrence rate, low bleeding risk, and is minimally invasive.13 The advantages of CDT for the patient include a reduction in thrombosis, dosage of lysate, hospitalization time, and complications. Additionally, CDT provides timely symptom relief and maintains venous patency and valve function to prevent PTS. Du et al reported that anticoagulation plus CDT can more effectively improve venous patency and prevent PTS than anticoagulation alone.14–16 CDT is usually used to treat acute femoral vein thrombosis, but few studies have reported the efficacy and safety of CDT, especially in the treatment of acute extensive lower extremity DVT. In contrast to proximal DVT, whether anticoagulation or other methods should be used for the treatment of distal DVT remains controversial.
Severe DVT is usually treated with catheter thrombolysis and mechanical thrombus clearance through anticoagulation. Meta analysis shows that intracatheter thrombolysis is more effective than standard anticoagulant therapy alone and can improve deep vein patency and significantly reduce the incidence of PTS. In our study, it was safe and effective to use a comprehensive treatment scheme to treat lower limb DVT, and the patients had no significant serious complications.17–19 With the development of interventional technology, multiple interventional thrombolytic therapies will be used together to restore venous patency and accelerate the thrombolytic process and effect, which is the future trend of interventional thrombolysis.
The recurrence rate of DVT is 2.6–29%. It is reported that the incidence of PTS after distal DVT is as high as 23%.20 Yang et al reported that the main causes of PTS were venous obstruction and venous valve insufficiency.21 Therefore, prevention is crucial. Auxiliary technologies such as ultrasound assisted CDT and percutaneous mechanical thrombectomy combined with CDT can more effectively and quickly accelerate thrombolysis, reduce the dosage of thrombolytic agents, and shorten the hospital stay.21–24 However, these treatments increase the financial burden on the patient. Our study shows that CDT treatment is a feasible, effective, and safe method. The overall patency was better than with standard treatment and did not significantly increase the economic burden on patients.
IVCF implantation is effective in preventing a fatal pulmonary embolism. While the indications and complications of IVCF implantation have always been a concern, more reasonable indications include symptomatic pulmonary embolism, large floating thrombosis in the iliofemoral vein, and contraindications of anticoagulation therapy.25 IVCF implantation can not only prevent a fatal pulmonary embolism, but also provides a safety guarantee for interventional catheter thrombolytic therapy.26 We believe that after thrombolysis, the stenosis of the deep vein lumen is more than 50%, and balloon dilation combined with stent implantation should be done with caution. The stent itself can cause damage to the inner wall of the blood vessel, produce an inflammatory reaction, or cause endothelial hyperplasia. Platelet aggregation can cause stenosis in the stent, which slows down blood flow and can cause thrombosis recurrence, which affects the long-term efficacy of the treatment.27 After catheter thrombolysis and endovascular stent implantation, it is necessary to continue anticoagulation and antiplatelet therapy and wear elastic stockings for an extended period to prevent thrombosis recurrence, promote venous valve function recovery, and reduce the incidence of PTS.28 In our follow-up there were two patients with recurrent DVT in their iliac vein stents, both of which were related to insufficient anticoagulation. Therefore, effective anticoagulant therapy and regular monitoring and re-examination of various indicators during foot therapy are the key to maintaining long-term patency and preventing thrombosis recurrence.
This study has some limitations. As a single center retrospective study and an initial project in our unit, the number of cases is relatively small. Moreover, our study also lacked a standard control group and comparative indicators to determine the specific advantages of our CDT treatment. Despite these limitations, our study results support the treatment of acute lower limb deep vein thrombosis with a combination of intravenous and healthy side femoral vein puncture and directed thrombolysis as a safe, effective, and minimally invasive method with a good therapeutic effect.
Conclusion
It is safe, effective, and minimally invasive to treat acute DVT of lower extremities through popliteal vein and healthy femoral vein puncture and deep vein seamless thrombolysis combined with endovascular angioplasty. We believe this treatment is worthy of routine clinical consideration among physicians.
Ethics Approval and Consent to Participate
The study was performed according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Hexi University affiliated Zhangye People’s Hospital (Zhangye, China). Written informed consent was obtained from the patient.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
All authors contributed significantly to the work reported, in terms of the conception, study design, execution, data acquisition, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by grants from Hexi University President Fund Innovation Team Project (CXTD2022012).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stubbs MJ, Mouyis M, Thomas M. Deep vein thrombosis. BMJ. 2018;360:k351. doi:10.1136/bmj.k351
2. Aydin E, Bademci MS, Kocaaslan C, Denli Yalvac ES, Oztekin A. Complications of iliofemoral deep venous thrombosis treatment with AngioJet pharmacomechanical thrombectomy system. J Vasc Surg Venous Lymphat Disord. 2020;8(3):496. doi:10.1016/j.jvsv.2019.11.017
3. Pikovsky O, Rabinovich A. Prevention and treatment of the post-thrombotic syndrome. Thromb Res. 2018;164:116–124. doi:10.1016/j.thromres.2017.07.008
4. Chiarello MA, Sista AK. Catheter-directed thrombolysis for submassive pulmonary embolism. Semin Interv Radiol. 2018;35(02):122–128. doi:10.1055/s-0038-1642041
5. Avgerinos ED, Chaer RA. The ATTRACTiveness of catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord. 2018;6(3):303. doi:10.1016/j.jvsv.2018.02.002
6. D’Auria S, Sezer A, Thoma F, et al. Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm Circ. 2020;10(1):2045894019898368. doi:10.1177/2045894019898368
7. Fleck D, Albadawi H, Shamoun F, Knuttinen G, Naidu S, Oklu R. Catheter-directed thrombolysis of deep vein thrombosis: literature review and practice considerations. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S228–S237. doi:10.21037/cdt.2017.09.15
8. Xing Z, Tang L, Zhu Z, Hu X, Kirchmair R. Effects of thrombolysis on outcomes of patients with deep venous thrombosis: an updated meta-analysis. PLoS One. 2018;13:e0204594. doi:10.1371/journal.pone.0204594
9. Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Primers. 2015;1(1):15006. doi:10.1038/nrdp.2015.6
10. Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320(15):1583–1594. doi:10.1001/jama.2018.14346
11. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743–770. doi:10.1016/j.emc.2017.06.003
12. Birhan A, Assefa T, Beyene A, Ndayishimiye P, Woldu MA. Outcome of acute deep venous thrombosis using standard treatment versus thrombolytics: a literature review. Int J Hematol Oncol Stem Cell Res. 2019;13:201–207.
13. Tarazi M, Bashir A, Khan K, Kakani N, Murray D, Serracino-Inglott F. A literature review and case series of DVT patients with absent IVC treated with thrombolysis. Ann Vasc Surg. 2020;67:521–531. doi:10.1016/j.avsg.2020.03.021
14. Du GC, Zhang MC, Zhao JC. Catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of proximal deep vein thrombosis – a meta-analysis. Vasa. 2015;44:195–202. doi:10.1024/0301-1526/a000430
15. Liu G, Zhao Z, Cui C, et al. Endovascular management of extensive lower extremity acute deep vein thrombosis with AngioJet rheolytic thrombectomy plus catheter-directed thrombolysis from contralateral femoral access. Phlebology. 2019;34:257–265. doi:10.1177/0268355518790407
16. Choi YJ, Kim DH, Kim DI, Kim HY, Lee SS, Jung HJ. Comparison of treatment result between anticoagulation alone and catheter-directed thrombolysis plus anticoagulation in acute lower extremity deep vein thrombosis. Vasc Specialist Int. 2010;35:28–33. doi:10.5758/vsi.2019.35.1.28
17. Loss L, Stefanopoulos S, Siddiq A, et al. Catheter directed lysis and thrombectomy are equally effective for extensive deep vein thrombosis. J Surg Res. 2019;244:540–546. doi:10.1016/j.jss.2019.06.083
18. Wang H, Qi X, Luo H, Zhang Q, Chen Y, Sun J. Catheter-directed thrombolysis through anterior tibial vein for treating acute extensive deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2018;6:681–688. doi:10.1016/j.jvsv.2018.04.013
19. Eckenrode G, Baltich nelson B, Belarmino A, et al. Meta-analysis and systematic review of interventional therapy versus anticoagulation for isolated femoropopliteal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2019;7:272–276. doi:10.1016/j.jvsv.2018.10.017
20. Schwarz T, Buschmann L, Beyer J, Halbritter K, Rastan A, Schellong S. Therapy of isolated calf muscle vein thrombosis: a randomized, controlled study. J Vasc Surg. 2010;52(5):1246–1250. doi:10.1016/j.jvs.2010.05.094
21. Yang B, Xu XD, Gao P, et al. Catheter-directed thrombolysis via small saphenous veins for treating acute deep venous thrombosis. Med Sci Monit. 2016;22:2972–2980. doi:10.12659/MSM.897016
22. Klein AJ, Shishehbor MH. Ultrasound-assisted catheter directed therapy (CDT) for pulmonary embolism versus standard CDT: sounds of a cry for data! Vasc Med. 1019;24:248–250. doi:10.1177/1358863X19838346
23. Rao G, Xu H, Wang JJ, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute pulmonary embolism: a multicenter comparison of patient-centered outcomes. Vasc Med. 2019;24:241–247. doi:10.1177/1358863X19838334
24. Engelberger RP, Schroeder V, Nagler M, et al. Enhanced thrombolysis by ultrasound-assisted catheter-directed thrombolysis and microbubbles in an in vitro model of iliofemoral deep vein thrombosis. Thromb Haemost. 2019;119(07):1094–1101. doi:10.1055/s-0039-1688973
25. Elias M, Elias A, Oropello J, et al. Outcomes and prognosis factors in patients with vena cava filters in a quaternary medical center: a 5-year retrospective analysis. J Intensive Care Med. 2019;27:0885066619890324.
26. Jerjes-Sanchez C, Rodriguez D, Navarrete A, et al. Inferior vena cava filters in pulmonary embolism: a historic controversy. Arch Cardiol Mex. 2017;87:155–166. doi:10.1016/j.acmx.2017.01.007
27. Goktay AY, Senturk C. Endovascular treatment of thrombosis and embolism. Adv Exp Med Biol. 2017;906:195–213.
28. Che H, Liu G, Yu Y, Sang G, Zhang X. Guidance of venous stent implantation after catheter-directed thrombosis in patients with acute left lower extremity deep venous thrombosis based on pressure gradient differences between the iliac vein and Inferior vena cava: a single-center retrospective study. Ann Vasc Surg. 2019;59:217–224. doi:10.1016/j.avsg.2018.12.088
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.